Long-term survival of dogs with stage 4 oral malignant melanoma treated with anti-canine PD-1 therapeutic antibody: A follow-up case report
Funding information: JSPS KAKENHI, Grant/Award Number: 21H04754
Abstract
A monoclonal antibody targeting programmed cell death-1 (PD-1) is one of the most promising treatments for human cancers. Clinical studies in humans demonstrated that the anti-PD-1 antibody provides a long-lasting tumour response. Previously, we established an anti-canine PD-1 therapeutic antibody (ca-4F12-E6), and the pilot clinical study demonstrated that the antibody was effective in dogs with oral malignant melanoma (OMM). However, two OMM cases were still undergoing treatment when the pilot study was published. Here, we describe the long-term follow-up of those two cases. Although both cases showed long-term survival with complete response (CR), the tumour response differed; the effect onset was slow in one case and a durable response was observed in the second case even after treatment discontinuation. Secondary malignant tumours occurred during treatment in both cases. This follow-up study revealed that ca-4F12-E6 maintains CR in dogs for more than 1 year. In addition, the pattern of tumour response was unique compared to conventional therapy. These results indicate that new evaluation criteria for tumour response may be necessary for immunotherapy in veterinary medicine. Long-term follow-up is necessary regardless of the short-term treatment responsiveness.
1 INTRODUCTION
Canine oral malignant melanoma (OMM) is one of the most malignant tumours due to the high metastatic rate to the lungs and sentinel lymph nodes. Canines with OMM without metastasis are effectively treated with a combination of surgery and radiation. The median overall survival times (MST) for stage I and II canine OMM are 758 and 278 days, respectively. However, stage III and IV canine OMM have poor prognoses (MST: 163 and 80 days, respectively), even with radiation therapy and surgical resection or systemic chemotherapy.1 Conventional chemotherapy exhibits limited efficacy in dogs with advanced OMM2; therefore, the development of an effective systemic treatment is needed.
Immunotherapy is one of the most promising systemic treatments for tumours. Programmed cell death 1 (PD-1) is a suppressive immunoreceptor expressed on activated antigen-specific T-cells which are exposed to various antigens. Tumours can escape host immunity via programmed death 1 ligand (PD-L1) expression, which lead to T-cell dysfunction. PD-1/PD-L1 are immune checkpoint molecules.3 Human clinical trials demonstrate that immune checkpoint inhibitors (ICIs) exhibit a remarkable clinical efficacy in a significant minority of melanoma patients.4, 5
In recent veterinary studies, immunohistochemical staining revealed that PD-L1 is expressed in more than 70% of canine OMM cases6-8; thus, canine OMM is a good candidate for ICI therapy, similar to human melanoma. However, only a few reports focus on the clinical relevance of ICIs in canine tumours. Igase et al and Maekawa et al showed that a tumour response is observed in approximately 20% of stage IV canine OMM cases treated with an anti-canine PD-L1 antibody or an anti-canine PD-1 antibody.6, 9 These treatments prolonged MST compared with the treatment response in historical controls. However, little information is available concerning long-term survival. In previous pilot clinical study,9 two OMM cases were still undergoing treatment with the anti-PD-1 antibody at the time the paper was submitted. Here, we describe the long-term follow-up results of these two cases.
