Toceranib phosphate in the treatment of canine thyroid carcinoma: 42 cases (2009-2018)
Abstract
Thyroid carcinoma is the most common endocrine malignancy in dogs. Thyroidectomy and radiation therapy control local disease, yet are not always feasible, and efficacious medical therapies need to be identified. Toceranib phosphate has been reported to provide clinical benefit (CB) in dogs with thyroid carcinoma, while its role in treatment-naïve thyroid tumours has not been well-described. The objective of this study was to describe the use of toceranib in the management of thyroid carcinomas in dogs in both the naïve-disease and prior therapy- settings. A medical record search identified 42 dogs diagnosed with thyroid carcinoma and treated with toceranib, of which 26 and 16 dogs were in settings of naïve-disease and after prior therapy, respectively. Twenty-three (88.4%) and twelve (75%) dogs experienced CB in the naïve and prior therapy settings, respectively. The median [95% confidence interval] progression free interval (PFI) for dogs in the naïve and prior therapy settings were 206 [106,740] and 1015 [92,1015] days, respectively. The median overall survival time (OST) for dogs in the naïve and prior therapy settings were 563 [246,916] and 1082 [289,1894] days, respectively. Overall, the data provided no evidence for differences in overall PFI (P > .20) or OST (P = .15) between settings. However, when asymptomatic at the time of diagnosis, dogs in the naïve setting showed poorer survival prognosis (estimated hazard ratio 17.2 [1.8, 163]) relative to dogs in the prior therapy setting. This study characterizes PFI, OST and CB with minimal AE in dogs with thyroid carcinoma treated with toceranib in both the naïve and prior therapy settings.
1 INTRODUCTION
Thyroid carcinoma is the most common endocrine malignancy in dogs.1-5 Local disease often accounts for the initial clinical signs in dogs with thyroid carcinoma, and either local progression and/or distant metastasis are reported as the cause of death.1, 6-9 Metastatic disease is reported in 16% to 38% of dogs at diagnosis, and 65% to 90% by the time of their death.1, 7-13 Therefore, there is a need to identify agents to provide clinical benefit in advanced-stage disease and reduce the risk of death as a result of disease progression.
Initial therapy for dogs diagnosed with thyroid carcinoma aims at local disease control, ideally thyroidectomy.1, 2, 9, 14, 15 Alternative or additional therapeutic interventions, such as external beam radiation therapy or radioactive iodine, are considered if surgery is infeasible, declined by the owner, or the dissected surgical margins are incomplete.15-21 These disease control measures may not address potential metastasis or the clinical complications of advanced-stage disease.
One therapeutic option is chemotherapy in the adjuvant setting, or as primary treatment for advanced stage disease.1, 2, 22-24 However, the existing literature does not demonstrate significantly improved outcomes.22, 23 Studies are necessary to better elucidate the role for conventional and/or novel chemotherapeutics in treating canine thyroid carcinoma.
In humans, several multikinase inhibitors have been utilized for the treatment of advanced-stage thyroid tumours.25-32 In veterinary medicine, the multikinase inhibitor toceranib phosphate* was approved by the Food and Drug Administration (FDA) after it was demonstrated to have biologic activity in the treatment of canine, recurrent or non-resectable, grade 2 or 3 mast cell tumours (MCTs).33-36 Additionally, responses were observed for a spectrum of solid tumour histotypes in a phase 1 study.36 Subsequent off-label investigations further supported a role for toceranib in the management of numerous canine malignancies.37-49 Moreover, the expression of multiple receptor tyrosine kinases (RTKs), including KIT, VEGFR, and PDGFR-α and -β, as well as activation of their downstream effector pathways, has been demonstrated in canine thyroid carcinoma.50-53
The primary objective of this study was to describe the use of toceranib in the management of thyroid carcinomas in dogs in both a naïve disease setting and in dogs that received prior therapies. Notably, evaluation of toceranib in the treatment of naïve-disease is lacking in the literature. Secondary objectives were to describe the adverse effects (AEs) in dogs being treated with toceranib for thyroid carcinoma, and to identify clinically-applicable potential prognostic factors.
