Acute phase proteins and biomarkers of oxidative status in feline spontaneous malignant mammary tumours
Funding information Fundação para a Ciência e a Tecnologia (FCT) of Ministério da Ciência, Tecnologia e Ensino Superior, Portugal, Grant/Award Number: Project UID/CVT/00772/2013; Ministerio de Economia y Competitividad, Spain, Grant/Award Number: Program Juan de la Cierva Incorporacion; Ministério da Ciência, Tecnologia e Ensino Superior; Fundação para a Ciência e a Tecnologia
Abstract
Acute phase proteins (APP) and biomarkers of oxidative status change in human and canine mammary tumours, however, they have not been studied in feline mammary tumours. The aims of this study were to investigate the APP and antioxidant responses in feline malignant mammary tumours, to evaluate their relation with tumour features, and to assess their prognostic value. Serum amyloid A (SAA), haptoglobin (Hp), albumin, butyrylcholinesterase (BChE), insulin-like growth factor1 (IGF1), paraoxonase1 (PON1), total serum thiols (Thiol), glutathione peroxidase (GPox) and total antioxidant capacity determined by different assays, including trolox equivalent antioxidant capacity assessed by two different methodologies (TEAC1/2), ferric reducing ability of plasma (FRAP), and cupric reducing antioxidant capacity (CUPRAC), were determined in serum of 50 queens with spontaneous mammary carcinomas and of 12 healthy female cats. At diagnosis, diseased queens presented significantly higher SAA and Hp, and lower albumin, BChE, GPox, TEAC1, TEAC2 and CUPRAC than controls. Different tumour features influenced concentrations of APP and antioxidants. Increases in serum Hp, and decreases in albumin, Thiol and FRAP were significantly associated with neoplastic vascular emboli, metastasis in regional lymph nodes and/or in distant organs. Distant metastasis development during the course of the disease was associated with increases in SAA and TEAC1. At diagnosis, decreased albumin was associated with a longer survival, and BChE <1.15 μmoL/mL.minute was associated with a shorter survival time on multivariate analysis. Feline malignant mammary tumours are associated with an APP response and oxidative stress, and different tumour features influence the inflammatory response and the oxidative damage. Furthermore, some of these analytes proved to have prognostic value.
1 INTRODUCTION
Mammary tumours are among the most frequent neoplasias in female cats.1 Approximately, 80% to 90% are malignant, and most of these present an aggressive behaviour and a poor prognosis.2-4 Reported survival times from diagnosis of queens with malignant mammary tumours vary significantly from a few days to several years.5, 6
Several studies characterized feline mammary tumours and evaluated related prognostic factors, most of them on clinical, histopathological and molecular features of these neoplasias.2, 7, 8 However, information on clinical value of serum biomarkers related with inflammation and oxidative stress in diagnosis, evolution of disease, response to treatment and in prognosis of feline mammary tumours is scarce.
Acute phase proteins (APP) are serum proteins which concentrations are altered in acute or chronic inflammatory conditions of different aetiology, including neoplastic diseases.9 The APP response is a very fast reaction, which develops before stimulation of the specific immune response, and in many cases even before the onset of clinical signs; consequently, can be considered one of the earliest markers of inflammatory processes or diseases.10 In the cat, serum amyloid A (SAA) is considered a positive major APP, haptoglobin (Hp) a positive moderate APP, and albumin is the main negative APP.9, 10 Butyrylcholinesterase (BChE) is considered a negative APP in dogs and cats, and insulin-like growth factor1 (IGF1) is considered a negative APP in dogs.11-13
The oxidative status of the organism is dependent of the balance between oxidant reactants and antioxidant defences.14 Oxidative stress develops when oxygen and nitrogen free radicals exceed the capacity of the antioxidant defences of the organism.15 The antioxidant response can be assessed by determination of individual analytes like paraoxonase 1 (PON1), total serum thiols (Thiol) and glutathione peroxidase (GPox), and/or by determination of the total antioxidant capacity (TAC) of the organism.16 The TAC represents the sum of the activities of the different antioxidants, and also the antioxidative effects provided by the interactions between antioxidants,17, 18 and can be determined by different methods, including trolox equivalent antioxidant capacity (TEAC), ferric reducing ability of plasma (FRAP), and cupric reducing antioxidant capacity (CUPRAC).19, 20
APP and analytes related with oxidative status have been increasingly studied as biomarkers of neoplastic diseases in human and in veterinary medicine, including in human breast cancer and in canine mammary tumours.21-26 However, to the author´s knowledge, there is no information on the APP response and the oxidative status in feline mammary tumours. Thus, the main aim of the present study was to investigate the possible changes in APP and oxidative biomarkers in queens with spontaneous malignant mammary tumours. In addition, this study aimed to evaluate changes in these analytes related to clinical and histological features of these tumours, and to assess their prognostic value.
2 MATERIALS AND METHODS
2.1 Animals and samples
Fifty female cats that were presented to three veterinary medical centres from Portugal between January 2011 and January 2016 because of the presence of single or multiple masses in the mammary glands, and that were submitted to mastectomy, were included in the study (Table S1). Queens that presented, at diagnosis, concomitant diseases, other types of tumours, history of previously diagnosed neoplasia (mammary or other tumours) or with benign mammary lesions were excluded from this research. Data from queens that developed other diseases or other malignant tumours during the follow-up period, and data from queens that were submitted to chemotherapy was only considered for determinations of APP and antioxidants at presentation, but was excluded from the survival analysis.
In all diseased animals included in the study, physical and clinical examination including haematology, serum biochemistry profile, lymph node evaluation, thoracic radiology, abdominal ultrasonography and histopathology of the excised mammary tumours were performed at admission and also in control visits when clinically indicated and at owners consent. Recommended post-surgical control protocol included clinical reevaluations at 1 and 3 months after surgery, and then at every 3 months until death of the animal, or when local disease or distant metastasis were detected. Identification and clinical parameters evaluated included age, breed, weight, neuter status, tumour size, tumour ulceration, regional lymph node neoplastic invasion, presence of distant metastasis, clinical stage, disease-free interval (DFI; interval of time from surgical treatment to detection of either local tumour recurrence or metastasis in the regional lymph nodes or other organs) and survival time from diagnosis.
Whole blood samples were collected in all the diseased queens included in the study before surgery, and whenever possible and clinically indicated in subsequent control visits, into ethylenediaminetetraacetic acid (EDTA) tubes (K3EDTA tubes, Aquisel, Barcelona, Spain) for haematology and into tubes without anticoagulant (Vacuette Z serum clot activator, Greiner Bio-One International GmbH, Kremsmünster, Austria) for serum biochemistry. Within 20 minutes after collection, these were centrifuged (10 minutes, 2000×g) and supernatant was used for analyses. Remaining serum samples were stored after use at −80°C. APP and antioxidants were determined in the remaining serum samples that were collected for clinical purposes; no blood samples were collected exclusively for this study. Lymph node evaluation was performed by lymph node palpation and cytology of the enlarged lymph nodes by fine needle aspiration; and thoracic radiology (three projections) and abdominal ultrasonography were performed for clinical staging at diagnosis, and during the follow-up period whenever possible and clinically indicated. Clinical staging was determined according to the modified World Health Organization (WHO) staging criteria (Table S2).27
Serum samples from 12 queens considered clinically healthy based on clinical history, physical examination and results of complementary diagnostic exams, and that required haematological analysis for elective surgical procedures, geriatric check-ups or determination of feline immunodeficiency virus (FIV) and feline leukaemia virus (FeLV) infection status, were used as controls (Table S1).
