Ultrasound is a poor predictor of early or overt liver or spleen metastasis in dogs with high-risk mast cell tumours
Abstract
Conflicting evidence exists regarding the importance of routine abdominal ultrasound (US) with hepatic and splenic fine needle aspiration (FNA) cytology during staging of canine mast cell tumours (MCT). The objective of this study was to correlate ultrasonographic and cytologic findings in dogs with strictly defined high-risk MCTs and to determine the influence on outcome. Our hypothesis was that US poorly predicts visceral metastasis in high-risk MCTs and that early metastasis is associated with improved outcome when compared to overt metastasis. US of liver and spleen correlated to cytologic results, categorized as no metastasis, early metastasis or overt metastasis. Of 82 dogs prospectively enrolled, 18% had early visceral metastasis and 7% had overt metastasis on cytology; 67% with visceral metastasis had regional LN metastasis. US was a poor predictor of metastasis with sensitivity, specificity, positive predictive value and negative predictive value for the spleen of 67%, 68%, 21% and 94%, respectively and for the liver of 29%, 93%, 56% and 82%, respectively. Median time to progression (TTP) for dogs with no metastasis, early metastasis and overt metastasis was not reached, 305 and 69 days, respectively (P < .001). Median survival time (MST) for the 3 groups were not reached, 322 and 81 days, respectively (P < .001). High Patnaik or Kiupel grade, early metastasis, overt metastasis and adequate local control were significantly associated with outcome. Early visceral metastasis was associated with poorer outcome compared to dogs without metastasis, however, a subset of dogs experienced long-term control.
1 INTRODUCTION
Canine mast cell tumours (MCTs) represent 16%-21% of cutaneous tumours and are the second most common malignancy in the UK.1-5 MCTs display variable clinical behaviour ranging from a localized mass to aggressive disease with distant metastasis. Consistent negative prognostic indicators include tumour recurrence, metastasis, rapid growth, ulceration, grade III Patnaik, high grade Kiupel, high risk locations (mucocutaneous sites, prepuce and scrotum) and high mitotic count (MC).6-32 Prior to determining treatment options, staging is recommended for tumours considered at high-risk for metastasis. Full staging includes haematology/biochemistry, regional lymph node (LN) fine needle aspiration (FNA) cytology, thoracic radiographs, and abdominal ultrasound (US) with hepatic and splenic FNA cytology.33 While current literature provides information on dogs with distant metastasis, precise staging data is often lacking clear documentation of whether visceral metastasis was classified on the basis of cytologic findings or US appearance alone.11, 18, 30, 34-38 When normal on US, liver and spleen may not be aspirated and several studies include patients without full staging.12, 19, 39-43
The utility of US to predict cytologic findings is controversial. One study demonstrated that sensitivity of US to detect liver and spleen metastasis was 0% and 43%, respectively.44 Another study suggested a sensitivity of 71% for both liver and spleen.45 A recent study reported that all dogs with cytologic confirmation of metastasis had abnormal ultrasound findings.27 Routine aspiration of the liver and spleen is also debatable. The presence of mast cells is not definitively diagnostic for metastasis as they can be present in healthy dogs and dogs with non-neoplastic conditions.28, 46-52 Cytologic guidelines for the designation of visceral MCT metastasis have been described.27 Using these criteria, dogs with distant metastasis were shown to have a poor outcome with a median survival time (MST) of 34 and 100 days compared to >733 and 291 days, respectively, for dogs without metastasis.27, 44 In the authors' experience, some dogs have cytologic findings that do not fit the criteria for overtly positive metastasis. These dogs have FNA results characterized by, a mild subjective increase in numbers of mast cells of normal morphology, not associated with connective tissue elements, where early metastasis cannot be ruled out. This cytologic finding therefore creates a challenge when determining treatment decisions.
The overall value of routine US and cytology of the liver and spleen in dogs with MCTs is unclear, because dogs with low-to-intermediate grade tumours without negative prognostic factors have a favourable prognosis after surgery, even following incomplete or narrow margins.20, 50, 53, 54 However, routine hepatic and splenic cytologic assessment is probably useful for dogs at high-risk for visceral metastasis.27, 44 Cytologic evidence of early metastasis may be prognostic and alter treatment decisions. The objective of this study was to prospectively correlate US and cytologic findings in dogs with high-risk MCTs, and to determine the outcome of dogs with early metastasis compared to dogs without metastasis and those with overt visceral metastasis. The hypothesis was that US is poorly predictive of hepatic or splenic metastasis when selecting for dogs at high-risk for MCT metastasis, and that dogs with cytologic evidence of early metastasis have improved outcomes compared to dogs with overt metastasis.
2 METHODS
2.1 Dogs
Dogs that presented to a referral oncology service between March 2013 and May 2017 for staging of high-risk MCTs diagnosed on cytology and/or histopathology were prospectively enrolled. The study was conducted following approval by the University veterinary ethical review committee. Written owner consent was obtained for all dogs. A subset of the cases had previously been published in a study on escalating vinblastine protocol in dogs with MCTs.55 Dogs were included if they had negative prognostic indicators consistent with biologically aggressive behaviour and if US and FNA cytology of liver and spleen were scheduled to be performed as part of full staging. Strict criteria for designation of “high risk” included the following negative prognostic indicators: grade III Patnaik and/or high grade Kiupel), mitotic count >5, LN metastasis, recurrent disease, recent rapid growth and ulceration, and high-risk location (mucocutaneous, preputial, scrotal or visceral).6-32 Rapid growth was defined as volume increase of >200% in the 30 days prior to clinical exam.
LN evaluation on cytology was performed using previously published guidelines and histopathological evaluation from 2015 onwards was performed according to the classification published in 2014.15, 32 Both HN2 and HN3 were documented as metastatic for the current study as these had been reported to be associated with outcome.