2 METHODS
2.1 Case selection
The medical record databases of five university teaching hospitals were searched for cases in which toceranib was utilized to treat dogs diagnosed with thyroid carcinoma. The records of eligible cases included the following information; (a) cytologic or histologic diagnosis of thyroid carcinoma, (b) details of prior, concurrent, or subsequent therapies, (c) toceranib dose and schedule, and (d) at least one documented response assessment to toceranib.
Additional information collated from the records included; signalment, clinical signs and diagnostics performed at diagnosis, longest diameter of tumour measurement (cm), tumour mobility, the results of baseline clinicopathologic testing, thyroxine (T4) values, baseline blood pressure measurement, stage of disease at time of toceranib initiation, dose and dose interval of toceranib, AEs, duration of toceranib therapy, response to therapy, date of disease progression following toceranib, anticancer therapies prior to toceranib, concurrent anticancer therapies, subsequent anticancer therapies, duration of follow-up, comorbidities, concomitant medications, date and cause of death. Referring veterinarians and/or owners were contacted for additional follow-up details where required.
Tumour stage at the time of toceranib initiation was retrospectively designated according to the WHO TNM classification system (Table 1).54 Tumour size at diagnosis was based on recorded calliper measurements, and/or cervical ultrasound measurements, and/or computed tomography (CT) measurements. The presence or absence of locoregional metastasis at the time of toceranib initiation was based on fine needle aspirate biopsy (FNA) and cytological evaluation, and/or cervical ultrasound and/or computed tomography (CT) findings interpreted as probable metastasis by a board-certified radiologist. The presence or absence of distant metastasis was also based on radiographic and/or CT findings interpreted as probable metastasis by a board-certified radiologist.
| Staging group | Primary tumour | Regional lymph nodes | Distant metastasis |
|---|---|---|---|
| I | T1 a, b | N0 | M0 |
| II | T0 | N1 | M0 |
| T1 a, b | N1 | M0 | |
| T2 a, b | N0 or N1 a | M0 | |
| III | T3 | Any N | M0 |
| Any T | N1 b or N2 b | M0 | |
| IV | Any T | Any N | M1 |
- Abbreviations: a, freely movable; b, fixed; M0, no evidence of distant metastasis; M1, evidence of distant metastases; N0, no lymph node involvement; N1, ipsilateral lymph node involvement; N2, bilateral lymph node involvement; T0, microscopic residual disease; T1, <2 cm; T2, 2 to 5 cm; T3 > 5 cm.
AEs were retrospectively graded according to the Veterinary Co-operative Oncology Group – Common Terminology Criteria for Adverse Events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapies in dogs and cats v1.1.55
Endpoints were clinical benefit (CB), progression-free interval (PFI), and overall survival time (OST). Response to therapy was determined by retrospectively applying the Response Evaluation in Solid Tumours in Dogs v1.0 criteria (cRECIST v1.0) (Table S1).56 This was based on records of repeated calliper measurements of the primary tumour, and/or repeated thoracic imaging. Dogs were deemed to have CB if a complete response (CR), partial response (PR), or stable disease (SD) was documented for a minimum of 10 weeks while receiving toceranib.56
2.2 Statistical analysis
For each treatment setting, initial patient status was described utilizing frequency tables and summary statistics. Initial status was compared between treatment settings using chi-square or Wilcoxon rank sum tests, as appropriate depending on the nature of the response.
Time-to-event variables (ie, PFI and OST) were compared between treatment settings utilizing Kaplan-Meier curves for survival distributions. Censoring criteria were (lack of) progression diagnosis and death for PFI and OST, respectively.
