Survival analysis in dogs with urinary transitional cell carcinoma that underwent whole-body computed tomography at diagnosis
Abstract
This retrospective study aimed to evaluate factors associated with survival and to compare characteristics between tumour localizations in dogs with urinary transitional cell carcinoma (TCC) that underwent whole-body computed tomography (CT) at diagnosis. Dogs with histologically confirmed TCC that received medical therapy between 2010 and 2017 were included; dogs that underwent surgery or radiotherapy for the primary tumour were excluded. According to the CT findings, primary tumour localization (classified into the Bladder, Urethra and Bladder and Urethra groups), prostate involvement, iliosacral lymphadenomegaly, sternal lymphadenomegaly and metastasis to the bone and lung were evaluated for survival analysis. CT at diagnosis revealed iliosacral lymphadenomegaly, sternal lymphadenomegaly, bone metastasis and lung metastasis in 47.7%, 18.5%, 24.6% and 35.4% of the 65 included dogs, respectively. The overall median survival time was 196 days. On multivariable analysis, TCC localization (hazard ratio [HR], 1.90; P = .037), bone metastasis (HR, 2.76; P = .013) and sternal lymphadenomegaly (HR, 3.56; P = .004) were significantly associated with survival. Compared to the Bladder group (n = 16), the Urethra group (n = 26) had higher metastasis rates to the bone (6.3% vs 42.3%; P = .045) and lung (6.3% vs 46.2%; P = .022). The survival time was shorter in the Urethra group than in the Bladder group (121.5 vs 420 days; P < .001), and it was similar only in female dogs (247 vs 420 days; P = .031). These findings suggest that whole-body CT could be valuable for predicting the prognosis in urinary TCC.
1 INTRODUCTION
Transitional cell carcinoma (TCC) is the most common urinary tract cancer in dogs, accounting for approximately 2% of all reported canine malignancies.1-3 Most dogs exhibit haematuria, stranguria and pollakiuria at diagnosis.4 Clinical signs such as pain and lameness associated with bone metastases are rarely present in dogs with TCC.3-6 Currently, because of frequent development in the trigone region, presence of undetectable microscopic tumour expansion within the bladder, and/or urethral involvement in dogs with TCC, systemic medical therapy is the mainstay of treatment.3-5 Most common medical therapies include administration of cyclooxygenase (COX) inhibitors alone, such as piroxicam, firocoxib or deracoxib; and administration of chemotherapeutic drugs, such as cisplatin, mitoxantrone, carboplatin, vinblastine or chlorambucil combined with COX inhibitors; the median survival time (ST) of dogs after these treatments ranged from 152 to 323 days for COX inhibitors alone and from 147 to 307 days for patients receiving a combination of chemotherapy and COX inhibitors.7-18
Metastases to the regional lymph nodes (LNs) and distant organs at diagnosis have been reported in 2% to 25% and 6% to 29% of dogs, respectively; moreover, at the time of death, nodal and distant metastases were seen in 26% to 48% and 50% to 58% of dogs, respectively.2, 4, 6, 8, 9, 13, 17, 18 Occasionally, skeletal metastasis can lead to severe uncontrolled bone pain which may directly lead to euthanasia. Bone metastasis has been detected in 9% to 11% of dogs at the time of death.1, 4, 19
A recent report showed that sternal LN metastasis in dogs with various malignant neoplasms was likely to occur in tumours originating from the urogenital system, including urinary TCC.20 In that study, 9 out of 25 (36%) cases of cytologically confirmed sternal LN metastasis were from urogenital neoplasms. In a large case series, 4 of 137 (3%) dogs with TCC were reported to have sternal LN metastasis among the various distant metastases, but the clinical significance of this finding as a prognostic factor remains unknown.4
Studies describing canine TCC arising from the urethra only are sparse. One study reported shorter median STs in dogs with urethral tumours (119 days) than in dogs with tumours localized cranially to the trigone (662 days).21 However, while most dogs that had lesions cranial to the trigone in this study received palliative surgery, the other dogs did not; this made the relationship between tumour locations and their outcomes difficult to interpret. In contrast, another study reported that dogs with prostatic involvement had a significantly shorter ST (109 days) than did dogs with urethral, trigonal or apically located tumours (300, 190 and 645 days, respectively).13 That study also reported no significant difference in STs between dogs with urethral involvement and those with tumours in all other locations. Moreover, the median ST was shorter in dogs with both bladder and urethral involvement (90 days) than in dogs with bladder or urethral tumours (290 days).6 These studies indicate that the outcome of patients diagnosed with TCC affecting the urethra alone can be variable and needs to be better defined; therefore, a comparison between TCCs of the bladder and urethra is clinically significant.