The anaesthetic protocol used in the diseased queens and in the queens from the control group that were submitted to elective surgical procedures consisted of premedication with butorphanol (0.2 mg/kg) or methadone (0.4 mg/kg), midazolam (0.2 mg/kg) and ketamin (5 mg/kg), induction with propofol (2-4 mg/kg), and maintenance with isoflurane (1%-2%). Meloxican (initial dose of 0.2 mg/kg, SC, maintenance dose of 0.05 mg/kg, PO, every 24 hours) and methadone (0.2-0.4 mg/kg, SC, every 4-6 hours) or buprenorphine (0.03 mg/kg, SC, every 6 hours) were used as analgesics in the post-surgical period. Post-surgical antibiotherapy with amoxicillin/clavulanic acid (12.5-20.0 mg/kg, PO, every 12 hours) was administered in all queens of the diseased (during 7-10 days) and control (during 5 days) groups. The post-surgical period was monitored in all the queens included in the study. The post-operative recovery was uneventful in all the queens of the control group. In the queens of the diseased group, occasional mild complications in the surgical wound were observed during the post-operative period, but all resolved within 2 weeks after surgery.
2.2 Histopathology
After surgery, mastectomy samples were fixed in 10% buffered formalin, routinely processed and 3-μm sections were cut and stained with haematoxylin and eosin. Histological classification of feline mammary tumours was independently performed by two pathologists based on the criteria of the WHO for the histological classification of mammary tumours of domestic animals.28
The histological parameters analysed included tumour type and grade, presence of neoplastic vascular invasion, presence of tumour necrosis and assessment of mitotic counts (determined in 10 high-power fields). Tumour resection margins were assessed in all cases, and classified as tumour infiltrated margins (<1 mm), narrow tumour-free surgical margins (≥1 mm to <3 mm) or as tumour-free surgical margins (≥3 mm). Queens with tumour-narrow free surgical margins or with tumour infiltrated margins were submitted to a second surgery to obtain tumour-free wide surgical margins whenever possible and clinically indicated. In queens with multiple tumours, the characteristics of the nodule with larger dimension and with higher histological grade were considered for statistical analysis.
Histological grade of mammary carcinomas was determined in accordance to the Nottingham histological grading system, based on the assessment of three histological features, namely tubule formation, mitotic counts and nuclear pleomorphism.29 Mammary carcinomas were classified as grade I (well differentiated), grade II (moderately differentiated) or grade III (poorly differentiated).
2.3 APP and antioxidants
Serum concentration of SAA, Hp, albumin, BChE, IGF1, PON1, Thiol, GPox and TAC were determined in all samples. Total antioxidant capacity was determined in all cases by four spectrophotometric methods, including FRAP, CUPRAC and TEAC determined by two methodologies, namely TEAC1 and TEAC2. Only serum samples free of haemolysis and lipaemia were used for analysis, since significant analytical interference with APP and antioxidants determinations have been reported.30-32
2.4 APP analysis
SAA concentrations were determined by a human turbidimetric immunoassay (LZ-SAA; Eiken Chemical Co., Tokyo, Japan) previously validated for use in cats.33 Serum Hp concentrations were determined by use of the haemoglobin-binding method with the use of a commercial kit (Tridelta Development Ltd., Kildare, Ireland). The method was previously validated for use in cats.34 Serum albumin was determined using a commercially available kit (Albumin OSR 6102; Olympus Life and Material Science Europe GmbH, Irish branch, Ennis, Ireland) following instructions of the manufacturer. Serum BChE concentrations were determined by spectrophotometry, using butyrylthiocoline as substrate, through a method previously validated for use in cats.34 Serum concentrations of IGF1 were determined using an automated solid-phase, enzyme-labelled immunochemiluminescent assay (Immulite System; Siemens Health Diagnostics, Malvern) previously validated for use in cats.34
2.5 Antioxidants analysis
Serum PON1 concentrations were determined by spectrophotometry, using p-nitrophenylacetate as substrate through a method previously validated for cats.34 Serum GPox concentrations were determined by a commercial kit (Ransel, Randox Laboratories Limited, Crumlin, UK) previously validated for use in cats.35 Total serum thiol concentrations were determined by a method that uses aromatic disulfides as reagents, previously validated for use in cats.36 The FRAP assay uses the ferric-tripyridyltriazine complex (Fe3+-TPTZ) as reagent,32, 37 through a method previously validated for cats.36 The CUPRAC was determined by a method that uses bathocuproinedisulfonic acid disodium salt as chelating agent,16, 38 previously validated for use in feline serum.36 The TEAC was determined by two different methodologies, namely TEAC using an acidic medium (TEAC1) and TEAC using the horse radish peroxidase (TEAC2), both methods previously validated for cats.35, 36, 39, 40
All analyses were performed on an automated biochemistry analyser (Olympus AU600, Olympus Diagnostica, GmbH).
2.6 Statistical analysis
Results are shown as medians with interquartile range (IQR) unless otherwise stated. Concentrations of APP and antioxidants with results lower than detection limit were set as equal to the detection limit for further statistical analysis. D'Agostino & Pearson omnibus normality test was used to assess normality. As most data were not normally distributed, a Kruskal-Wallis test followed by the Dunns multiple comparison test were used. Differences in concentrations of the analytes between cats with mammary tumours at presentation and healthy control cats were assessed. In addition, the possible changes in analytes according with clinical and histological features of the neoplasms, namely tumour size, tumour ulceration, regional lymph node neoplastic invasion, presence of distant metastasis, clinical staging, tumour histological type and grade, presence of neoplastic vascular invasion, presence of necrosis and mitotic counts, were evaluated in the population of cats with mammary tumours, and also in comparison with healthy cats. Spearman correlation coefficient was used to determine correlations between concentrations of APP and parameters of oxidative status and size of tumour. Furthermore, in the diseased queens, differences between concentrations of APP and biomarkers of oxidative status at presentation and at different time points in the evolution of the disease were also assessed. The Cox proportional hazards model was used to investigate the prognostic value of serum analytes in overall survival by adjusting for confounding factors. Results of the multivariate analyses are presented as hazard ratios (HR) with 95% confidence intervals (CI). Overall survival was plotted using the Kaplan-Meier method, with statistical analysis by means of the Log-rank test. In order to discover the best cut-off point for those variables that were influenced by clinical outcome, different values were assessed, and the value that separates most significantly Kaplan-Meier curves of two resulting groups were selected. Statistical analysis was performed using Graph Pad Prism (version 6, GraphPad Software Inc., California) and SPSS (version 19.0, SPSS, Illinois). A P value <.05 was used to determine the level of statistical significance.