Proportional hazards models were fitted to PFI and OST. The linear predictor included treatment setting, and considered the following explanatory variables, namely sex, age, tumour stage and tumour side, presence of clinical signs at the time of treatment decision, and all 2-way interactions. Purposeful selection of explanatory variables into the model containing the fixed effect of treatment setting was conducted using stepwise selection at a 10% significance level.
Variables such as concurrent therapy, supportive medication, and toceranib frequency regimen were not considered in the model as their realization followed (as opposed to preceded) subgroup assignment, and thus may interfere with associations involving treatment setting. For the final model selected for inference, the proportional hazards assumption was evaluated; there was no evidence to question such assumption. Point estimates and confidence intervals for proportional hazard ratios are provided.
Statistical analyses were conducted utilizing statistical software SAS (Version 9.4, Cary, NC), specifically procedures FREQ, LIFETEST and PHREG.
3 RESULTS
3.1 Patient population
The medical record search identified 47 dogs diagnosed with thyroid carcinoma and treated with toceranib, between January 2009 and July 2018. Forty-two dogs fulfilled the inclusion criteria. Three dogs were excluded from further evaluation because of insufficient follow-up information.
Two dogs were excluded because they received toceranib in the setting of microscopic disease.
Of 42 dogs, 26 and 16 were treated with toceranib in a naïve disease vs prior therapy setting. Of the dogs that received prior therapy, 8/16 had undergone thyroidectomy, 8/16 had received radiation therapy, 2/16 had undergone both thyroidectomy and radiation therapy, and 2/16 had received adjuvant cytotoxic chemotherapy (one dog received two cycles of alternating doxorubicin and carboplatin, one dog received carboplatin).
The study population was demographically diverse (Table 2). Clinical signs at diagnosis were recorded for all dogs (Table S2). Most dogs treated in the naïve setting presented with clinical signs (65.3%), compared with a minority of dogs in the prior therapy setting (P = .036). For the 19 dogs lacking clinical signs at diagnosis, a palpable mass in the ventral cervical region was noted upon presentation for unrelated reasons, prompting FNA and cytologic evaluation, resulting in the diagnosis of thyroid carcinoma. Miscellaneous comorbidities and concurrent medications were also recorded for the study population.
| Parameter | Naïve disease | Prior therapy | Significance |
|---|---|---|---|
| P-value | |||
| Age (years) | n = 26 | n = 16 | .41 |
| Median | 10 | 9 | |
| Range | 5-14 | 3-12 | |
| Sex | n = 26 | n = 16 | .61 |
| Males | 15 | 8 | |
| Females | 11 | 8 | |
| Weight (kg) | n = 26 | n = 16 | .96 |
| Median | 22.9 | 27.5 | |
| Range | 5.5-40.2 | 5.4-41.8 | |
| Clinical signs at diagnosis | n = 26 | n = 16 | .036* |
| Yes | 17 | 6 | |
| No | 8 | 12 | |
| Tumour size (cm) | n = 21 | n = 10 | .58 |
| Median | 5.7 | 5.1 | |
| Range | 1.4-10.5 | 1.5-16 | |
| Tumour mobility | n = 22 | n = 16 | NAa |
| Fixed | 17 | 10 | |
| Mobile | 5 | 5 | |
| Unknown | 4 | 3 | |
| Thyroxine (T4) level (nmol/L) | n = 12 | n = 11 | .92 |
| Median | 18.66 | 19.31 | |
| Range | 8.5-153.2 | 5.2-90.1 |
- a NA; not available—too few observations to perform formal testing.
- * Statistically significant difference at P ≤ .05.
3.2 Diagnosis and staging
Thyroid neoplasia of epithelial or neuroendocrine origin was diagnosed in all dogs by cytology, and confirmed by histopathology in a subset of seven. Of the seven available histopathologic diagnoses, there were three thyroid follicular carcinomas and one of each of the following: thyroid adenocarcinoma, thyroid follicular carcinoma with squamous differentiation, malignant carcinoma (presumed thyroid in origin), and thyroid carcinosarcoma. Twelve dogs had bilateral thyroid tumours, 12 left-sided, 10 right-sided, and two ectopic. In six dogs the tumour location and extent was not specified. Tumour mobility was described for 35 tumours; 27 were fixed and 8 freely mobile. These characteristics were either not significantly different between subgroups or there was inadequate information to enable formal statistical analysis (Table 2).