2.2 Staging
Staging included physical examination with MCT measurement where present, haematology, serum biochemistry, regional LN evaluation and FNA cytology, three view thoracic radiographs, and abdominal US with FNA cytology of liver and spleen. All palpable regional LNs were sampled; non-palpable LNs were sampled where feasible with US-guidance. If surgical removal was elected, the regional LN was excised and submitted for histopathology. US with FNA of the liver and spleen was performed by board-certified specialists in diagnostic imaging. Sedation was used to facilitate imaging and FNA. Liver and spleen appearance on US was characterized as nodules, masses, altered echogenicity, enlargement or normal appearance. FNA of the liver/spleen was attempted regardless of the US findings and when abnormalities were present, both abnormal and normal regions were aspirated. FNA was performed using a 23-gauge needle attached to a 5 mL syringe without any active aspiration at the time of sampling to avoid blood contamination, based on radiologist preference. Slides were stained with May-Grünwald Giemsa and cytologic evaluation was performed by one of two board-certified pathologists. Prior to sampling the liver and spleen, manual platelet counts were assessed and congenital coagulopathies were ruled out based on clinical history. If there was a history of liver disease, partial thromboplastin time and activated partial thromboplastin time were assessed in house.
2.3 Cytologic interpretation of liver and spleen
Dogs were assigned to 3 groups based on a modification of previous recommendations and included: no metastasis, early metastasis and overt metastasis.27 Criteria used can be seen in Table 1.
| Liver | Spleen | |
|---|---|---|
| No metastasis | One or less individualized well-granulated mast cells per 100 hepatocytes | No or rare and scattered individualized well-granulated mast cells |
| Early metastasis | 2-4 individualized well-granulated mast cells per 100 hepatocytes | Subjective mildly increased number of individualized well-granulated mast cells not associated with connective tissue elements |
| Overt metastasis | Clusters or ≥5 well differentiated mast cells per 100 hepatocytes | Clusters or subjective large number of individualized well-granulated mast cells or cells with atypical morphology |
2.4 Data collection
Data collected for all dogs included age, sex, breed, weight, date of diagnosis, previous and/or concurrent MCTs, tumour size, grade (Patnaik and Kiupel), localized clinical signs, systemic clinical signs, LN assessment, US appearance of liver and spleen, cytology results of liver and spleen, duration from diagnosis to staging, treatment (systemic treatment, surgery, radiation therapy), surgical margins, whether adequate local control was achieved, time to progression or metastasis (TTP), survival time (ST), and cause of death. Adequate local control at our institution at the time of prospective study design was defined as surgery with wide margins (≥ 3 mm for grade I-II Patnaik or low grade Kiupel, ≥5 mm for grade III Patnaik or high-grade Kiupel) or incomplete or narrow surgical margins followed by definitive-intent radiation therapy (RT) of the primary tumour site and regional LN bed where feasible.54, 56-59 Chemotherapy with escalating vinblastine and prednisolone was recommended for all dogs diagnosed with MCT at high-risk for metastasis following local control.55 TTP was defined as time from date of diagnosis to the date of progression of the primary tumour, local recurrence or development of metastasis. The development of de novo MCTs was not documented as progression unless greater than five MCTs appeared rapidly (within 14 days) in the face of therapy, in which case skin metastasis was presumed. Survival time was documented as time from date of diagnosis to date of death. Recommended follow-up included recheck examination once every 3 months for 18 months, followed by once every 6 months for 18 months, followed by yearly revisits. At the time of recheck, physical examination, regional LN measurement +/− FNA cytology, and US and with liver/spleen FNA were recommended.
2.5 Statistical analysis
Statistical analyses were performed using the R Statistical System (R Core Team 2015).60 Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated to evaluate US in relation to cytology results. TTP and MST were calculated using Kaplan-Meier curves and 95% confidence intervals. For TTP, dogs were censored if they were alive without evidence of progressive disease at the time of last follow-up. For MST, dogs were censored if they were alive at the time of last follow-up regardless of the status of disease progression and if they died due to MCT-unrelated causes. Cox-proportional hazards regression model was used to determine if high-risk location, MCT recurrence, rapid growth and ulceration, grade III Patnaik, high-grade Kiupel), high MC, LN metastasis, early hepatic or splenic metastasis, overt hepatic or splenic metastasis, surgery, adequate loco-regional control or chemotherapy had a significant impact on outcome. The proportional hazard assumption was assessed by graphical examination of the Schoenfeld residuals for each variable against time and a null hypothesis test that there is not a time dependent trend in these residuals (using the cox.zph function in the R survival package). Multivariable analysis was performed to evaluate for factors related to outcome within groups and between groups. For this analysis, variables were grouped into primary tumour characteristics (rapid growth and ulceration, high risk location, recurrence, grade III Patnaik or Kiupel high grade, high MC), metastasis status (no metastasis, LN metastasis, early liver or spleen metastasis, overt liver or spleen metastasis) and treatment variables (surgery of the primary tumour +/− regional LN, adequate local control, chemotherapy). A P-value of <.05 was considered statistically significant.