2 MATERIALS AND METHODS
The detailed design of this clinical trial has been described previously.9 The objectives of that study were to assess the safety, antitumor activity and overall survival time (OS) in dogs receiving anti-canine PD-1 antibodies. Between 21 April 2017 and 8 April 2020, 30 dogs with various treatment-resistant, refractory or advanced-stage tumours were enrolled. Seventeen cases were OMM stage IV. When the pilot study was prepared for publication on 8 April 2020, treatment of two OMM cases (Case 28 and Case 30) was ongoing.9 The tumour response was assessed by the investigators according to the response evaluation criteria for solid tumours in dogs (cRECIST v1.0).10 The OS was defined as the time from the initial treatment until death or at the end of this study (18 February 2022). The canine therapeutic monoclonal antibody ca-4F12-E6 was administered at 3 mg/kg as an intravenous infusion for 1 h every 2 weeks for each 10-week treatment cycle. Treatment was discontinued if the owner withdrew the dog from the study due to unacceptable adverse events, disease progression or deterioration of the dog's condition. Historical controls included 23 stage IV OMM cases treated with standard regimens, human tyrosinase DNA vaccine or oncolytic virotherapy at Yamaguchi Animal Medical Center (YUAMEC) from 1 March 2016 to 4 February 2020, as described previously.9
3 RESULTS
Case 28 is a 9-year-old male Pug with a history of stage IV OMM with metastasis to the lung after surgery and external beam radiation therapy for the primary oral mass. At the time of study inclusion, the primary oral tumour was well-controlled, but four lung metastatic lesions were identified by computed tomography. Two of the four masses were defined as target lesions and the others were defined as non-target lesions. On day 267 (the time of the manuscript submission9), four treatment cycles were completed. One of the target lesions gradually increased while all other lesions regressed. At that time, case 28 was judged to be progressive disease (PD). However, with continuous treatment, the enlarged target lesion regressed by day 352 (Figure 1). After six cycles of treatment (day 422), complete response (CR) was achieved, and the dog survived for >2 years (day 954) since the beginning of the clinical trial with continuous treatment. During the anti-PD-1 antibody treatment, mast cell tumours developed on the skin on days 201 and 453 and were removed by surgery. At the owner's request, we decided to continue the anti-PD-1 antibody treatment. On day 840, a new tumour mass was found near the oral mucosa, where the primary melanoma had been located. The mass was surgically removed and the pathology was determined. Nonepithelial tumour cells were detected, but unlike the primary melanoma, melanin production was not observed in the tumour cells. In addition, melanoma markers, including PNL2 and Melan A, were negative by immunohistochemistry. Although the possibility of melanoma recurrence was not completely excluded, the new oral tumour appears to be different from the original melanoma.
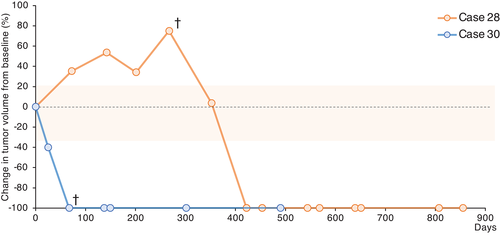
Case 30 was a 12-year-old male Labrador Retriever with a history of treatment-naïve stage IV OMM. The primary oral mass and lung metastasis were defined as the target and non-target lesions, respectively, at the first visit. After the first treatment cycle with ca-4F12-E6, complete tumour regression of the target lesion was observed (at day 67). The lung metastases regressed. However, the lung metastases could still be observed by x-ray. After the second treatment cycle, the oral mass remained in complete regression and the lung metastases sizes decreased more. However, during the third cycle of treatment, hyperproteinemia (total protein, 12.3 g/dl; reference interval [RI], 5.0–7.2 g/dl) and hypercalcemia (14.5 mg/dl; RI, 9.3–12.1 mg/dl) occurred, although the blood albumin concentration (3.5 g/dl; RI, 2.6 4.0 g/dl) was normal. Multiple myeloma was suspected, however, the owner did not want further examinations, such as bone marrow aspiration, and decided to stop any further treatment including the clinical trial. The dog did not return to the hospital until 6 months later. On day 301, hyperproteinemia continued but no recurrence of the oral mass was observed and the lung metastases were completely regressed (Figure 1). The dog did not return to the hospital again and died of weakness on day 490. According to the necropsy, melanoma cells were not detected in the oral cavity, lung, lymph node or liver. In contrast, the bone marrow, spleen, abdominal lymph node and liver were infiltrated with plasma cells. In addition, myocardial fibrosis and membranous glomerulonephritis caused by hyperglobulinemia were detected. Therefore, the cause of death in case 30 may be the progression of a plasma cell tumour and cardiovascular disease.
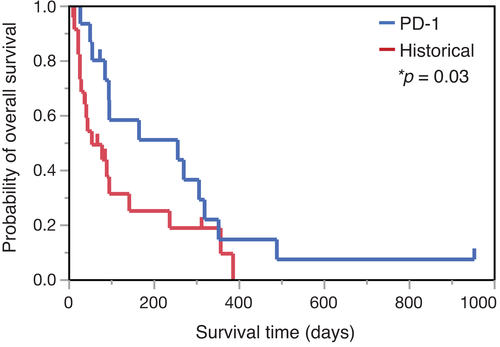
Kaplan–Meier curves for OS in dogs with stage 4 OMM were updated with the data from these two cases (Figure 2), both of which were censored in our previous clinical study.9 Previously, the OS for cases 28 and 30 were reported to be 267 and 93 days, respectively. In the present follow-up study, case 28 was still alive on day 954 and case 30 died on day 490.