Out of the 42 total dogs, 22 in the naïve setting and 15 in the prior therapy setting had baseline thoracic radiographs taken; of these, 16/22 and 6/15, respectively, showed radiographic changes indicative of pulmonary metastasis. Four dogs, all in the prior therapy setting, did not have any pulmonary nodules at the time of initial diagnosis, but later had radiographic evidence of progressive pulmonary metastatic disease, contributing to the reason for commencing toceranib.
Seventeen dogs underwent a CT scan of the head/neck, +/− thorax (n = 8), +/− abdomen (n = 3). There was one dog with a concurrent intranasal mass, one with extradural and vertebral lesions that were later identified as thyroid carcinoma metastasis on cytology, and one with a concurrent right anal sac mass. Of the dogs that underwent a CT scan, there were four that had multiple pulmonary nodules, one with a solitary soft tissue pulmonary mass and large right pancreatic mass, and six with retropharyngeal lymph node enlargement identified. The dog with a concurrent right anal sac mass also had multiple hepatic and splenic nodules, and intra-abdominal lymphadenopathy that were all identified to have neuroendocrine morphology on cytology.
Eleven dogs in the naïve and six in the prior therapy setting had cervical ultrasound at diagnosis. Presumptive locoregional metastasis was identified in four in the naïve and one in the prior therapy setting. Three dogs had ipsilateral medial retropharyngeal lymph node enlargement, one dog had ipsilateral and contralateral medial retropharyngeal lymph node enlargement, and one dog had ipsilateral cervical lymph node enlargement. Two dogs underwent ultrasound-guided FNA of the enlarged lymph node(s), and the cytologic evaluation confirmed metastasis. One dog developed lymph node metastasis later, contributing to the reason for commencing toceranib.
WHO stage at the time of diagnosis and toceranib initiation was retrospectively determined for all dogs (Table 3). At diagnosis, 22 dogs (84.6%) in the naïve, and six (37.5%) in the prior therapy setting had metastatic disease. At the time of toceranib initiation,18 dogs (69.2%) in the naïve and 10 (62.5%) in the prior therapy setting had presumed pulmonary metastasis based on thoracic imaging. Additional locations for metastasis, confirmed by FNA or tissue biopsy, included lymph node(s) (n = 5), liver (n = 2), pancreas (n = 1), spleen (n = 1), L1 vertebral body (n = 1), and cervical subcutaneous tissues (n = 1).
| Prior therapy | Significance | ||
|---|---|---|---|
| WHO TNM stage | Naïve disease (n = 26) | (n = 16) | P-value |
| I-III | 6 | 6 | .13 |
| IV | 20 | 10 |
Records of baseline clinicopathologic analyses were available for 23 dogs in the naïve and 15 in the prior therapy setting. Most dogs had blood work performed regardless of clinical signs or subgroup allocation. Concurrent urinalyses were recorded for 29 dogs, with a urine protein creatinine ratio (UPC) available for six dogs. Eight of these dogs had inadequately concentrated urine and five were proteinuric based on either dipstick analysis or UPC. Baseline T4 values were recorded for 24/42 dogs (Table 2). Two dogs were considered hyperthyroid, with a T4 value exceeding 45.0 nmol/L; one in each setting. Two dogs were hypothyroid, with a T4 value less than 10.0 nmol/L; one in each setting.
3.3 Treatment
Twenty-six dogs received toceranib in the naïve setting. Of these dogs, one was included in a prior survey of toceranib activity in dogs with solid tumours.37 The median time between diagnosis and toceranib initiation for these dogs was zero days (range, 0-81 days). The median starting dose was 2.8 mg/kg (range, 1.5-3.6 mg/kg). Twenty dogs commenced toceranib according to an every other day (EOD) dosing schedule, and six dogs according to a Monday, Wednesday, Friday (MWF) dosing schedule.