3 RESULTS
Eighty-two dogs met the inclusion criteria and were enrolled. One dog previously received targeted therapy with masitinib for a MCT, however, this medication was discontinued 1 month prior to referral because of the development of a metastatic MCT. None of the other dogs had received any chemotherapy or targeted therapy prior to referral. Represented breeds and clinical characteristics and outcome are summarized in Tables 2 and 3, respectively. Of note, rapid growth and ulceration occurred simultaneously in dogs affected. Two cases presented with clinical signs at time of diagnosis. One dog had a rapidly growing, bruised and ulcerated, 8.0 cm diameter mass in the inguinal area with no evidence of LN or visceral metastasis. Clinical signs included hyporexia and lethargy. The other dog had a 4.5 cm ulcerated rapidly growing mass on the pinna (high grade Patnaik and Kiupel with a high MC), with LN and visceral metastasis at time of diagnosis and clinical signs included diarrhoea with frank blood, lethargy and weakness. Sixty-one dogs (74%) presented with cutaneous MCT, 12 dogs (15%) presented with subcutaneous MCT, 8 dogs (10%) presented with mucocutaneous MCT and 1 dog (1%) presented with visceral MCT (Table 4). Haematology and serum biochemistry were performed in all dogs. Thoracic radiography was performed in 74 cases (90%) and none revealed lymphadenopathy or pulmonary metastasis. All regional LNs were assessed (for MCTs on the caudal half of the body inguinal and medial iliac LNs, for MCTs on the hind limbs also popliteal LN, for MCTs on the cranial half of the thorax and front limbs prescapular and axillary LNs, for MCTs of the neck mandibular and prescapular LNs, for MCTs on the head both left and right mandibular LNs). Thoracic radiographs were available in the majority of cases to assess intra-thoracic lymph nodes. Peripheral lymph nodes were assessed on palpation, however, for axillary, inguinal and medial iliac lymph nodes ultrasound was used. All lymph nodes assessed were sampled where possible, however, axillary, inguinal and medial iliac lymph nodes were difficult to sample when normal in size. Regional lymphadenomegaly was documented in 33 dogs (40%). Sixty-one dogs (74%) had at least one regional LN assessed cytologically (n = 55) and/ or histologically (n = 48). Overall 41 dogs (50%) were diagnosed with metastasis (Table 5).
| Breeds | Number of dogs |
|---|---|
| Labrador | 37 |
| Cross breed | 11 |
| Boxer | 7 |
| Staffordshire Bull Terrier | 6 |
| Pug | 4 |
| Shar-Pei | 2 |
| Jack Russel Terrier | 2 |
| Springer Spaniel | 2 |
| Bearded Collie | 1 |
| Golden Retriever | 1 |
| Yorkshire Terrier | 1 |
| Lhasa Apso | 1 |
| Cocker Spaniel | 1 |
| Portuguese Water Dog | 1 |
| Dalmatian | 1 |
| Scottish Terrier | 1 |
| Rottweiler | 1 |
| Native American Indian Dog | 1 |
| German Shepherd | 1 |
| Overall dogs (n = 82) | Dogs without metastasis (n = 61) | Dogs with early metastasis (n = 15) | Dogs with convincing liver/spleen metastasis (n = 6) | |
|---|---|---|---|---|
| Labradors (%) | 37 (45) | 24 (39) | 9 (60) | 4 (67) |
| M (%) | 39 (48) | 29 (47) | 9 (60) | 1 (17) |
| MN (%) | 26 (32) | 18 (30) | 8 (53) | 0 (0) |
| F (%) | 43 (52) | 32 (52) | 6 (40) | 5 (83) |
| FN (%) | 33 (41) | 26 (43) | 3 (20) | 4 (67) |
| Mean age (median) | 7.57 (7.7) | 7.16 (7.5) | 8.46 (8.2) | 9.55 (9.8) |
| Median weight (kg) | 28.3 | 26.4 | 35.5 | 30.65 |
| High risk location (%) | 22 (27) | 18 (30) | 4 (27) | 0 (0) |
| SC location (%) | 12 (15) | 7 (11) | 3 (20) | 2 (33) |
| Median size MCTs (cm) | 2 | 2 | 3 | 4.3 |
| Recurrent MCT (%) | 10 (12) | 4 (7) | 5 (33) | 1 (17) |
| Rapid growth/ulceration (%) | 38 (46) | 25 (41) | 8 (53) | 5 (83) |
| Systemic clinical signs (%) | 2 (2) | 1 (2) | 0 (0) | 1 (17) |
| Grade assessed (%) | 64 (78) | 53 (87) | 8 (53) | 3 (50) |
| P (%) | 61 (74) | 50 (82) | 8 (53) | 3 (50) |
| High (%) | 25 (30) | 19 (31) | 4 (27) | 2 (33) |
| K (%) | 51 (62) | 41 (67) | 7 (47) | 3 (50) |
| High (%) | 26 (32) | 20 (33) | 3 (20) | 3 (50) |
| High P or K (%) | 34 (41) | 27 (44) | 4 (27) | 3 (50) |
| MC analysed | 69 (84) | 55 (90) | 11 (73) | 3 (50) |
| High (%) | 27 (33) | 19 (31) | 5 (15) | 3 (50) |
| Previous MCTs (%) | 13 (16) | 7 (11) | 3 (20) | 3 (50) |
| Concurrent MCTs (%) | 7 (9) | 6 (10) | 0 (0) | 1 (17) |
| Median time from diagnosis to staging (days) | 20 | 20 | 16 | 15 |
| Abnormal CBC/BC (%) | 43 (52) | 28 (46) | 10 (67) | 5 (83) |
| LN assessed by cytology or histology (%) | 61 (74) | 43 (70) | 13 (87) | 5 (83) |
| Lymphadenomegaly | 33 (40) | 23 (38) | 5 (33) | 5 (83) |
| Metastatic LN (%) | 41 (50) | 27 (44) | 9 (60) | 5 (83) |
| Thoracic radiographs (%) | 73 (89) | 57 (93) | 11 (73) | 5 (83) |
| Abnormalities (%) | 14 (17) | 12 (20) | 2 (13) | 0 (0) |
| Abnormal hepatic US | 9 (11) | 4 (7) | 4 (27) | 1 (17) |
| Abnormal splenic US | 29 (35) | 18 (30) | 7 (47) | 4 (67) |
| Surgery (%) | 73 (89) | 58 (95) | 12 (80) | 3 (50) |
| Complete margins (%) | 36 (44) | 29 (48) | 5 (33) | 2 (33) |
| Definitive RT (47.5-48 Gy) | 13 (16) | 11 (18) | 2 (13) | 0 (0) |
| Palliative RT (25 Gy) | 1 (2) | 1 (2) | 0 (0) | 0 (0) |
| Adequate local control | 50 (61) | 41 (67) | 7 (47) | 2 (33) |
| Vinblastine/prednisolone | 53 (65) | 40 (66) | 10 (67) | 3 (50) |
| Progressive disease | 31 (38) | 16 (26) | 9 (60) | 6 (100) |
| Median TTP | Not reached | Not reached | 305 | 69 |
| Died (%) | 38 (46) | 24 (39) | 9 (60) | 5 (83) |
| Died due to MCT (%) | 29 (35) | 16 (26) | 8 (53) | 5 (83) |
| MST | Not reached | Not reached | 322 | 81 |
| Still alive (%) | 44 (54) | 37 (61) | 6 (40) | 1 (17) |
| Median follow-up (range) | 597 (66-1505) | 614 (92-1505) | 457 (141-973) | 66 (66) |
- Abbreviations: BC, serum biochemistry; CBC, complete blood count; F, female; K, Kiupel; LN, lymph node; MCT, mast cell tumour; M, male; MC, mitotic count (high >5); MST, median survival time; N, neutered; P, Patnaik; SC, subcutaneous; RT, radiation therapy; TTP, time to progression; US, ultrasound.