3 RESULTS
Characterization of the diseased (n = 50) and healthy control (n = 12) cats is presented in Table S1. Most of the animals included in the study were domestic short-hair cats (47 out of the 50 diseased cats, and 11 out of the 12 controls), and approximately half in each group were intact and half were neutered at presentation. Most of the queens of the diseased group had history of contraceptives administration. No statistically significant differences were detected between body weight of the two groups of cats (P = .886), but control cats were significantly younger than diseased queens (P = .006). Most of the diseased queens presented tubulopapillary (n = 23) or solid (n = 16) adenocarcinomas. The group of queens with mammary carcinomas presented a median DFI of 6 months, and a median survival time (MST) of 11 months.
3.1 APP and antioxidants at presentation
Data, presented as medians and IQR, of serum concentrations of APP and antioxidants in queens with malignant mammary carcinomas at presentation and controls are presented in Table 1. Serum concentrations of the positive APP, SAA and Hp were significantly higher, and of the negative APP, albumin and BChE were significantly lower in cats with malignant mammary tumours than in healthy control cats. Queens with mammary carcinomas also presented serum GPox and TAC determined by TEAC1, TEAC2 and CUPRAC methods significantly lower than controls. Although the difference with controls was not significant, a tendency to decrease was also detected in serum Thiol concentrations of the diseased queens (P = .059).
| Mammary carcinoma group | Control group | P | |
|---|---|---|---|
| SAA (μg/mL) | 0.4 (0.38-15.2) | 0.38 (0.38-0.38) | .023 |
| Hp (g/L) | 3.8 (2.7-5.5) | 3.1 (2.7-3.6) | .027 |
| Albumin (g/dL) | 2.8 (2.4-3.0) | 3.1 (2.8-3.2) | .019 |
| BChE (μmol/mL·min) | 1.5 (1.0-1.8) | 2.4 (1.6-2.6) | .002 |
| IGF1 (ng/mL) | 300 (220-423) | 280 (129-427) | .549 |
| PON1 (IU/mL) | 3.5 (2.8-4.1) | 3.8 (2.8-4.8) | .402 |
| Thiol (mmol/L) | 0.175 (0.111-0.230) | 0.257 (0.119-0.313) | .059 |
| GPox (IU/mL) | 2.8 (1.8-5.2) | 6.1 (4.8-6.8) | .001 |
| TEAC1 (mmol/L) | 0.474 (0.405-0.638) | 0.662 (0.583-0.714) | .002 |
| TEAC2 (mmol/L) | 0.340 (0.300-0.403) | 0.438 (0.398-0.459) | <.001 |
| FRAP (mmol/L) | 0.392 (0.326-0.421) | 0.463 (0.356-0.470) | .192 |
| CUPRAC (mmol/L) | 0.372 (0.321-0.402) | 0.466 (0.415-0.491) | <.001 |
- Abbreviations: BChE, butyrylcholinesterase; CUPRAC, cupric reducing antioxidant capacity; FRAP, ferric reducing ability of plasma; GPOx, glutathione peroxidase; Hp, haptoglobin; IGF 1, insulin-like growth factor 1; PON1, paraoxonase 1; SAA, serum amyloid A; TEAC1 and TEAC2, trolox equivalent antioxidant capacity, methods 1 and 2; Thiol, total serum thiols.
- Significant differences are presented in bold.
3.2 APP and antioxidants at presentation in accordance to clinical and histopathological features of mammary tumours
Data, presented as medians and IQR, of serum concentrations of APP and antioxidants of cats with malignant mammary carcinomas at presentation in accordance to clinical parameters—tumour size, tumour ulceration, regional lymph node neoplastic invasion, presence of distant metastasis and clinical staging; and histological parameters - tumour type and grade, presence of neoplastic vascular invasion, presence of necrosis and mitotic counts are presented in Tables 2-5. Correlations between the size of the tumour and concentrations of APP and antioxidants are presented in Table S3.
| n | SAA (μg/mL) | Hp (g/L) | Alb (g/dL) | BChE (μmol/mL·min) | IGF1 (ng/mL) | ||
|---|---|---|---|---|---|---|---|
| Tumour size | <2 cm | 14 | 0.38 (0.38-0.60) | 3.27 (2.67-3.50)† | 2.94 (2.66-3.10) | 2.00 (1.60-2.30)† | 387.0 (281.5-467.5) |
| ≥2 cm | 36 | 0.70 (0.38-35.63)*** | 4.77 (3.03-5.62)*† | 2.77 (2.25-2.97)* | 1.30 (0.75-1.66)**† | 272.0 (194.0-372.0) | |
| Ulceration | Positive | 19 | 0.80 (0.38-29.50)*** | 5.27 (3.80-5.62)**‡ | 2.60 (2.18-2.94)** | 1.30 (1.05-1.65)** | 286.0 (211.5-371.5) |
| Negative | 31 | 0.38 (0.38-3.10)** | 3.28 (2.51-4.81)‡ | 2.87 (2.69-3.06) | 1.60 (0.95-2.10)* | 323.0 (223.0-423.0) | |
| Ln. invasion | Positive | 27 | 0.70 (0.38-12.0)*** | 4.76 (2.92-5.54)* | 2.81 (2.28-2.99) | 1.35 (0.93-1.80)* | 306.0 (231.5-412.5) |
| Negative | 23 | 0.40 (0.38-47.5)** | 3.44 (2.74-5.47) | 2.87 (2.47-3.00) | 1.50 (1.00-2.20)* | 279.5 (210.3-423.0) | |
| Distant mets. | Positive | 10 | 0.38 (0.38-16.65)** | 4.77 (4.20-5.52)* | 2.84 (2.39-2.91)* | 1.30 (0.60-1.45)** | 332.0 (250.3-565.0) |
| Negative | 40 | 0.40 (0.38-7.90)*** | 3.53 (2.74-5.50) | 2.85 (2.40-2.99) | 1.60 (1.03-2.00)* | 299.5 (218.5-420.0) | |
| Clin. staging | I | 7 | 0.38 (0.38-0.50) | 3.04 (2.71-3.40) | 3.01 (2.80-3.17) | 2.20 (1.53-2.50) | 412.0 (307.3-527.3) |
| II | 3 | 0.40 (0.38-66.50)* | 3.75 (3.00-5.47) | 2.92 (2.67-2.99) | 1.40 (0.80-2.30) | 338.0 (221.3-440.5) | |
| III | 30 | 0.40 (0.38-15.20)*** | 3.85 (2.66-5.69) | 2.76 (2.26-2.95)* | 1.60 (1.00-1.80)* | 279.5 (188.0-363.0) | |
| IV | 10 | 0.38 (0.38–16.65)** | 4.77 (4.20–5.52)* | 2.84 (2.39–2.91)* | 1.30 (0.60–1.45)** | 332.0 (250.3–565.0) |
- Abbreviations: Alb, albumin; BChE, butyrylcholinesterase; Clin, clinical; DFI, disease-free interval; Hp, haptoglobin; IGF1, insulin-like growth factor 1; IQR, interquartile range; Ln, lymph node; Mets, metastasis; MST, median survival time; SAA, serum amyloid A.