Sixteen dogs had therapy for thyroid carcinoma prior to toceranib initiation. The median time between diagnosis and toceranib initiation for this setting was 84.5 days (range, 0-1176 days). One dog with stage II disease had a thyroidectomy and was initiated on toceranib in the adjuvant setting, after a definitive diagnosis was obtained. Six dogs commenced toceranib because of PD following prior therapy. The reason for toceranib therapy was not specified for the remaining 10 dogs, although all of these dogs had measurable disease at toceranib initiation. The median starting dose was 2.7 mg/kg (range, 2.1-3.0 mg/kg). Seven dogs received toceranib according to an EOD dosing schedule, and nine dogs in a MWF dosing schedule.
The toceranib dosing schedule differed between settings, with dogs in the naïve setting more frequently receiving toceranib EOD rather than MWF (P = .01).
Six dogs in the naïve and three dogs in the prior therapy setting received concurrent anticancer therapy, including metronomic cyclophosphamide (n = 4), carboplatin (1-3 doses, n = 2), doxorubicin (1 dose, n = 1), and palliative radiation therapy (2-5 fractions, n = 3).
3.4 Response to toceranib
All dogs treated with toceranib in the naïve setting had a response recorded. One dog (3.8%) experienced a CR, 11 (42.3%) PR, 11 (42.3%) SD, resulting in a CB rate of 88.4%. Three dogs (11.5%) had PD. Eighteen discontinued toceranib because of disease progression. Seven dogs discontinued toceranib for reasons unknown. One dog being treated with toceranib and concurrent carboplatin chemotherapy had SD at the time of data collection.
All dogs treated in the prior therapy setting had a response recorded. One dog (6.3%%) experienced a CR, three (18.7%) PR, and eight (50%) SD, resulting in a CB rate of 75%. Four dogs (25%) had PD. Four dogs in the prior therapy setting discontinued toceranib because of PD. One dog discontinued toceranib because of gastrointestinal toxicity, this dog had SD at the time of toceranib discontinuation. Ten dogs discontinued toceranib for reasons unknown. One dog was still taking toceranib at the time of data collection and continued to have SD.
3.5 Time-to-event outcomes: Kaplan-Meier survival curves
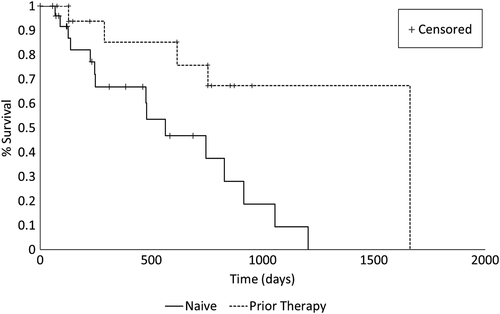
The estimated median overall PFI for dogs in the naïve and prior therapy settings were 206 (95% CI: [109, 790]) and 1015 [92, 1015] days, respectively. There was no evidence for differences between settings in the overall survival curves for PFI (P = >.20) (Figure 1). The probability of progression free by 500 days was estimated at .32 (SE [SE] ±0.11) and .64 (SE ±0.13) for naïve and prior therapy settings, respectively. The estimated median OST for dogs in the naïve and prior therapy settings were 563 [246, 916] and 1082 [289, 1894] days, respectively and there was no evidence for differences between settings in survival curves for OST (P = .15) (Figure 2). The probability of survival by 1000 days was estimated at .19 (SE ±0.11) and .71 (SE ±0.12) for the naïve and prior therapy settings, respectively.
