The association between symmetric dimethylarginine concentrations and various neoplasms in dogs and cats
Abstract
Following the introduction of the symmetric dimethylarginine (SDMA) immunoassay, cases were reported where the SDMA concentration was markedly increased above the reference interval (RI) with neither concurrent increases in serum creatinine (Cr) concentrations nor clinical signs of kidney disease. Many of these animals were also concurrently diagnosed with cancer, most commonly lymphoma. The purpose of the study was to evaluate the association of increased SDMA in dogs and cats with lymphoma and other cancers as compared with age- and breed-matched non-tumour controls. In this retrospective case–control study, serum chemistry results from 1804 tumour cases, and age- and breed-matched non-tumour control animals were used. Matched-pair odds ratios between animals diagnosed with neoplasms and non-tumour controls for dichotomized SDMA values were determined by tumour type. SDMA concentrations were significantly higher in dogs and cats with lymphoma (p < .0001) compared with non-tumour controls. The odds ratio for increased SDMA concentrations in dogs with lymphoma was 10.0 (95% CI, 5.98–16.72) and for cats with lymphoma was 3.04 (95% CI 1.95–4.73). A significant number of canine and feline lymphoma cases had an increased SDMA concentration not associated with an increased Cr concentration (p < .001). Canine and feline lymphoma patients have an increased odds of having a SDMA concentration above the RI at diagnosis. Further characterization and evaluation of dogs and cats with lymphoma is required to help understand the mechanism(s) and the clinical significance of these alterations.
1 INTRODUCTION
Symmetric dimethylarginine (SDMA) is a biomarker of excretory renal function. Serum SDMA concentrations have been shown to be well correlated to glomerular filtration rate (GFR) in dogs and cats.1-3 Over 90% of the SDMA produced is excreted through renal clearance.4, 5 In dogs and cats with renal disease, SDMA can increase above the reference interval (RI) earlier in the course of disease than serum creatinine (Cr).2, 3, 6 With the commercial introduction of the SDMA test, occasional reports from practicing veterinarians were made to the laboratory describing cases where the SDMA concentration was markedly increased above the RI (≥1.24 μmol/L) with neither concurrent increases in Cr concentrations nor reported clinical signs of kidney disease. In reviewing these cases, a number of these animals were concurrently diagnosed with cancer, most commonly lymphoma.
Kidney dysfunction in cases of both Hodgkin's and non-Hodgkin's lymphoma has been reported in human patients, including acute kidney injury (AKI), neoplastic infiltration of kidney parenchyma, and a wide range of neoplastic-induced nephropathies.7-10 Reductions in estimated GFR, along with clinical signs ranging from no renal impairment to acute renal failure have been reported in human patients.8-10 Lymphoma in dogs is morphologically similar to human non-Hodgkin's lymphoma, with extranodal disease occurring in the skin, digestive tract, lungs, spinal cord, spleen, tonsils and eyes,11, 12 Renal infiltration and neoplastic-induced clinical renal disease have also been reported in end-stage multicentric lymphoma.13, 14 The majority of feline lymphomas are extranodal with gastrointestinal lymphoma predominating and nasal and renal lymphoma also considered common.15-18
SDMA is a byproduct of the intranuclear arginine methylation by; specifically, the type II protein arginine methyltransferase (PRMT) enzymes, PRMT5 and PRMT9.19, 20 PRMT5 is the main enzyme responsible for the symmetric dimethylation of arginine, is ubiquitously expressed and is responsible for methylation of histone proteins and a number of transcription factors, playing a large role in the regulation of transcription.21-23 Proteolysis of the methylated proteins usually occurs at a constant rate, and SDMA is released from the cell into circulation.24 In mouse studies, PRMT5 plays a role in primordial germ cell, keratinocyte, muscle, and nerve cell differentiation, and is essential for B cell development and antibody responses.21, 25 PRMT5 ensures cell survival, proliferation, and/or differentiation. Over-expression of PRMT5 has been reported in a number of human tumour types, including leukaemia and non-Hodgkin's lymphoma, and is associated with increased tumour cell survival in lymphoma and solid organ tumours.21, 26-29
Because of the reports of unusual SDMA and Cr levels seen in animals with cancer and the findings of renal dysfunction associated with lymphoma, a retrospective study to evaluate the association between increased SDMA in dogs and cats with various cancers, including lymphoma, was conducted.