- Significant differences are presented in bold: *P < .05 with controls, **P < .01 with controls, ***P˂.001 with controls, †P < .05 between tumours <2 cm and tumours ≥2 cm, ‡P < .01 between ulcerated and non-ulcerated tumours.
| n | SAA (μg/mL) | Hp (g/L) | Alb (g/dL) | BChE (μmol/mL·min) | IGF1 (ng/mL) | ||
|---|---|---|---|---|---|---|---|
| Histopathology | Tubulopapillary | 23 | 0.38 (0.38-0.95)** | 3.44 (2.66-4.76) | 2.82 (2.56-2.93)* | 1.30 (1.00–1.80)* | 258.5 (205.3-396.5) |
| Solid | 16 | 0.38 (0.38-25.55)** | 4.99 (3.14-6.32) | 2.86 (2.06-3.09) | 1.75 (1.30-2.05) | 371.5 (297.5-420.0) | |
| Cribriform | 5 | 0.70 (0.38-122.1)** | 3.43 (2.88-5.23) | 2.87 (1.98-3.10) | 1.80 (1.00-2.75) | 375.5 (176.3-542.5) | |
| Other tumours | 6 | 8.80 (0.38-98.40)*** | 5.51 (2.77-6.10) | 2.95 (2.23-3.01) | 1.40 (0.70-1.60)* | 292.5 (229.0-448.0) | |
| Gradea | I | 4 | 0.70 (0.38-1.03)* | 3.09 (2.38-6.59) | 2.91 (2.79-2.94) | 1.00 (0.18-2.13) | 219.5 (168.5-313.3) |
| II | 14 | 0.40 (0.38-57.0)*** | 3.47 (2.79-5.46) | 2.74 (2.54-3.02) | 1.60 (1.10-2.20) | 358.5 (221.5-433.0) | |
| III | 26 | 0.38 (0.38-3.00)** | 4.64 (2.66-5.43)* | 2.84 (2.30-2.91)* | 1.50 (1.00–1.80)* | 303.0 (217.3-391.8) | |
| Vasc. Invasion | Positive | 24 | 0.38 (0.38-1.20)** | 4.39 (3.27-5.43)* | 2.78 (2.23-2.87)**† | 1.30 (1.00-1.63)** | 279.5 (217.3-420.0) |
| Negative | 26 | 0.90 (0.38-86.18)*** | 3.48 (2.68-5.60) | 2.92 (2.59-3.06)† | 1.60 (0.90-2.20)* | 318.0 (214.0-423.0) | |
| Necrosis | Positive | 38 | 0.38 (0.38-8.80)*** | 4.64 (3.27-5.63)*‡ | 2.78 (2.37-2.96)* | 1.40 (1.10-1.80)* | 302.5 (212.8-425.5) |
| Negative | 12 | 0.50 (0.38-62.70)*** | 2.80 (2.03-3.88)‡ | 2.94 (2.62-3.06) | 1.50 (0.50-2.20)* | 286.0 (232.0-399.0) | |
| Mitotic counts | 0–9 | 7 | 2.00 (0.38-57.68)** | 3.85 (2.10-6.37) | 2.76 (2.02-3.00) | 1.25 (0.40-1.53)* | 304.0 (265.3-363.0) |
| 10-19 | 10 | 0.70 (0.40-6.88)*** | 3.57 (3.27-6.71) | 2.93 (2.79-2.98) | 1.35 (0.43-1.93)* | 244.0 (200.5-407.0) | |
| ≥20 | 33 | 0.38 (0.38-2.65)** | 4.31 (2.74-5.52) | 2.73 (2.28-2.98)* | 1.60 (1.10-1.95) | 323.0 (211.8-430.5) |
- Abbreviations: Alb, albumin; BChE, butyrylcholinesterase; DFI, disease-free interval; Hp, haptoglobin; IGF1, insulin-like growth factor 1; IQR, interquartile range; MST, median survival time; SAA, serum amyloid A; Vasc., vascular.
- a According with the Nottingham grading system (Elston and Ellis29), histological grade was not determined for mucinous and squamous cell carcinomas.
- Significant differences are presented in bold: *P < .05 with controls, **P < 0.01 with controls, ***P < .001 with controls, †P < .05 between cats with and without neoplastic vascular invasion and ‡P < .05 between tumours with and without necrosis.
| n | TEAC1 (mmol/L) | TEAC2 (mmol/L) | FRAP (mmol/L) | CUPRAC (mmol/L) | Thiol (mmol/L) | GPox (IU/mL) | PON1 (IU/mL) | ||
|---|---|---|---|---|---|---|---|---|---|
| Tumour size | <2 cm | 14 | 0.43 (0.30-0.52)** | 0.39 (0.34-0.46) | 0.40 (0.32-0.41) | 0.39 (0.35-0.45) | 0.22 (0.17-0.26) | 2.44 (2.02-5.13) | 4.07 (3.67-4.99)† |
| ≥2 cm | 36 | 0.51 (0.41-0.65)* | 0.34 (0.30-0.37)*** | 0.37 (0.32–0.41) | 0.37 (0.32-0.39)*** | 0.15 (0.10-0.19)* | 3.19 (1.72-5.23)** | 3.36 (2.72-3.72)† | |
| Ulceration | Positive | 19 | 0.57 (0.46-0.68)*‡ | 0.33 (0.30-0.34)** | 0.37 (0.30-0.42)* | 0.36 (0.33-0.39)*** | 0.15 (0.10-0.18)* | 4.50 (1.80-5.68)* | 3.38 (2.70-3.72) |
| Negative | 31 | 0.43 (0.38-0.53)***‡ | 0.35 (0.27-0.42)* | 0.40 (0.33-0.42) | 0.37 (0.32-0.42)** | 0.20 (0.11-0.26)* | 2.52 (1.73-3.91)** | 3.61 (2.84-4.61) | |
| Ln. invasion | Positive | 27 | 0.46 (0.40-0.66)* | 0.34 (0.29-0.34)** | 0.37 (0.32–0.41) | 0.35 (0.31-0.38)*** | 0.15 (0.10-0.24)* | 2.52 (1.65-4.65)** | 3.41 (2.65-3.88) |
| Negative | 23 | 0.49 (0.38-0.63)* | 0.34 (0.31-0.42)* | 0.39 (0.32–0.42) | 0.38 (0.32-0.42)** | 0.19 (0.14-0.22) | 3.01 (1.78-5.64)* | 3.60 (2.92-4.32) | |
| Distant mets. | Positive | 10 | 0.57 (0.44-0.64) | 0.31 (0.26-0.34)** | 0.37 (0.34-0.40) | 0.38 (0.31–0.38)** | 0.16 (0.12-0.23) | 3.87 (2.91-5.20) | 2.81 (2.65-3.52) |
| Negative | 40 | 0.46 (0.40-0.67)** | 0.34 (0.30-0.41)* | 0.39 (0.32–0.41) | 0.36 (0.34-0.41)*** | 0.18 (0.11-0.22) | 2.44 (1.52-4.85)* | 3.60 (2.86-4.46) | |
| Clin. staging | I | 7 | 0.46 (0.16-0.64) | 0.42 (0.38-0.47) | 0.40 (0.30-0.40) | 0.43 (0.42-0.45) | 0.22 (0.21-0.25) | 2.85 (2.43-4.72) | 3.81 (3.42-5.15) |
| II | 3 | 0.53 (0.37-0.68) | 0.42 (0.32-0.58) | 0.42 (0.36-0.53) | 0.39 (0.34-0.42) | 0.15 (0.10-0.25) | 0.92 (0.46-5.30)* | 3.54 (2.47-3.99) | |
| III | 30 | 0.45 (0.40-0.68)* | 0.34 (0.29-0.38)** | 0.37 (0.32–0.41) | 0.35 (0.33-0.40)*** | 0.15 (0.10-0.20) | 2.31 (1.57-5.18)** | 3.59 (2.87-4.61) | |
| IV | 10 | 0.57 (0.44–0.64) | 0.31 (0.26–0.34)** | 0.37 (0.34–0.40) | 0.38 (0.31–0.38)** | 0.15 (0.12-0.18) | 3.83 (2.72-5.23) | 2.81 (2.65–3.52) |
- Abbreviations: Clin, clinical; CUPRAC, cupric reducing antioxidant capacity; DFI, disease-free interval; FRAP, ferric reducing ability of plasma; GPox, glutathione peroxidase; IQR, interquartile range; Ln, lymph node; Mets, metastasis; MST, median survival time; PON1, paraoxonase 1; TEAC1 and TEAC2, Trolox equivalent antioxidant capacity, methods 1 and 2; Thiol; total serum thiols.