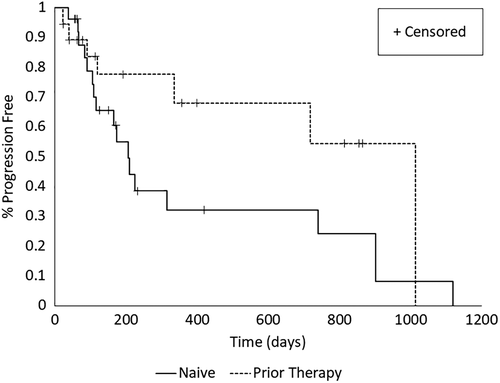
In the naïve disease setting, death attributed to PD was observed for 16 dogs, although details of PD were lacking for half of these dogs. In the absence of a death record, survival was censored in 10 dogs that were either lost to follow up (n = 6) or alive (n = 4) at the time of last assessment. Median time to survival censorship of 271.5 days (range, 69-687 days).
In the prior therapy setting, death was observed in eight dogs, five of which had undergone solely prior thyroidectomy, one solely radiation therapy, and two combination surgery and radiation therapy. In the absence of a death record, survival was censored in nine dogs that were either lost to follow-up (n = 5) or alive (n = 4) at the time of last assessment. Median time to survival censorship of 762 days (range, 146-952 days).
3.6 Time-to-event outcomes: Proportional hazards modelling
Proportional hazards with a stepwise model selection were used to refine inference on the outcomes and their association with disease settings. Potential prognostic explanatory variables (eg, sex, age, tumour stage, tumour location, presence of clinical signs) and all 2-way interactions for inclusion were considered. To prevent overcontrol bias, other variables (eg, type of prior therapy, concurrent therapy, supportive medication) were not considered for model selection as the realization of these variables happened at the time of subgroup assignment.57
For overall PFI, none of the potential prognostic explanatory variables considered showed any evidence of association with PFI (P = .15), as determined using stepwise selection. Moreover, there was no evidence for any difference between treatment settings in overall PFI (P > .20), as also indicated by Kaplan Meier survival curves.
For OST, a significant interaction was identified between treatment setting and presence of clinical signs at the time of subgroup assignment (P = .0008). Specifically, the hazards ratio (HR) for dogs in the naïve setting relative to those in the prior therapy treatment setting was estimated at 17.2 (95% CI: [1.8, 163.0]), provided that dogs were asymptomatic at the time of toceranib treatment. In contrast, if dogs showed clinical signs at the time of toceranib treatment, there was no evidence for a difference in survival time between settings (estimated HR 0.37 [0.10, 1.3]). These results indicated that asymptomatic dogs showed a poorer survival prognosis if they underwent toceranib treatment in the naïve disease setting compared with the prior therapy setting. By contrast, if dogs showed clinical signs at diagnosis, there was no evidence for differences in survival time between the treatment settings. There was no evidence of any association between survival time and any of the other proposed explanatory variables at a 5% level of significance (P > .15).
3.7 Adverse events
The most commonly reported AEs leading to a dose reduction and/or supportive medications were gastrointestinal (Table 4). A total of 15/26 and 10/16 dogs in the naïve and prior therapy settings, respectively, required a dose modification, whereas 22/26 and 14/16 dogs of the respective settings received supportive medications. Out of those dogs receiving supportive medication, 24/36 and 12/36 commenced toceranib according to an EOD and a MWF regimen, respectively. Supportive medications initiated during toceranib treatment included; famotidine (n = 14), omeprazole (n = 8), and metronidazole (n = 4), maropitant (n = 2), enalapril (n = 4), benazepril (n = 2), amlodipine (n = 1), and carprofen (n = 1). One dog developed hypothyroidism and was initiated on levothyroxine (0.02 mg/kg PO q12h).