4 DISCUSSION
Recent advancements in systemic immunotherapy affect canine OMM,6, 11-13 although CR is rare in cases of advanced-stage disease. In this follow-up study, we demonstrated that the anti-PD-1 antibody ca-4F12-E6 resulted in CR in two stage 4 OMM cases, which lasted for more than 1-year. However, so far, no prognostic markers have been identified for tumour shrinkage or long-term survival in dogs treated with ICIs. Previous clinical study of anti-canine PD-L1 antibody suggested that high levels of plasma C-reactive protein (CRP, cutoff value: 2.55 mg/dl) and the low lymphocyte to monocyte ratio (LMR, cutoff value: 1.41) were correlated to poor prognosis in canine OMM.6 However, in the present two cases, these blood biomarkers were not predictors of long survival time (data not shown). Nevertheless, further studies will be necessary to investigate the utility of blood biomarkers as prognostic predictors in canine OMM cases.
The pattern of tumour response to ICIs is quite different from conventional therapies, such as chemotherapy and other kinds of molecular-targeted therapies. The tumour response to ICI treatment is long-lasting in responsive patients. In long-term follow-up studies in humans, survival curves for OS after anti-PD-1 antibody treatment exhibited a tail plateau, which is not observed after conventional therapy.14-16 In Figure 2, the median OS in the anti-PD-1 treatment group was prolonged for 257 days (95% confidence interval [CI] 56–320), while the OS for the historical control group was 55 days (95% CI 27–143). Although the objective response rate for anti-PD-1 therapy in dogs was approximately 20% in our previous study, the survival times for cases 28 and 30 are consistent with the concept of long-term survival, as shown in human patients.
In case 28, one of the target masses had gradually increased while the others had regressed at the time of submission of our previous paper,9 similar to a mixed response in humans.17 Although case 28 was identified as a PD, further anti-PD-1 therapy induced complete tumour regression (Figure 1). This transient increase in the tumour lesion may represent pseudoprogression, which is occasionally observed in human studies.18, 19 An atypical tumour response, such as pseudoprogression, was observed in approximately 10% of human melanoma patients treated with pembrolizumab.20 Pseudoprogression is more likely to occur before 3-months.20 More importantly, the definition of pseudoprogression is not clear in human medicine. Therefore, the categorisation of this case as pseudoprogression is unclear.
Case 30 showed interesting treatment responsiveness. During the third cycle of treatment, we had to discontinue the treatment due to hyperglobulinemia despite a partial response (PR) tumour response. Nevertheless, when case 30 came back a year later, all the tumours were in CR. This suggests that once a good tumour response is observed, anti-ICI therapy provides long-term clinical benefits even after discontinuation. Similarly, human clinical studies of ICIs revealed that most patients who discontinued treatment due to adverse events still achieved prolonged CR or continued lack of progression during long-term follow-up.21-23 This long-term response may be due to the generation of memory adaptive antitumor immunity in different populations of immune cells.24, 25
The long-term survival induced by ICIs could increase the possibility of developing secondary cancer by prolonging life-span. Administration of ICIs is associated with a decreased incidence of secondary cancer, based on a large exhaustive database.26 However, some case reports describe secondary cancers in patients treated with ICIs.27-30 In the present study, case 28 developed mast cell tumours several times and a nonepithelial tumour other than melanoma. Case 30 had possible multiple myeloma during the treatment of OMM. In these dogs, the tumours were resistant to anti-PD-1 therapy. However, further clinical trials in dogs with various types of tumours are needed to understand the responsiveness of these tumours to anti-PD-1 therapy.
In conclusion, this follow-up study suggests that the therapeutic efficacy of the anti-PD-1 antibody ca-4F12-E6 can be maintained as a CR for more than 1 year. However, treatment with the anti-PD-1 antibody ca-4F12-E6 exhibited mixed responses or slow onset effects, suggesting that the usual use of cRECIST v1.010 to determine the efficacy of therapy may be unsuitable. The use of immune-related response criteria (irRC), immune-related RECIST (irRECIST) or immune RECIST (iRECIST) has been proposed for determining the effect of immunotherapy in medicine,31 and we suggest that a similar evaluation method is necessary for veterinary medicine. However, since each tumour histology has a different temporal progression characteristic and the time frame for “conformation of response” will vary depending on the progression time for a particular histology, the irRECIST criteria for that histology needs to be modified temporally. Therefore, further clinical study is required to develop new response criteria for canine cancers. Furthermore, long-term follow-up is also important, even if CR is not obtained.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the all YUAMEC clinical staff and the laboratory members for helping us succeed the present study. Finally, we thank the dogs and their owners for making this study possible.
FUNDING INFORMATION
This work was supported by JSPS KAKENHI Grant Number 21H04754.
CONFLICT OF INTEREST
Takuya Mizuno received research funding from Nippon Zenyaku Kogyo Co., Ltd. The remaining authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.