2 MATERIALS AND METHODS
2.1 Database
The anatomic and clinical pathology databases of a commercial diagnostic laboratory (IDEXX Laboratories, Inc., Westbrook, ME, USA) were used in this study. All canine samples submitted between 1 January and 31 July 2016 and feline samples submitted between 1 January and 31 December 2016 were accessed and searched in this study. All pathology and blood samples used in this retrospective study were obtained by a practicing veterinarian during the normal diagnostic workup and monitoring of the patients in her or his care with owner consent. All rights necessary to perform this study were granted to IDEXX Laboratories under the terms of the applicable service agreements. The study was approved by the IDEXX Laboratories Animal Welfare Review Committee and complies with its guidelines. To ensure privacy, additional demographic information on the pet owner, or veterinarian who submitted the sample were not collected.
2.2 Case definition
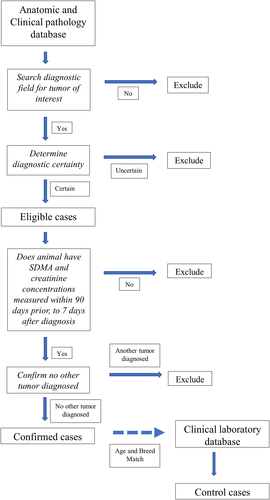
A flow diagram of the case selection process if provided in Figure 1. Newly diagnosed cases of the following canine and feline tumour types were included: canine lymphoma/lymphosarcoma (multicentric), canine lipoma (subcutaneous), canine hemangiosarcoma (visceral/retroperitoneal), canine mammary carcinoma (primary), canine mammary adenocarcinoma (primary), feline lymphoma/lymphosarcoma (multicentric/visceral), and feline mast cell tumour (visceral). All cases were diagnosed by a board-certified anatomic or clinical pathologist following excisional biopsy and histopathology, or fine-needle aspiration and cytology, respectively.
2.3 Selection of cases
A free-text word search of the diagnostic interpretation field of the pathology report was conducted using an on-line, open-source analytics and visualization platform.30 Search terms used for identification of potential cases are listed in Supporting Information Table S1. All cases were required to include the tumour type of interest, along with the tumour inclusion terms within the diagnostic interpretation section of the pathology report. Tumours not matching these criteria were excluded from analysis. In an effort to include only cases with definitive pathologic diagnoses, cases were excluded if any terms of diagnostic uncertainty (e.g., suspect, consistent, presumptive, emerging, concerning, probable, possible, favour, suspicious, supportive or suggestive) were included in the diagnostic interpretation.31, 32 Reports that included the terms recur, recurrent, or recurrence were also excluded as evidence of prior sampling. Cases were then matched to their clinical laboratory database and only animals with a serum chemistry with SDMA and Cr concentrations measured on the same blood sample within the period of 3-months prior to 7 days after the date of diagnosis were used for further analysis.
The pathology report and accession record from each case animal were then reviewed to confirm that all inclusion criteria were met. Cases were excluded if more than one tumour type was identified on the pathology report or evidence of a previous biopsy or cytology confirming a diagnosis of cancer was found in the animal's accession record. If more than one measure for SDMA and Cr occurred during the 3-month period prior to or the 7-day period after diagnosis, result closest to the date of diagnosis was used in the analysis.
2.4 Selection of controls
All dogs and cats having SDMA and Cr concentrations measured on the same sample in an IDEXX reference laboratory during the study period were eligible. Control dogs and cats were randomly age- and breed-matched to cases on a one-to-one basis. Breed was matched exactly. Cases where breed was not listed were matched to control dogs or cats where breed was not listed. In cases where age could not be matched exactly, a dog or cat of the same breed with the closest age within 6 months (above or below) was selected. Control dogs were only matched for sex for canine mammary adenocarcinoma and canine mammary carcinoma cases. Controls were not matched to neuter status. Each control animal's accession record was then reviewed to confirm signalment and inclusion criteria.
2.5 Serum chemistry
SDMA was determined using a commercially available high throughput immunoassay (IDEXX SDMA Test, IDEXX Laboratories, Inc.). An increased SDMA concentration was defined as >0.69 μmol/L for both dogs and cats (SDMA reference interval for dogs and cats: 0–0.69 μmol/L). Cr concentrations were determined by a colorimetric method, Jaffe's reaction using picrate at alkaline pH (Beckman Coulter, Inc. Diagnostics Division, Brea, CA, 92821, United States). An increased Cr concentration was defined as >132.6 μmol/L for dogs and 203.3 μmol/L for cats (Cr reference interval for dogs: 44.2–132.6 μmol/L; Cr reference interval for cats: 79.6–203.3 μmol/L).