| Location | No metastasis (n) | Early metastasis (n) | Overt metastasis (n) |
|---|---|---|---|
| Cutaneous | 48 | 9 | 4 |
| Subcutaneous | 7 | 3 | 2 |
| Mucocutaneous | 6 | 2 | 0 |
| Visceral | 0 | 1 (intestine) | 0 |
| n | |
|---|---|
| Dogs with at least one enlarged ln | 33 |
| Enlarged lns | 45 |
| Dogs with at least one ln assessed on cytology | 55 |
| Lns assessed on cytology | 99 |
| Dogs with ln mets on cytology | 32 |
| Lns positive for mets on cytology | 43 |
| Dogs with at least one ln assessed on histopathology | 48 |
| Lns assessed on histopathology | 72 |
| Dogs with ln mets on histopathology | 34 |
| Lns positive for mets on histopathology | 37 |
| Lns HN0a | 26 |
| Lns HN1a | 9 |
| Lns HN2a | 18 |
| Lns HN3a | 19 |
| Dogs with lns assessed on cytology and/or histopathology | 61 |
| Dogs with metastatic lns on cytology and/or histopathology | 41 |
| Dogs with lns assessed on cytology and histopathology | 41 |
| Dogs with metastasic lns on cytology and histopathology | 23 |
| Dogs with non-metastastic lns on cytology and histopathology | 13 |
| Dogs with discordant ln results between cytology and histopathologyb | 4 |
- Abbreviations: ln, lymph node; mets, metastasis.
- a Ln metastasis status according to previously published classification.32
- b At least one ln negative on cytology, but positive for metastasis on histopathology.
All dogs underwent US with FNA of the spleen, however, FNA of the liver was not performed in 4 dogs because its location deep within the cranial abdomen was not safely accessible despite sedation. Cytology of the liver was non-diagnostic in 3 dogs while all splenic samples were diagnostic. A median 2 samples were taken each from the liver and spleen (range 1-4). Variable degrees of cellularity and haemodilution were present, however, most slides were of diagnostic quality except for one dog with three out of four smears non-diagnostic and one other dog with two out of four smears non-diagnostic. No complications were documented following hepatic or splenic aspiration. US abnormalities were detected in the spleen and liver in 29 (35%) and 9 (11%) dogs, respectively (Table 6). Cytologic evaluation of the liver and/or spleen was consistent with no metastasis in 61 dogs (74%), early metastasis in 15 dogs (18%) and overt metastasis in 6 dogs (7%). Seventeen dogs had hepatic metastasis (15 early and 2 overt metastasis); 12 of which had a normal appearing liver on US (11 early and 1 overt metastasis). Nine dogs had splenic metastasis (3 early and 6 overt metastasis), 3 of which had dogs a normal spleen on US (1 early metastasis and 2 overt metastasis). LN metastasis was present in 27 (44%) of the 61 dogs without metastasis to the liver or spleen, in 9 (60%) of the 15 dogs with early metastasis and in 5 (83%) of the 6 dogs with overt metastasis. Overall, 14 of the 21 dogs (67%) with visceral metastasis had regional LN metastasis. Fourteen of the 41 dogs (34%) with LN metastasis had distant visceral metastasis.
| Spleen | All dogs (n = 82) | Dogs without metastasis (n = 74) | Early metastasis (n = 3) | Overt metastasis (n = 6) | |
|---|---|---|---|---|---|
| Single nodule (%) | 6 (7) | 3 (4) | 2 (67) | 1 (17) | |
| Splenic mass (%) | 2 (2) | 1 (1) | 0 (0) | 1 (17) | |
| Multiple nodules (%) | 10 (12) | 8 (11) | 0 (0) | 2 (33) | |
| Diffusely altered echogenicity (%) | 14 (17) | 12 (16) | 0 (0) | 2 (33) | |
| Splenomegaly (%) | 5 (6) | 5 (7) | 0 (0) | 0 (0) | |
| Multiple abnormalities (%) | 7 (9) | 6 (8) | 0 (0) | 2 (33) | |
| Normal (%) | 54 (66) | 51 (69) | 1 (33) | 2 (33) | |
| Liver | All dogs (n = 76) | Dogs without metastasis (n = 59) | Early metastasis (n = 15) | Overt metastasis (n = 2) | |
| Single nodule (%) | 2 (3) | 0 (0) | 2 (13) | 0 (0) | |
| Multiple nodules (%) | 4 (5) | 1 (2) | 2 (13) | 1 (50) | |
| Diffusely altered echogenicity (%) | 4 (5) | 1 (2) | 2 (13) | 1 (50) | |
| Hepatomegaly (%) | 2 (3) | 1 (2) | 1 (7) | 0 (0) | |
| Multiple abnormalities (%) | 3 (4) | 0 (0) | 2 (13) | 1 (50) | |
| Normal (%) | 67 (88) | 55 (74) | 11 (73) | 1 (50) |
The sensitivity, specificity, PPV, and NPV for splenic US in detecting metastasis were 67% (CI 0.30-0.93), 68% (CI 0.57-0.79), 21% (CI 0.08-0.4) and 94% (CI 0.84-0.99), respectively. Repeating these calculations for dogs with overt splenic metastasis only as true positives minimally affected these values. The sensitivity, specificity, PPV, and NPV for liver US in detecting metastasis (only including dogs with diagnostic liver cytology) were 29% (CI 0.10-0.56), 93% (CI 0.83-0.98), 56% (CI 0.21-0.86) and 82% (CI 0.70-0.90), respectively. If only dogs with overt metastasis were used as true positives, the sensitivity, specificity, PPV and NPV were 50%, 89%, 13% and 98%, respectively.