| AE | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Gastrointestinal | ||||
| Anorexia | 11 | 5 | - | 1 |
| Diarrhoea | 14 | 3 | 1 | 1 |
| Nausea | 1 | - | - | - |
| Vomiting | 6 | 1 | 1 | - |
| Constitutional | ||||
| Fever | 1 | - | - | - |
| Weight loss | 5 | - | - | - |
| Lethargy | - | - | 1 | - |
| Hematologic | ||||
| Anaemia | 2 | - | - | - |
| Neutropenia | 10 | - | - | - |
| Biochemical | ||||
| Azotemia | 3 | - | - | - |
| Increased BUN | 2 | 3 | - | - |
| Increased ALP | 5 | 1 | 3 | - |
| Increased ALT | 2 | 2 | - | - |
| Increased AST | 3 | 1 | - | - |
| Hyperbilirubinemia | 1 | - | - | - |
| Increased CK | 2 | - | - | - |
| Hypoalbuminemia | 2 | - | - | - |
| Increased cholesterol | 1 | - | - | - |
| Hypokalemia | 1 | - | - | - |
| Hypocalcemia | 1 | - | - | - |
| Cardiovascular | ||||
| Hypertension | 9 | 1 | - | - |
| Musculoskeletal | ||||
| Lameness | - | 2 | - | - |
| Renal | ||||
| Proteinuria | 1 | 5 | 1 | - |
- Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; CK, creatinine kinase.
4 DISCUSSION
In veterinary medicine, the potential survival benefit of chemotherapy for canine thyroid carcinoma remains hypothetical. Prior retrospective studies have reported the utilization of conventional chemotherapies for the treatment of thyroid carcinoma in dogs.22-24, 58-61 Measurable tumour responses between 10 and 54% were reported, but significant improvements in PFIs and OSTs were not demonstrated.22-24, 58-61 There is also a small study reporting the administration of 9-cis-retinoic acid, a first generation retinoid, for the treatment of canine thyroid tumour.62 Overall, no studies have demonstrated improved measures of outcome. As the natural disease is acknowledged to be slowly progressive, SD is difficult to evaluate as representing CB. However, in certain clinical settings, such as advanced-stage disease, SD can be desirable.
The objective of this study was to describe the use of toceranib in the management of thyroid carcinomas in dogs in both naïve disease and prior therapy settings. The selection criteria and investigators' desire to document response likely contributed to bias towards inclusion of dogs with more advanced stage disease. Despite this, the majority of dogs (83.3%) treated with toceranib experienced CB.
The CB observed in this study would be expected from previous studies, which have suggested that dogs with thyroid tumours experienced CB with toceranib.36, 37, 58 Following the initial clinical trials for toceranib in dogs, observations of biologic activity lead to a study evaluating toceranib use for a spectrum of tumour types, including 15 thyroid carcinomas.36, 37 Eighty percent experienced CB, all dogs with thyroid tumours had measurable disease and majority received prior therapy.37 In another study, CB was documented in seven dogs with thyroid carcinoma treated with toceranib.38 Finally, in a phase I study evaluating combination toceranib and carboplatin therapy, two cases of thyroid carcinoma were included and both experienced CB.58 A unique aspect of our study was separation of dogs treated in a naïve disease setting from those treated with toceranib following prior anticancer therapies. Importantly, preliminary analysis indicated no evidence for any difference in PFI (P > .20) between the two treatment settings. However, a proportional hazards modelling approach indicated that asymptomatic dogs at the time of diagnosis had a poorer survival prognosis if treated under the naïve setting compared with the prior therapy treatment setting.
The benefit of toceranib for thyroid carcinoma in the setting of microscopic disease was not addressed in this study, and has yet to be determined. The authors attempted to delve into this issue, but the medical records obtained only identified two dogs with microscopic disease. In clinical practice, dogs with more advanced stage disease are often treated more aggressively, and as mentioned this is an assumed bias in this study. Thus, the benefit for toceranib in the adjuvant setting remains unknown. In veterinary medicine, the use of toceranib in the adjuvant setting for treatment of both osteosarcoma and hemangiosarcoma has not improved outcomes.63, 64 Given that CB in the macroscopic disease setting does not always translate into extended OST, this issue requires further investigation.