- Significant differences are presented in bold: *P < .05 with controls, **P < .01 with controls, ***P˂.001 with controls, †P < .05 between tumours ˂2 cm and tumours ≥2 cm and ‡P < .05 between cats with and without ulcerated tumours.
| n | TEAC1 (mmol/L) | TEAC2 (mmol/L) | FRAP (mmol/L) | CUPRAC (mmol/L) | Thiol (mmol/L) | GPox (IU/mL) | PON1 (IU/mL) | ||
|---|---|---|---|---|---|---|---|---|---|
| Histopathology | Tubulopapillary | 23 | 0.47 (0.41-0.57)** | 0.34 (0.31-0.41)* | 0.40 (0.35-0.42) | 0.38 (0.35-0.39)** | 0.17 (0.11-0.23) | 2.40 (1.03-4.48)** | 3.64 (2.71-3.98) |
| Solid | 16 | 0.47 (0.27-0.64)** | 0.34 (0.29-0.36)** | 0.37 (0.30–0.40) | 0.35 (0.31-0.40)** | 0.16 (0.11–0.23) | 3.09 (1.90-5.80) | 3.39 (2.92-4.09) | |
| Cribriform | 5 | 0.55 (0.44-0.68) | 0.38 (0.32–0.41) | 0.33 (0.32-0.41) | 0.36 (0.33-0.43) | 0.18 (0.10-0.36) | 3.55 (2.36-5.24) | 4.00 (2.78-4.95) | |
| Other tumours | 6 | 0.47 (0.36-0.67) | 0.34 (0.27–0.42)* | 0.32 (0.27-0.53) | 0.31 (0.25-0.41)*** | 0.19 (0.11-0.27) | 3.45 (2.13-5.81) | 3.38 (2.74-4.00) | |
| Gradea | I | 4 | 0.42 (0.14-0.44)** | 0.27 (0.23-0.33)** | 0.41 (0.41-0.42) | 0.35 (0.34-0.36)* | 0.14 (0.09-0.19) | 1.79 (0.75-3.58)* | 3.80 (3.07-3.87) |
| II | 14 | 0.46 (0.30-0.58)** | 0.34 (0.33–0.43) | 0.40 (0.35-0.43) | 0.39 (0.34–0.42)* | 0.18 (0.14–0.22) | 2.14 (0.92-3.27)** | 3.89 (3.38-4.77) | |
| III | 26 | 0.51 (0.40-0.64)* | 0.34 (0.30–0.41)** | 0.37 (0.32-0.40) | 0.38 (0.32–0.40)** | 0.17 (0.11-0.25) | 3.28 (2.20-5.69) | 3.36 (2.70-3.78) | |
| Vasc. invasion | Positive | 24 | 0.47 (0.40-0.62)* | 0.33 (0.29-0.37)** | 0.35 (0.33–0.40)* | 0.37 (0.31–0.38)*** | 0.15 (0.12-0.20)* | 2.91 (1.73-4.50)** | 3.32 (2.64-3.73) |
| Negative | 26 | 0.47 (0.37-0.67)** | 0.34 (0.32–0.42)* | 0.40 (0.32-0.43) | 0.36 (0.34–0.42)** | 0.18 (0.10–0.25) | 2.51 (1.74-5.85)* | 3.69 (3.11-4.46) | |
| Necrosis | positive | 38 | 0.49 (0.40-0.65)** | 0.34 (0.30-0.36)***† | 0.37 (0.32–0.41)* | 0.37 (0.32–0.39)*** | 0.15 (0.11–0.22)* | 2.91 (1.86-5.58)** | 3.54 (2.71-4.59) |
| negative | 12 | 0.45 (0.30-0.53)* | 0.39 (0.33–0.43)† | 0.40 (0.30-0.53) | 0.36 (0.32–0.43)* | 0.21 (0.14-0.25) | 2.56 (1.62-3.59)** | 3.57 (2.85-3.89) | |
| Mitotic counts | 0–9 | 7 | 0.47 (0.30-0.57)* | 0.34 (0.31-0.54) | 0.40 (0.32-0.52) | 0.35 (0.33–0.39)* | 0.13 (0.04-0.16)* | 0.97 (0.57-2.15)***‡ | 3.06 (2.43-3.53) |
| 10–19 | 10 | 0.44 (0.33-0.46)* | 0.31 (0.27-0.38)* | 0.41 (0.40-0.44) | 0.36 (0.34-0.39)* | 0.15 (0.12-0.21)* | 2.21 (1.01-3.62)** | 3.86 (3.55-4.57) | |
| ≥20 | 33 | 0.50 (0.40–0.65)* | 0.34 (0.30–0.41)* | 0.35 (0.32–0.40) | 0.38 (0.31–0.42)** | 0.18 (0.10–0.25)* | 3.27 (2.11-5.46)*‡ | 3.59 (2.75-4.33) |
- Abbreviations: Clin, clinical, CUPRAC, cupric reducing antioxidant capacity; DFI, disease-free interval; FRAP, ferric reducing ability of plasma; GPox, gluthatione peroxidase; IGF1, insulin-like growth factor 1; IQR, interquartile range; Ln, lymph node; Mets, metastasis; MST, median survival time; PON1, paraoxonase 1; TEAC1 and TEAC2, Trolox equivalent antioxidant capacity, methods 1 and 2; Thiol, total serum thiols; Vasc, vascular.