Fifty dogs (61%) were considered to have received adequate local therapy. Thirteen dogs (16%) received definitive RT (Table 7). Adjuvant RT was not pursued in 6 of 7 dogs with narrow margins and 16 of 28 dogs with incomplete margins. Margin evaluation was not available in two dogs. Fifty-three dogs (65%) received vinblastine-prednisolone chemotherapy, with 38 dogs completing the protocol. Sixty-six percent, 67%, and 50% of dogs without, with early, and with overt visceral metastasis, respectively, received chemotherapy. Four dogs (5%) received prednisolone alone.
| RT | Dogs (n) |
|---|---|
| RT following incomplete excision | 12 |
| RT following narrow excision | 1 |
| RT protocol 16 × 3 Gy | 12 |
| RT protocol 19 × 2.5 Gy | 1 |
| LN bed irradiated following excision of metastatic LN | 10 |
| LN irradiated prophylactically | 1 |
| Manual planning | 10 |
| CT-guided RT planning | 3 |
- Abbreviations: CT, computed tomography; LN, lymph node; RT, radiation therapy.
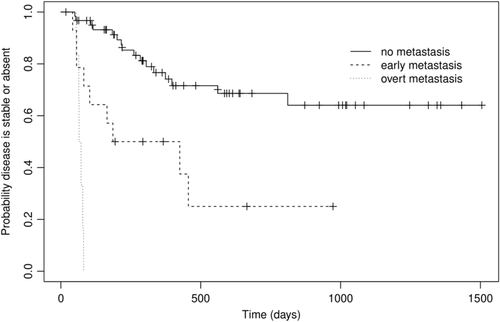
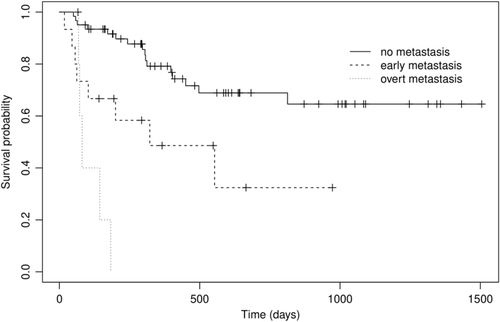
The median TTP for dogs with no metastasis, early metastasis and overt metastasis was not reached (observed range 50-811), 305 (42-465) and 69 (55-81) days, respectively (P < .001) (Figure 1). The MST for dogs with no metastasis, early metastasis and overt metastasis was not reached (observed range 50-813), 322 (18-553) and 81 (68-183) days, respectively (P < .001) (Figure 2). Observed median follow-up for the dogs was 597 days (range 66-1505 days). At the time of writing, 38 dogs (54%) were still alive at last point of follow-up, or censored due to death for a reason unrelated to MCT. The nine censored dogs (11%) were euthanised for various reasons including: vestibular syndrome, large cell lymphoma, intra-cranial meningioma, acute gastro-enteritis, carotid body tumour, metastatic melanoma, bone tumour, pyometra and degenerative myelopathy. Three dogs in the early metastasis group (20%) were censored more than 500 days following diagnosis; two of those dogs had adequate local control and all three were treated with chemotherapy.
Forty-one dogs (50%) had repeat FNA of liver/spleen at least once during a recheck visit following completion of treatment. Of the 21 dogs with visceral metastasis at initial staging, 9 dogs had repeat FNA and only 4 had cytologic evidence of metastasis (one overt); Eight of the nine dogs had received chemotherapy. Of the 61 dogs without metastasis on initial staging, 32 dogs (52%) were reassessed. Two dogs developed early metastasis and two dogs developed overt visceral metastasis. All dogs with metastasis at recheck were euthanised within 6 months of detection of metastasis.
For the 12 dogs with subcutaneous MCTs risk factors included: recurrence only (n = 1); LN metastasis only (n = 3); rapid growth and ulceration (n = 2); high grade and high MC (n = 2); rapid growth and ulceration with LN metastasis (n = 1); recurrence and high grade with LN metastasis (n = 1); recurrence, rapid growth and ulceration with LN metastasis (n = 1); recurrence, rapid growth and ulceration, high MC and LN metastasis (n = 1). Grade was not available in seven dogs; two dogs did not have surgery and the pathologist did not grade subcutaneous tumours for the remaining five dogs. MC was provided in all cases where surgery was performed. Five of those 12 dogs had evidence of visceral metastasis (three early, two overt).
Seven dogs presented with concurrent MCTs, with only one dog having visceral metastasis (overt). None of these concurrent tumours demonstrated any negative prognostic factors. In the dog with visceral metastasis the primary tumour had a high grade Kiupel MCT with a high MC which led to inclusion in the study.