2.6 Statistical analysis
Descriptive statistics were performed on signalment parameters. Normality was tested by the Shapiro–Wilk test. SDMA and Cr concentrations were compared by cancer type between cases and controls using the Wilcoxon signed rank test. The Wilcoxon rank sum test was used to compare the distributions of SDMA between cases and controls. Matched-pair odds ratios (ORs) between animals recently diagnosed with neoplasm and age- and breed-matched controls for dichotomized SDMA values (i.e., within or above the RI) were determined by tumour type.33 All calculations were performed using JMP software (JMP, Version 13. SAS Institute., Cary, NC, 27513, United States).
3 RESULTS
Following case review, 6704 potential cases were identified of which 1803 qualified for entry into the study: 307 canine lymphoma/lymphosarcoma cases, 212 canine lipoma cases, 230 canine hemangiosarcoma cases, 388 canine mammary adenocarcinoma cases, 387 canine mammary carcinoma cases, 224 feline lymphoma/lymphosarcoma cases, and 55 feline mast cell tumour cases. Summary statistics for each tumour type are shown in Supporting Information Table S2. For all tumour types, the average time from measurement of SDMA and Cr to diagnosis was ≤15 days prior to diagnosis.
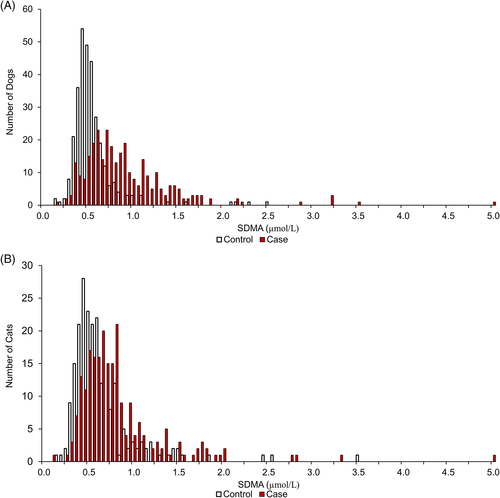
To determine if there was a relationship between SDMA and/or Cr and tumour status, SDMA and creatinine measures were compared between cases and controls for each cancer type. SDMA concentrations were significantly higher in dogs and cats with lymphoma (p < .0001) (Table 1). The distribution of SDMA for cases and control are illustrated in Figure 2. SDMA concentrations were significantly lower in dogs with lipoma, mammary adenocarcinoma and mammary carcinoma (p ≤ .008) when compared with controls. Creatinine concentrations were significantly lower in dogs with mammary adenocarcinoma and mammary carcinoma and in cats with lymphoma (p < .0001) when compared with controls (Table 1). Results in United States Conventional Units are shown in Supporting Information Table S3 and Figure S1.
| Tumour type | SDMA μmol/L (range) | Cr μmol/L (range) | ||||
|---|---|---|---|---|---|---|
| Case animals | Control animals | p value | Case animals | Control animals | p value | |
| Canine hemangiosarcoma | 0.54 (0.1–1.53) | 0.49 (0.2–2.97) | .136 | 79.6 (17.7–265.2) | 79.6 (26.5–875.2) | .105 |
| Canine lipoma | 0.44 (0.1–1.24) | 0.49 (0.15–2.03) | .004 | 79.6 (26.5–229.8) | 79.6 (26.5–415.5) | .099 |
| Canine lymphoma | 0.79 (0.15–4.94) | 0.49 (0.15–2.47) | <.0001 | 79.6 (17.7–406.6) | 79.6 (35.4–919.4) | .897 |
| Canine mammary adenocarcinoma | 0.44 (0.1–1.33) | 0.49 (0.1–2.82) | .006 | 61.9 (26.5–327.1) | 70.7 (26.5–724.9) | <.0001 |
| Canine mammary carcinoma | 0.44 (0.1–2.22) | 0.49 (0.05–4.94) | .008 | 61.9 (26.5–229.8) | 79.6 (17.7–574.6) | <.0001 |
| Feline lymphoma | 0.69 (0.1–4.94) | 0.54 (0.15–3.46) | <.0001 | 114.9 (44.2–1007.8) | 123.8 (53.0–795.6) | <.0001 |
| Feline visceral mast cell tumour | 0.64 (0.25–1.93) | 0.54 (0.25–4.35) | .566 | 132.6 (53.0–716.1) | 132.6 (44.2–539.3) | .826 |
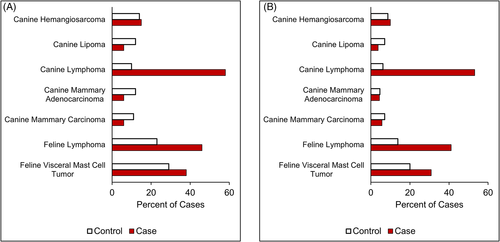
The ORs and 95% confidence intervals (CI) for increased SDMA concentrations and tumour type are found in Table 2. In both cats and dogs with lymphoma, the odds of an increased SDMA concentration within the period around diagnosis were significantly higher than in matched controls (p < .001). The OR for lymphoma in dogs or cats was >3. Additionally, a significant number of feline and canine lymphoma cases had an increased SDMA concentration not associated with an increased Cr concentration (92/224 for cats, 163/307 for dogs, p < .001); Cr measures remained within the RI (Figure 3, Supporting Information Table S4). Conversely, canine lipoma, mammary adenocarcinoma and carcinoma cases had decreased odds of an increased SDMA concentration (OR <0.5, p ≤ .013). There was no association between an increased SDMA concentration and canine hemangiosarcoma or feline visceral mast cell tumour.