- a According with the Nottingham grading system (Elston and Ellis29), histological grade was not determined for mucinous and squamous cell carcinomas.
- Significant differences are presented in bold: *P < .05 with controls, **P < .01 with controls, ***P˂.001 with controls, †P < .05 between cats with and without tumour necrosis, ‡P < .05 between tumours with mitotic counts ≤9 and tumours with mitotic counts ≥20 in 10 hpf.
Serum concentrations of SAA and Hp were significantly positively correlated (ρ = 0.278, P = .048; ρ = 0.304, P = .032, respectively), and albumin, BChE and IGF1 (ρ = −0.277, P = .049; ρ = −0.437, P = .002; ρ = −0.448, P = .001, respectively) were significantly negatively correlated with tumour dimensions, however, the correlation coefficients were weak to moderate. Concentrations of SAA and Hp were significantly higher, and of albumin and BChE were significantly lower in queens with tumours ≥2 cm than in control queens. Moreover, concentrations of Hp were significantly higher, and of BChE were significantly lower in queens with tumours ≥2 cm than in queens with tumours <2 cm. In addition, size of tumour was significantly negatively correlated with serum PON1, and queens with tumours ≥2 cm presented PON1 significantly lower than queens with tumours <2 cm. Serum TEAC2, CUPRAC and Thiol were significantly lower, and GPox was significantly higher in queens with tumours ≥2 cm than in healthy queens.
Queens with ulcerated tumours showed significantly higher serum Hp and TEAC1 concentrations than queens with non-ulcerated tumours and than controls; and also significantly lower albumin and FRAP than controls.
Queens with neoplastic vascular emboli presented significantly lower albumin than queens without evidence of neoplastic vascular invasion. No other significant differences in any of the evaluated analytes were detected between queens with and without neoplastic vascular invasion, metastasis in the regional lymph nodes or metastasis in distant organs. However, compared with healthy controls, diseased queens with neoplastic vascular invasion and cats with metastasis in distant organs presented significantly higher Hp and lower albumin, and queens with metastasis in regional lymph nodes had significantly higher Hp. Also compared with healthy controls, serum Thiol was significantly lower in queens with vascular invasion and in cats with metastasis in regional lymph nodes; and serum FRAP was lower in animals with neoplastic vascular invasion.
No significant differences were detected in concentrations of APP or antioxidants between diseased queens in different clinical stages. However, when compared with controls, stages higher than I showed higher SAA values, stages higher than II showed lower values for albumin, BChE, TEAC2 and CUPRAC, and stage IV showed higher values for Hp.
Cats with tubulopapillary adenocarcinomas presented significantly lower albumin, BChE, Gpox, TEAC1, TEAC2 and CUPRAC than controls. Significant decreases in serum TEAC1, TEAC2 and CUPRAC were also detected in queens with solid carcinomas; and in BChE, TEAC2 and CUPRAC were detected in animals of the group of other carcinomas when compared with controls. Queens with mammary carcinomas with higher histological grade presented significantly higher Hp and lower albumin and BChE than controls, while serum GPox tended to decrease in those with lower histological grade.
Queens with tumour necrosis had significantly higher Hp and lower TEAC2 than queens without evidence of necrosis; and also significantly higher Hp and lower albumin, Thiol, TEAC2 and FRAP than controls.
Concentrations of albumin and TEAC2 decreased in queens with tumours with higher mitotic counts, while concentrations of BChE and GPox decreased in tumours with lower proliferation activity.
3.3 APP and antioxidants at follow-ups
Differences in concentrations of APP and antioxidants (presented as medians and IQR) in queens with mammary carcinomas without distant metastasis at presentation, and concentrations at the time of diagnosis of distant metastasis are presented in Table S4 (n = 11). Serum concentrations of SAA (P = .035) and TEAC1 (P = .024) were significantly higher at time of detection of distant (thoracic or abdominal) metastasis than at diagnosis.
3.4 Overall survival
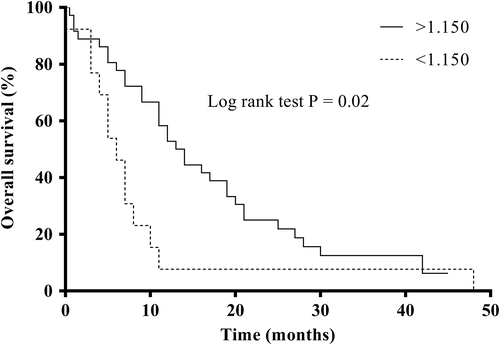
Cox regression hazards model was applied to estimate the impact of APP and antioxidants (determined at diagnosis) on overall survival (Table S5). The multivariate analysis revealed that concentrations of albumin (P = .038) and of BChE (P = .002) at diagnosis were independent predictors of mortality. Queens with decreased serum albumin concentrations at diagnosis presented a longer survival time than queens with serum albumin in the reference range. Moreover, the Kaplan-Meier curve of serum BChE (Figure 1) showed that concentrations of BChE at diagnosis lower than 1.15 μmoL/mL.minute were significantly related to a shorter survival. Diseased queens with serum BCHE <1.15 μmoL/mL.minute had a MST of 9.0 months (95% CI: 2.4-15.6 months), while cats with BChE >1.15 μmoL/mL.minute presented a MST of 16.9 months (95% CI: 12.7-21.0 months) (Figure 1). A tendency for a shorter survival time was also observed in diseased queens with increased SAA (P = .061), and decreased IGF1 (P = .070) and TEAC2 (P = .066).
4 DISCUSSION
Inflammation and oxidative damage have been associated with several tumours in humans and animals.8, 41, 42 Furthermore, development of an APP response and of oxidative stress were previously described in human breast cancer and in canine mammary tumours.21-26 APP and parameters of oxidative status were also proved to be clinically useful biomarkers in feline oncologic diseases,43-45 however, to the best of our knowledge, this is the first study to comprehensively evaluate the APP and the antioxidant responses of feline spontaneous malignant mammary tumours.