Twelve dogs had previously been diagnosed with other MCTs prior to enrolling in the study. Five of those dogs had evidence of visceral metastasis (two of which overt metastasis). None of those previous MCT were associated with documented negative prognostic indicators.
On univariable analysis (Table 8), rapid growth and ulceration, Patnaik grade III, Kiupel high grade, high MC, liver or spleen metastasis, surgical removal, and adequate local control were prognostic for TTP. Univariables associated with MST included rapid growth and ulceration, Patnaik grade III, Kiupel high grade, high MC, liver or spleen metastasis and adequate local control. On multivariable analysis (Table 9), within the primary tumour characteristics group, only Patnaik grade III or Kiupel high grade was significantly associated with TTP or MST (P = .02 and P = .04, respectively). Within the metastatic group, both early visceral metastasis and overt metastasis were negatively associated with TTP (P = .003 and P < .001, respectively) and MST (P = .01 and P < .001, respectively). Of the treatment variables, adequate local control was associated with both TTP (P = .001) and MST (P = .01). As four dogs did not have liver FNA and three additional dogs had non-diagnostic liver samples, statistical analysis assessing association between metastasis status and outcome was repeated not including those seven dogs, and significant findings were unchanged.
| Prognostic variables | TTP analysis | Hazard ratio | MST analysis | Hazard ratio |
|---|---|---|---|---|
| Growth and ulceration | P < .001* | 1.34 | P < .001* | 1.87 |
| High-risk location | P = .6 | 1.12 | P = .5 | 0.14 |
| Recurrence | P = .1 | 0.49 | P = .2 | 0.71 |
| Lymph node metastasis | P = .1 | 0.55 | P = .07 | 0.71 |
| Grade III (Patnaik) | P < .001* | 4.38 | P < .001* | 6.3 |
| High grade (Kiupel) | P = .001* | 1.70 | P = .001* | 2.11 |
| Mitotic index >5 | P < .001* | 1.80 | P < .001* | 1.84 |
| Visceral metastasis | P < .001* | 5.27 | P < .001* | 4.60 |
| Vinblastine/prednisolone chemotherapy | P = .5 | 1.11 | P = .4 | 1.38 |
| Surgery | P = .04* | 2.86 | P = .1 | 2.38 |
| Adequate local control | P < .001* | 3.91 | P = .003* | 3.49 |
| Prognostic variables | TTP analysis | Hazard ratio | MST analysis | Hazard ratio |
|---|---|---|---|---|
| Primary tumour | ||||
| High grade P and/or K | 0.02* | 4.9 | 0.04* | 4.3 |
| Growth and ulceration | 0.10 | 2.45 | 0.06 | 3.03 |
| Recurrence | 0.38 | 2.00 | 0.26 | 2.49 |
| High risk location | 0.26 | 2.00 | 0.26 | 1.98 |
| Metastasis status | ||||
| LN mets | 0.75 | 1.13 | 0.36 | 1.45 |
| Early mets | 0.003* | 3.58 | 0.01* | 2.92 |
| Overt mets | <0.001* | 29.37 | <0.001* | 10.69 |
| Treatment | ||||
| Surgery | 0.66 | 0.81 | 0.79 | 0.87 |
| VBL | 0.38 | 1.42 | 0.93 | 1.03 |
| Adequate local control | 0.001* | 0.21 | 0.01* | 0.34 |
- Abbreviations: K, Kiupel; LN, lymph node; mets, metastasis; P, Patnaik; VBL vinblastine.
4 DISCUSSION
As we hypothesized, US was a poor predictor of cytologic metastasis of liver or spleen as previous literature has suggested,44, 45 yet dogs with early metastasis had improved outcome compared to those with overt metastasis. Sensitivity of US was unacceptably low for both liver and spleen, whereas specificity was high at 93% for hepatic appearance. For both liver and spleen, NPVs were high, which may have been influenced by the low prevalence of distant metastasis in our population.
The consequences of being assigned as having visceral metastasis are tremendous, as poor survival has been shown with MSTs ranging from 34 to 110 days.27, 44, 61 It influences treatment options in our institution, wherein palliative treatment is considered with more weight compared to definitive treatments in cases of visceral metastasis, given the poor outcome for dogs with overt metastasis. On the other hand, dogs with false negative results may undergo intensive treatment protocols involving chemotherapy and definitive RT with associated toxicities rather than palliative treatments focused on improving or maintaining quality of life. Considering these consequences, assigning cases to a stage based on US findings alone or aspirating liver and spleen only when sonographically abnormal may not be justified.
As full abdominal US is time-consuming and often contributes to the cost, where finances could be preserved for the pursuit of treatment, it is important to ensure that this technique in dogs with high-risk MCTs is defensible. A brief US to assess any regional lymph nodes that are difficult or cannot be palpated (non-peripheral) and to guide US-guided sampling of the liver and spleen may be more useful. Comprehensive abdominal US could still be considered to rule out concurrent disease; however, this could be reserved for dogs with systemic signs, where concurrent disease is likely or would influence therapeutic options, or for owners wishing to pursue complete imaging. Of note, similar findings were recently reported for dogs undergoing abdominal CT for MCT staging, in which cytologic findings did not correlate well to CT changes and sampling was recommended in the absence of abnormal CT findings.62
Early visceral metastasis was significantly associated with outcome and dogs with early visceral metastasis have the potential for long-term control. As it can be difficult to differentiate neoplastic from reactive mast cells, the relevance of mildly increased numbers of mast cells in the liver and/or spleen was questionable, especially given the range of survival in this small group. It is possible that biopsies may provide meaningful information to clarify the likelihood of metastasis; however, this adds a layer of risk to the dog and is not standard practice. Alternatively, additional work needs to be done to improve detection of neoplastic vs reactive mast cells; IHC (eg, CD25) and/or molecular testing (eg, c-kit mutational status) may have promise if validated in the dog as reliable markers.63-65 Because an association with outcome was detected in our population, it suggests that in dogs with high-risk MCTs, at least a proportion of dogs have metastatic mast cells. This is further supported by the fact that at least four dogs with early metastasis continued to have cytologic evidence of metastasis on restaging. Despite the inferior outcome for dogs with early metastasis compared to dogs without distant metastasis, the median TTP was 305 days with a range of up to 973 days. This data justifies the pursuit of definitive treatment with adequate local control and chemotherapy when early metastasis is identified. It is possible that the dogs with extended survival had increased non-metastatic mast cells in the spleen and liver, especially given that five dogs with early metastasis had no evidence of mast cell disease on restaging. Alternatively they may have enjoyed a favourable response to chemotherapy because had fewer metastatic mast cells or if they had mast cells more responsive to chemotherapy, however, the use of chemotherapy in this group of dogs with high-risk MCTs was not associated with improved outcome.