| Cancer type | N | OR (95% CI) | p |
|---|---|---|---|
| Canine lymphoma | 307 | 10.00 (5.98–16.72) | p < .001 |
| Feline lymphoma | 224 | 3.04 (1.95–4.73) | p < .001 |
| Feline visceral mast cell tumour | 55 | 1.63 (0.67–3.92) | p = .275 |
| Canine hemangiosarcoma | 230 | 1.11 (0.66–1.87) | p = .691 |
| Canine mammary carcinoma | 387 | 0.49 (0.28–0.84) | p = .009 |
| Canine mammary adenocarcinoma | 388 | 0.41 (0.231–0.71) | p = .001 |
| Canine lipoma | 212 | 0.39 (0.18–0.85) | p = .013 |
4 DISCUSSION
This study evaluated the association between SDMA concentrations and various tumour types in dogs and cats at the time of diagnosis. Although the tumours studied do not represent all those seen in clinical practice, they were chosen based on anecdotal reports of an association with increased SDMA concentrations, tumour mass and growth rates, and the likelihood of metastasis.11, 15, 16, 34-39
Increased SDMA in the absence of increased creatinine at the time of diagnosis is seen in approximately 50% of canine and feline lymphoma patients. The association was not seen in other tumour types in our study. Similar findings have been observed in a case series of dogs with lymphoma, where 72% of untreated dogs had disproportionately increased serum SDMA concentrations compared to other renal biomarkers40 Additionally, a recent prospective study of human patients with haematological malignancies, including non-Hodgkin's lymphoma, also found significant increase in SDMA concentrations in patients where renal function was not significantly affected.41 To date, there have been no reports of increased SDMA concentrations associated with lymphoma in cats.
Cases of canine lipoma, canine mammary adenocarcinoma, and canine mammary carcinoma had significantly lower concentrations of SDMA compared to controls. This difference in SDMA concentrations is most likely driven by the higher number of control dogs having both increased concentrations of SDMA and Cr (Supporting Information Table S4) and not due to decreased SDMA production by dogs with these tumours. The decreased OR for animals with these tumours was an unexpected finding and is not readily explained. Lipomas were originally chosen to represent a common benign tumour of dogs, and no association with SDMA or Cr concentrations was anticipated. A previous study found that dogs with mammary tumours had a higher rate of glomerulopathies on histopathology even without azotemia; unfortunately, SDMA was not measured.42 The association between renal disease and canine mammary tumours warrants further study.
Three possible etiologies could explain the increased SDMA concentrations seen in approximately 50% of dogs and cats with lymphoma: (1) reductions in GFR due to the infiltration of the kidney parenchyma by tumour cells or tumour-induced nephropathies; (2) overproduction of SDMA directly by the tumour or due to tumour factors stimulating protein turn-over; (3) or, less likely, reduced clearance of SDMA by the kidneys. Lymphomatous infiltration of the kidneys has been reported in one third of human patients with diffuse lymphoma at autopsy.7 However, most of the patients did not show clinical signs of renal disease and renal infiltration is not identified or suspected before death.7, 8 Renal infiltration and neoplastic-induced clinical renal disease has been reported to occur in end-stage multicentric lymphoma in dogs.13, 14 Kidney biopsies were not performed on the animals in this study, so we cannot rule out microscopic invasion of the kidneys and cannot evaluate what effect such potential invasion would have on serum SDMA or kidney function. Further study with more complete characterization of renal parameters and biopsies would be beneficial to understanding whether microscopic renal invasion or other renal pathologies contribute to increased SDMA in dogs and cats with lymphoma.