Even in human oncology, it is recognized that the response to molecularly-targeted agents observed in a macroscopic disease setting may not translate into as prolonged PFI and/or OST when utilized for microscopic disease.65-67 Recent meta-analyses examined the potential benefit of several receptor tyrosine kinase inhibitors (RTKIs) for neoplasms in the macroscopic disease setting and merely demonstrated variable trends towards improved outcomes.65-67 In summarizing their findings, the investigators uniformly recognized the need for further work to define optimal dosage regimens.65-67
Our study performed robust statistical analyses for potential prognostic associations. Previous reports of relevant prognostic factors for canine thyroid carcinoma have been inconsistent. Factors reported to be prognostic include size or volume of the primary tumour, number of lobes affected, tissue invasiveness, vascular invasion, histologic subtype and grade, and duration of clinical signs prior to diagnosis.8, 12-14, 22, 23 In our study, the only explanatory variable of prognostic value for OST was the presence of clinical signs at diagnosis. Interestingly, asymptomatic dogs treated with toceranib in the naïve disease setting had a poorer survival prognosis (ie, increased risk of death) compared with those in the prior therapy setting, as indicated by a hazards ratio significantly greater than the null value one. Although it is not possible to evaluate in this study, intervention with toceranib may have alleviated clinical signs in dogs that were symptomatic, yet may not have delayed tumour progression. Future studies of toceranib in various settings would ideally include an untreated control group, in order to elucidate toceranib's effect on PFI and OST in various treatment settings.
Gastrointestinal AEs were the most commonly reported in this population of dogs, which is consistent with previous reports of toceranib administration in dogs.36-49 No novel AEs were reported in our study. One concern was the development of iatrogenic hypothyroidism. Toceranib has previously been reported to alter thyroid hormone equilibrium in dogs.68 One dog in our study reportedly developed hypothyroidism. However, this dog also completed a course of hypofractionated radiation therapy 24 months prior to the documentation of hypothyroidism. A prior study reported hormonal dysfunction in 47.6% of dogs with both definitive and hypofractionated radiotherapy for thyroid carcinoma, which developed a median of 6 months following protocol completion.69 Hence, conclusions may not be drawn regarding the cause of hypothyroidism in our case.
The limitations of this study included biases inherent to retrospective clinical studies, such as an inability to randomize dogs into treatment settings and subsequent potential confounders, as well as a lack of treatment and follow-up standardization. In our study, diagnosis was predominantly based on cytologic evaluation. Since histologic subtype has been identified as a potential prognostic factor for dogs with thyroid tumours, absence of this information may have implications for interpretation of results in this study.12
A major limitation of the present study was the use of concurrent anticancer therapies in nine (21.4%) of the dogs that met the inclusion criteria. As mentioned previously, both palliative radiation therapy and cytotoxic chemotherapies have been previously described to result in measurable thyroid tumour responses in retrospective studies.16-19, 22-24, 58-61 Therefore, the CB of toceranib is less clear for this subset of dogs. Despite this limitation, the majority of the dogs included received toceranib as a single agent and experienced CB.
Dogs in our study also received a variety of supportive medications that potentially improved clinical signs and minimized AEs, thereby influencing treatment duration and outcomes. Finally, the limited number of dogs posed concerns regarding poor statistical power (ie, inability to detect real differences), although this was the largest study on record to characterize the use of toceranib for the management of thyroid carcinoma in dogs.
5 CONCLUSIONS
Majority of dogs treated with toceranib demonstrated CB with minimal AEs in both the naïve-disease and prior therapy settings. There was no evidence for any difference in PFI between the two treatment settings, although asymptomatic dogs treated in the naïve-disease setting had poorer survival prognosis compared with those that received prior anticancer therapies. To the best of the author's knowledge, this is the largest study to date characterizing the use of toceranib for the management of thyroid carcinoma in dogs. Further research is warranted, specifically on prospective, controlled randomized trials, to better delineate the benefit of toceranib for dogs with thyroid carcinoma in a spectrum of clinical settings.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.