The MST for dogs with overt metastasis was 81 days (compared to 322 days for dogs with early metastasis and > 614 days for the dogs without visceral metastasis). This is similar to what has previously been reported in literature, with survival ranges of 34-119 days reported.27, 44, 61 The majority of cases mentioned in the literature above had received systemic treatment, similar to the 62% of dogs in our study that received chemotherapy.
Of the primary tumour characteristics, metastasis and treatment variables, only the presence of grade III or high grade Kiupel, early metastasis, overt metastasis and adequate local control were significantly associated with both TTP and MST on multivariable analysis. It is important to highlight that this study was not designed to perform robust multivariable statistical analysis and in order to evaluate a number of prognostic factors, we would need 10 times as many events as prognostic variables to detect statistical significance.66 Even with comprehensive multivariable analysis on large case numbers, it is challenging to interpret the results as there is a complex relationship between primary tumour characteristics, metastatic status and treatment variables. For example, dogs with overt visceral metastasis achieved adequate local control less commonly (33% vs 67% of the dogs without metastasis). This is possibly due to more aggressive biologic behaviour, however, this cannot be concluded with certainty as definitive treatment options are less likely to be pursued in the face of visceral metastasis. However, the study was designed to clearly define “increased numbers of mast cells” and to follow dogs with high-risk tumours to evaluate if definitive treatment is rational in dogs with early visceral metastasis.
As aspiration of the liver and spleen were previously demonstrated to be safe with no obvious complications27, 44; repeat imaging was not routinely performed the same day following sampling to rule out intra-abdominal haemorrhage. No complications were seen in this study.
Fifty percent of the dogs with high risk MCTs had LN metastasis, 18% had early liver and/or spleen metastasis and 7% had overt metastasis. The LN metastatic rate in this study was high, which reflects the population of high-risk MCTs recruited for this study as was seen in previous literature.12, 30, 39, 43 The rate could have been biased as LN metastasis was one of the inclusion criteria. Early metastasis to the liver and/or spleen has not been previously associated to outcome to the authors' knowledge, and the rate of early metastasis using our criteria was higher than expected at 18%. Variable rates of distant visceral metastasis at time of diagnosis have been reported in previous publications, ranging from 0% to 19%, but studies utilized different inclusion criteria and cytologic evaluation of liver and/or spleen was not always performed.1, 18, 21, 27, 28, 39, 40, 42, 50, 67, 68 Combining both early and overt liver/spleen metastatic rates in this study, 25% of dogs had distant metastasis, supporting the routine use of hepatic and splenic cytology in the staging of dogs with high risk MCTs.
LN metastasis was not significantly associated with outcome in this study. This does not definitively mean that it is not prognostic in the population overall, as the inclusion criteria were designed to include dogs with at least one negative prognostic factor. Whether LN metastasis is always a negative prognostic indicator is controversial as multiple studies show a negative impact on outcome11, 12, 18, 39; however, other publications show that if treated appropriately a metastatic LN does not necessarily implicate a worse prognosis.36, 43, 69 Seven percent of dogs had overt distant metastasis with five of the six dogs having concurrent LN metastasis. Of the 15 dogs with early visceral metastasis, 47% did not have LN metastasis in the majority of LNs assessed on cytology and/or histopathology. Based on literature of sentinel LNs, those assessed might not have been the draining LNs.70 Despite the lack of sentinel lymph node mapping in this study, multiple regional lymph nodes, all considered to be probable draining lymph nodes based on anatomic knowledge, were assessed in all cases with aspirates obtained wherever possible. Seventy-four percent of dogs had at least one LN sampled. Localisation and excision of normal sized axillary and inguinal lymph nodes can be challenging and it was not routine practice to prescribe thoracoscopic surgery, thoracotomy, or exploratory laparotomy to remove normal sized sternal and medial iliac lymph nodes. As sentinel lymph node mapping was not performed and not all dogs had lymph nodes assessed on cytology and/or histopathology, it is likely that the lymph node metastatic rate was underestimated.70, 71 Dogs that did not have lymph nodes sampled were excluded for statistical analysis when assessing for association between LN metastatic status and outcome. Based on a recent study, there may be value in either removing regional lymph nodes at risk for metastasis or prophylactically treating them in dogs considered at high-risk for metastasis.69, 72 Sentinel LN mapping may therefore play a prominent role in determining more locally invasive procedures to remove additional tissue.73 Visceral metastasis without a detectable metastasis to LNs has been reported in previous literature; therefore, not having a metastatic LN does not completely remove the need to assess liver/spleen for metastasis.27, 28, 44-52, 55, 60, 61
Seven dogs lacked diagnostic liver cytology, therefore additional analysis was performed to exclude these dogs, which did not alter our findings. This study demonstrates that in most (but not all) dogs, liver samples can be taken safely with sedation and that few cytologic samples may be non-diagnostic. Repeat aspiration of the liver was offered to all clients prior to treatment for complete staging if non-diagnostic samples were obtained; however, repeat sampling was declined. In the author's opinion an attempt at cytology of liver should always be made based on the low sensitivity of ultrasound, however, considering the high NPV, it may be reasonable to assume that liver is negative for metastasis rather than positive when determining the therapeutic plan.