Alternately, overproduction of SDMA in tumour cells could explain the increased SDMA in the absence of increased Cr noted in approximately half of the lymphoma patients. The over-expression of PRMT5 has been associated with increased cell proliferation and survival in both solid and blood lymphoid cancers.27-29, 43 Higher serum or plasma SDMA has been reported in human cancer patients,41, 44 and is associated with poor survival in patients with acute myeloid leukaemia.41 Recently, over-expression of PRMT5 in canine lymphoma tissues compared to normal or hyperplastic lymphoid tissue has also been demonstrated.45 Additionally, in vitro cultured canine lymphoma cells lines that overexpress PRTM5 have increased SDMA and have reductions in intracellular SDMA with PRMT5 inhibition.43, 46, 47 To our knowledge, there have been no studies that evaluate both SDMA serum concentrations and concurrent tumour PRMT5 expression in humans, laboratory or pet animals. Further study would be needed to evaluate whether overproduction of SDMA by tumour tissue plays a role in the serum SDMA elevations seen in dogs and cats with lymphoma or other PRMT5 over-expressing tumours.
Selective reduction of SDMA clearance caused by changes in glomerular charge and size selectivity would be a less likely explanation for the increased SDMA values seen in these animals. In diabetic patients, alterations in glomerular charge and size selectivity have been shown to result in a decrease in selectivity, not an increase, resulting in microalbuminuria.48-50 The authors know of no mechanism through which glomerular selectivity could be increased.
There are a number of limitations with this study. Case selection was limited to evaluation of the diagnostic interpretation, and only cases with a high likelihood of diagnostic certainty were included in this analysis. This selection bias likely eliminated additional tumour cases from the analysis. Cases diagnosed by aspiration and cytology were not required to have confirmation by histopathology, considered the gold standard for diagnosis. Although all cases were diagnosed by a board-certified pathologist, this may have resulted in a few cases misclassified. As this is a retrospective big data study, we were also limited by the lack of clinical information for each case or control animal. We were unable to determine if clinical conditions that are known to affect GFR and the concentration of Cr (e.g., hydration status, concurrent diseases, cachexia) were present. Muscle scores or the level of muscle wasting were not available for these animals. Decreased muscle mass in these animals could have resulted in the low Cr relative to the increased SDMA concentrations. Unfortunately, urine specific gravity data was not available for most cases, which would have been helpful for assessing renal impairment. Urine samples were submitted within the diagnostic time window for <20% of the canine cases and <15% of the feline cases.
In this study we have demonstrated that canine and feline lymphoma patients have an increased odds of a SDMA concentration above the RI at diagnosis compared to patients with other tumour types. ORs represent the strength of the association between a risk factor (tumour) and outcome (SDMA concentration above 69 μmol/L). Further characterization and evaluation of dogs and cats with lymphoma is required to help understand the mechanism(s) and the clinical significance of these alterations; however, there is potential that SDMA may have clinical utility in identifying early renal impairment in lymphoma patients or as a biomarker for lymphoma progression, prognosis or response to therapy. Investigating any association between PRMT5 tumour expression and serum SDMA concentration or association between renal tumour infiltration and serum SDMA concentration could provide valuable insight into the likelihood of SDMA overproduction as a causal factor for increased concentration in these cases and elucidate possible associations with tumour cell lineage. Ultimately, additional studies are needed to evaluate the presence of renal injury and determine renal function by measuring GFR to inform our understanding of the underlying mechanisms affecting serum SDMA concentrations in dogs and cats with lymphoma. Further exploration of the relationship between PRMT5 overexpression and SDMA is an intriguing area for future research which could result in improved understanding of lymphoma pathophysiology, as well as novel applications for SDMA, namely as a cancer biomarker.
CONFLICT OF INTEREST
The authors have an affiliation to the commercial funders of this research, as employees of IDEXX Laboratories. The work presented in this study was supported by IDEXX Laboratories, Inc. Westbrook, ME, USA (https://www.idexx.com/en/about-idexx/).
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article