The APP response was evaluated through an APP profile, including a positive major (SAA), a positive moderate (Hp) and three negative (albumin, BChE and IGF1) APP. The evaluation of APP profiles that include at least one positive major, one positive moderate and one negative APP are recommended in order to better differentiate between pathological states and to obtain information on the evolution of the disease.46 The oxidative status was assessed through determination of serum concentrations of three individual antioxidants (PON1, Thiol and GPox) and of TAC by different methods (TEAC1, TEAC2, FRAP and CUPRAC). The evaluation of TAC in biological samples by different methods has been recommended, because they are based on diverse chemical reactions and consequently provide different information, allowing a better characterization of the antioxidant status of the organism.19, 20, 47 Furthermore, in the present study, TEAC was determined by two different methods. Both TEAC assays evaluate the capacity of antioxidants in a sample, mainly albumin, ascorbic acid, urate, α-tocopherol and bilirubin to reduce the radical 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) in the cation form (ABTS+), however, diverge in the method of its synthesis; in the TEAC1 method, the ABTS is oxidized to ABTS+ using hydrogen peroxide (H2O2) in an acidic medium, while in the TEAC2 method, ABTS is oxidized through the use of the enzyme horse radish peroxidase.48, 49 Overall the results obtained in the two assays are complementary.20, 36
The present study revealed that feline spontaneous malignant mammary tumours are associated with an APP response and with development of oxidative stress, since significant changes in concentrations of APP and antioxidants were detected in diseased queens when compared with controls.
The group of queens with malignant mammary tumours (n = 50) presented, at diagnosis, significantly higher serum concentrations of SAA and Hp, and significantly lower serum albumin and BChE than controls. Similar data was also reported in female dogs with mammary tumours.22, 23, 26 However, canine mammary tumours were considered weak inducers of APP, unless if they were of big dimensions, of specific histopathological types or were associated with metastasis, ulceration or secondary inflammation.22, 23, 50, 51 In the present study, the size of the tumour also influenced the development of an APP response, however, significant changes were also observed in smaller tumours. Furthermore, significant changes in APP were also associated with other tumour characteristics assessed in this study such as presence of ulceration, neoplastic emboli in lymphatic vessels, metastasis in regional lymph nodes and distant organs, histological type and grade, necrosis and higher proliferative activity, suggesting that mammary tumours may induce a stronger APP response in cats than in dogs. In general, the APP response was higher in tumours that presented features associated with a worst prognosis, suggesting that a systemic inflammatory process is related with more severe and complicated cases of feline malignant mammary tumours.
IGF1 was also associated with pathogenesis of canine malignant mammary neoplasia,52, 53 and was implicated in proliferation of mammary tissues in feline fibroadenomatous change.54 However, in our study, no significant differences in serum concentrations of IGF1 were detected between diseased queens at diagnosis and controls. Moreover, no significant differences in serum IGF1 were detected between the different groups of diseased queens according with the clinical and histopathological parameters evaluated, or between the different groups and controls.
In this study, queens with mammary adenocarcinomas presented, at diagnosis, concentrations of antioxidants lower than controls. In dogs, reported data concerning antioxidants in mammary adenocarcinomas are contradictory. Some studies found significantly lower serum concentrations of antioxidants in diseased females than in controls, which is in line with our results, whereas others revealed significantly higher concentrations of antioxidants in serum, blood cells hemolysates and in tumour tissue.21, 26, 55-57 These differences could be because of the different methods and assay conditions used to measure antioxidants, or because changes in antioxidant status can occur depending of the state and severity of the disease.40, 47, 58, 59 Our results suggest that, similar to the APP response, feline mammary adenocarcinomas also induce a stronger oxidative damage than canine malignant mammary tumours.
Tumour size, mainly if determined by tumour diameter, is considered as one of the most important prognostic factors of feline malignant mammary tumours.5, 8, 27 In our study, size of tumour was related to the concentrations of APP, contrarily to what is described in dogs, with exception of increased C-reactive protein (CRP) in female dogs with tumours bigger than 5 cm.22, 23 Significant increases in concentrations of APP were detected in queens with bigger tumours, and also significant positive and negative correlations were detected between size of tumour and positive and negative APP, respectively. Nonetheless, the correlation coefficients observed were weak to moderate, which could suggest that despite likely dependent to each other, other factors than tumour size may influence serum APP activity. Size of tumour also influenced significantly the concentrations of antioxidants. These results suggest that tumour dimensions are related with the systemic inflammatory response and with the oxidative damage associated with malignant mammary tumours in the feline species.
As in dogs,22, 23 tumour ulceration was associated with significant changes in concentrations of APP in our study, and was also associated with significant changes in antioxidants, showing that tumour ulceration is also implicated in the inflammatory response and in the oxidative damage of feline mammary cancer. Interestingly, contrarily to what was expected, serum TEAC1 was significantly higher in queens with ulcerated tumours than in cats without tumour ulceration. Significant increases and decreases in serum TAC were previously reported in different diseases in humans and animals.40, 47, 58, 59 As previously stated, these differences could be because of the different methods and assay conditions used to measure antioxidants, or because changes in antioxidant status can occur depending of the state and severity of the disease. We hypothesize that concentrations of antioxidants increase in the early stages of the disease process to counteract the increase in oxidants, and posteriorly a depletion in antioxidants occur because of a persistent state of oxidative stress. With the progression of the disease, the concentration of some antioxidants decrease early while others decrease later, and consequently, based on the antioxidants evaluated in each method, results in serum TAC may vary.
Increases in circulating CRP, SAA and Hp have been detected in female dogs with mammary cancer with metastasis in regional lymph nodes and/or distant organs (clinical stages IV and V) when compared with diseased females without metastasis and also with controls in the study of Tecles et al22 however, no significant differences in CRP or Hp were detected between diseased dogs with and without lymph node metastasis in the study of Planellas et al.23 In the feline species, an in vitro study showed that SAA promotes invasion of mammary carcinoma cells.60 In addition, higher concentrations of enzymatic antioxidants, including GPox, were described in neoplastic tissues of bitches with mammary tumours in clinical stages III and IV when compared with animals with mammary tumours of clinical stages I and II.57 In the present study, serum Hp at diagnosis was higher in cases of neoplasms with vascular invasion, with metastasis in regional lymph nodes and with metastasis in distant organs, suggesting that it might be clinically useful in assessment of metastatization of feline mammary tumours. In cases of vascular invasion also serum concentrations of albumin, thiol and FRAP were lower than in controls; and in addition, serum thiol and albumin were lower in cases of metastasis in regional lymph nodes and other organs, respectively, suggesting that these analytes might also be of clinical use in assessment of metastization of feline mammary cancer. Interestingly, serum TEAC1 was significantly lower in queens without distant metastasis than controls, while diseased cats with distant metastasis presented TEAC1 concentrations similar to control animals.
Furthermore, despite no significant differences in concentrations of APP were detected between groups of diseased queens according to the clinical stage, as detected in canine mammary tumours in the study of Tecles et al22 a concentrations of SAA and Hp increased, and of albumin and BChE decreased in diseased queens in higher clinical stages. Diseased cats in higher clinical stages also presented lower serum TEAC2 and CUPRAC than controls. The changes in APP and antioxidants according with the clinical stage reflect the influence of tumour size and the presence/absence of metastasis in regional lymph nodes and/or in distant organs in concentrations of these analytes. Nevertheless, results concerning queens with metastasis in distant organs (clinical stage IV) should be interpreted with caution, since only 10 queens of the diseased group presented metastasis in thoracic or abdominal organs at diagnosis. Further studies, with a higher number of female cats with mammary cancer with distant metastasis should be performed to clarify the clinical value of these parameters in queens with advanced disease.