Adequate local control was defined at the start of the study, recognizing that there is no clear standard of what defines loco-regional radiation therapy boundaries or exactly what margin is required to control grade 3 MCTs.56 However we were consistent with current literature in the standards set forth at the start of the study.53, 56, 72
Labradors were overrepresented at 45% of the canine population in this study. This is markedly higher than multiple previous reports that included 11%-27% Labradors.11, 12, 19, 28, 30, 31, 34, 35, 37, 39, 41, 74 In two studies that used similar inclusion criteria to ours, Labradors represented 41% and 37% of their study populations, supporting the possibility that Labradors are not only more prone to developing MCTs but are also more likely to develop high-risk MCTs.18, 44 A recent study did not identify the Labrador Retriever as a breed with an increased risk of developing higher grade MCTs, however, the study did not take other prognostic variables into consideration.75 Epidemiologic studies would be required to evaluate the proportion of Labradors in the overall population in this region and in our referral hospital prior to drawing any conclusions.
Only dogs with high-risk MCTs that fit to our specific criteria were included in the study. It is possible that some dogs were not included in the study even though they had negative prognostic factors that we were unaware of, particularly as we did not have tumour size for all dogs if they were referred following treatment at the referring practice.61 Size has previously been reported as prognostic for dogs with MCTs, however, was not evaluated as a prognostic indicator in this study as recent rapid growth was more easily assessed, even in cases that sought initial surgery with the referring practice.61 Size was not consistently recorded for cases which did not initially seek treatment with oncology, and as MCTs can shift size dramatically, we felt that recent rapid growth was more reliable. Additionally, others have suggested that rapid growth rather than size might be a more accurate predictor of prognosis.8 Additional studies could also include size, to better determine if it is an independent negative factor.19, 21, 28, 56
It is currently difficult to conclude a true visceral metastasis rate in low-risk MCTs (defined as MCTs lacking negative prognostic indicators as used in the current study) as previous literature did not consistently clarify all negative factors nor report consistent hepatic or splenic aspiration cytology.12, 28, 31, 35, 40, 41, 76 In multiple studies assessing metastasis in different grade MCTs, all dogs with visceral metastasis had at least one negative prognostic factor.38, 44, 53, 61 A separate study is needed to clearly identify the risk of visceral metastasis in low-risk dogs, or dogs lacking the high-risk features defined here or in other literature.
Even though previous literature is suggestive that subcutaneous MCTs are less biologically aggressive77, 78; 12 dogs with subcutaneous tumours were included in our study based on our negative prognostic factor requirement for inclusion. Indeed, 42% of these dogs demonstrated evidence of visceral metastasis. This suggests that dogs with subcutaneous MCTs that present with negative prognostic factors benefit from full staging including cytology of the liver and spleen.
Dogs with a history of previous MCTs or concurrent MCTs did not demonstrate any negative prognostic factors for those tumours. Of the dogs with previous MCTs 42% had visceral metastasis. Of the dogs with concurrent MCTs, only 14% had visceral metastasis. Based on previous literature it is clear that dogs that develop a MCT are at risk of developing more MCTs in the future and it has been suggested that these are most likely de novo tumours rather than cutaneous metastasis.19, 21, 29 The authors used an arbitrary 14-day duration and a requirement for five new MCTs to differentiate de novo tumour from metastasis; however, true differentiation is unlikely this simple and molecular testing to identify specific mutations (eg, c-kit) may provide more definitive information.63, 79 As the dogs with previous MCTs that were included in this study did not have any negative prognostic indicators at the time, we considered the new MCT requiring referral to be de novo. In the dog with multifocal MCTs with overt visceral metastasis at time of diagnosis, the authors considered the abundance of lesions most likely representative of mast cell metastasis, but we cannot rule out that these all represented de novo tumours. This dog developed progressive cutaneous MCTs despite chemotherapy with a TTP of 65 days.
One limitation is that multiple diagnostic imagers, anatomic pathologists and clinical pathologists were involved in this study and not all samples were reviewed by the same individuals. Grading was not available for all cases and second opinion histopathology was not possible for this study. While we contend that our data is reflective of clinical practice, where multiple pathologists may be responsible for interpreting cases, the study could have be strengthened if assessment was standardized, particularly to evaluate for agreement in the designation of “early” metastasis, and for determination of post-surgery, formalin-fixed tumour diameter. Another limitation was that not all dogs were initially evaluated by the specialty service and negative prognostic factors may have been unreported by the owner or referring veterinarian. While we chose to be strict in our inclusion criteria for high risk, it is likely that our criteria was not all encompassing and may have missed some eligible cases.
5 CONCLUSION
US findings to detect liver and spleen metastasis is poorly indicative of metastasis, even when only dogs defined as high-risk for visceral metastasis were evaluated. US-guided FNA of the liver and spleen should be performed in all high-risk MCTs, regardless of the US appearance or the regional LN status. The routine use of complete US is questionable unless there are clinical signs or concurrent disease suspected. In this group of 82 dogs considered high-risk for MCT metastasis, 18% of dogs had cytologic evidence of early metastasis, while 7% had cytologic evidence of overt metastasis to liver and/or spleen. Dogs with early metastasis had improved TTP and MST compared to dogs with overt metastasis, and 20% of dogs in the early metastasis group experienced survival times >500 days. Additional study is required to understand the complex relationship between multiple prognostic variables and the presence of early visceral metastasis, as well as to investigate methods to more definitively differentiate metastatic from reactive mast cells.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.