APP and biomarkers of oxidative status have proved to be clinically useful biomarkers in monitoring evolution and/or response to treatment of different feline diseases.36, 61 However, the evolution of these analytes during the course of feline malignant mammary tumours has not been studied. According to our results, increases in serum concentrations of SAA and TEAC1 during the course of disease in queens with a previous malignant mammary tumour might suggest development of metastasis in distant organs. However, these results should also be interpreted with caution, because of the small sample size (n = 11). Although presence of lymphovascular and regional lymph node neoplastic invasion was assessed, the present study was not designed to detect micrometastasis in distant organs. Thus, future studies, with a higher number of animals and with the use of more sensitive imaging diagnostic exams should be undertaken in order to elucidate the clinical significance of these biomarkers in feline carcinogenesis, and in monitoring evolution and response to treatment of feline malignant mammary tumours.
When APP and antioxidants levels were assessed in relation to the histological type of the tumour, significant differences were observed between diseased and control queens, in accordance with previous research in dogs.23, 51 However, no differences were observed between groups of queens with different types of tumours. These changes probably reflect differences in the inflammatory response and in the oxidative damage associated with the different histological types; but future studies are necessary in order to better characterize the inflammatory and the oxidative histological features of the different tumour types.
Tumour proliferative activity and histological grade have been described as important prognostic factors of canine and feline malignant mammary tumours.7, 8, 62, 63 In our study, significant changes in concentrations of APP and antioxidants were detected in queens with malignant mammary tumours according with the proliferative activity (assessed by determination of mitotic counts) and the histological grade, showing that these features also influence the inflammatory and the antioxidant responses of feline mammary cancer. In dogs, significantly higher serum FRAP was described in females with malignant mammary tumours of low grade malignancy than in healthy controls, although no significant differences were detected between tumours of high grade malignancy and controls, and between tumours of high- and low-grade malignancy.26 Nevertheless, we failed to detect differences in serum FRAP between the different histological grades.
Tumour necrosis is reported to stimulate a pro-inflammatory tumour microenvironment, which enhances the tumour-promoting potential.41 Our results revealed that tumour necrosis was associated with significant changes in concentrations of APP and of antioxidants, probably reflecting the changes in tumour microenvironment, and suggesting that the presence of tumour necrosis also influences the inflammatory and the oxidative responses.
In the present study, queens with mammary adenocarcinomas were significantly older than queens of the control group. This difference is related with the fact that feline mammary tumours are more frequent in middle aged to older queens,3, 44 while the control group included queens presented for elective ovariohysterectomy and assessment of the FIV and FeLV infection status, which are frequently performed at younger ages. Increasing age was associated with higher concentrations of SAA (but not of other APP) in felines, which was attributed to the higher incidence of subclinical diseases in geriatric cats.9 Information about the influence of age in the oxidative status of cats is scarce, but apparently is more significant in males than in females.14 However, the reported influence of age in concentrations of APP and antioxidants9, 14 is not sufficient to explain the magnitude of differences in concentrations of APP and antioxidants between diseased and control queens obtained in this study. On the other hand, in some cases, differences between groups or subgroups were statistically significant but the median values of the groups or subgroups were similar. Although, this fact could reflect limited utility of these biomarkers in the clinical setting, the generated data contribute to the knowledge about pathogenesis of feline malignant mammary tumours.
Several serum and tissue biomarkers of inflammation and oxidative status, including APP and serum antioxidants, have been related to prognosis of human breast cancer.64-66 Similar information concerning canine and feline mammary tumours is scarce.67, 68 The multivariate analysis of the impact of APP and antioxidants, determined at diagnosis, on overall survival of queens with mammary carcinomas revealed that serum concentrations of albumin and BChE were independent predictors of mortality. Interestingly, results concerning albumin showed that diseased cats with concentrations in the reference range at diagnosis had 20.2 times more chances of a shorter survival than queens with lower concentrations. Pre-treatment concentrations of albumin have also been implicated in survival of women with breast cancer, however, as expected and in contrast to our results, physiologic concentrations have been associated with a better prognosis.69, 70 Kaplan-Meier curves showed that pre-treatment serum BChE concentrations lower than 1.15 μmoL/mL.minute were significantly related to a shorter survival time. These results provide important clinical information concerning prognosis, and might be important in treatment decision of feline mammary cancer. Increases in concentrations of SAA and decreases in IGF1 were associated with a worst prognosis in women with breast cancer,71, 72 while increased IGF1 was associated with a poor prognosis in dogs with mammary tumours.53 In our study, a tendency to a shorter survival was also observed in queens with increased SAA and decreased IGF1 and TEAC2. Future studies are required to confirm or deny these results.
During the study period, only a small number of queens were presented because of presence of mammary hyperplasias/dysplasias and benign mammary tumours, and for that reason were not included in this research. The lack of information concerning the APP and the antioxidant responses in queens with benign mammary lesions and benign mammary tumours is a limitation of this study, since detection of serum biomarkers that could aid in differentiation of benign from malignant mammary tumours before mastectomy and histopathology would have a major clinical relevance in practice. Further studies should be performed to evaluate the differences in the APP response and in the oxidative status between queens with mammary gland hyperplasias/dysplasias, benign and malignant mammary tumours.
In this study, values of SAA lower than the detection limit (DL) of the assay (0.38 μg/mL)33 were set as equal to DL for statistical analysis. It should be pointed out that this approach could have resulted in bias in the statistical results. Nevertheless, from a clinical point of view, concentrations of SAA under the DL of the assay indicate absence of systemic inflammation, independently of their expression, and, thus, have no major influence on clinical interpretation of the data.
In conclusion, the present study revealed that feline spontaneous malignant mammary tumours are associated with APP response and oxidative stress. At diagnosis, increases in serum Hp, and decreases in albumin, Thiol and Frap may suggest mammary tumour metastization; and increases in SAA and TEAC1 during the course of the disease may suggest development of metastasis in distant organs. Furthermore, serum concentrations of albumin and of BChE at diagnosis were independent predictors of mortality. Overall, these data indicate the utility of different serum biomarkers of inflammation and oxidative stress in clinical diagnosis, management and monitoring of the queens with malignant mammary tumours.
ACKNOWLEDGMENTS
Financial support was provided by the Program “Juan de la Cierva Incorporacion” of “Ministerio de Economia y Competitividad”, Spain, through a postdoctoral grant; and by the “Fundação para a Ciência e a Tecnologia (FCT)” of “Ministério da Ciência, Tecnologia e Ensino Superior”, Portugal, through the project UID/CVT/00772/2013. The present study was approved by the Scientific Council of the School of Agricultural and Veterinary Sciences of the University of Trás-os-Montes and Alto Douro, Portugal.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
HV, AT, JJC, JP and ACSF conceived and designed the study; HV, AT, JJC, ACF, SM, AR, AC, PDP, CPR, LF, FT, RC, JP and ACSF collected, analysed and interpreted data; HV, AT, JJC, JP and ACSF drafted the manuscript; all authors revised the manuscript and approved the final version of the manuscript.