The current screening approach using diagnostic imaging in dogs with urinary TCC relies mainly on abdominal ultrasonography and radiography of the thorax and skeletal bones. Although ultrasonography has proven useful in investigating the bladder in detail, evaluation of the urethra is typically limited to the proximal urethra because shadowing from the pubis obscures the caudal intrapelvic urethra.22 For the same reason, the iliosacral lymphatic centre routinely identified includes the medial iliac LNs, but not more caudally positioned LNs such as the internal iliac and sacral LNs.23 Computed tomography (CT) has become widely available in veterinary practice and is being used for patient workup. This modality could overcome the aforementioned limitations when staging urinary TCCs. It has been widely recognized that CT is more sensitive than thoracic radiography for detecting pulmonary nodules in dogs.24-26 More recently, CT has been shown to be superior to ultrasonography in detecting more nodes within the iliosacral lymphatic centre and in identifying iliosacral lymphadenomegaly.27, 28 Furthermore, whole-body CT was reported to be effective in detecting skeletal metastasis in dogs with TCC.19 On the basis of these findings, primary tumours and metastasis to various sites, such as the LN, bone and lung, could be accurately evaluated using CT; however, to the authors' knowledge, no studies have described the findings of whole-body CT at diagnosis in dogs with TCC.
The aim of this retrospective analysis was to determine factors, including signalment, tumour localization and regional or distant metastasis, that influence ST in dogs with urinary TCC undergoing whole-body CT at diagnosis. Another purpose was to compare the characteristics of urethral- and bladder-origin TCCs.
2 MATERIALS AND METHODS
2.1 Cases
Dogs that were histopathologically diagnosed with urinary TCC were identified retrospectively by using the Gifu University Animal Medical Centre's medical record database between April 2010 and September 2017. Urinary bladder and urethral TCCs were assessed and classified in accordance with the World Health Organization classification system, and patients with prostate adenocarcinoma, that had the morphological features of tubular and acinar formation, were excluded.29-31 All specimens were evaluated by a single board-certified pathologist (H. S.) who was unaware of the patients' outcome. Dogs were included if they met the following criteria: (a) patients underwent whole-body CT and ultrasonography at diagnosis; (b) they received medical therapy; and (c) they had a minimum follow-up period of 6 months if they were alive. Dogs were excluded if they received surgery or radiotherapy for the primary TCC, or if they had received prior COX inhibitor treatment before diagnosis. Patient information collected from the medical records included signalment, body weight, clinical signs at diagnosis, physical examination, results of diagnostic testing (complete blood count, serum biochemistry profile and urinalysis), date of diagnosis, date of treatment initiation, treatment protocol, outcome and, when available, cause of death. Diagnostic testing abnormalities were defined as values deviating from the provided normal reference ranges used at the Gifu University Animal Medical Centre. Cause of death was classified as: related to primary TCC (such as urinary obstruction, acute renal failure or uraemia), related to TCC except for the primary tumour (such as metastasis or cancerous cachexia), or other diseases unrelated to TCC that was clearly identified. Follow-up information for dogs was obtained from medical records and telephone or fax contact with either the referring veterinarians or owners.
2.2 CT scan procedures
The dogs were positioned in sternal recumbency under sedation with medetomidine, midazolam and butorphanol. Subsequently, CT scans were acquired using a 16-detector row CT unit (Alexion TSX-034A, Toshiba Medical Systems, Tochigi, Japan). The technical parameters for CT were as follows: rotation time, 0.75 second; slice thickness, 2 mm; field of view, 160 to 340 mm; matrix dimensions, 512 × 512; reconstruction interval, 0.5 to 1 mm; detector pitch, 15; collimator pitch, 0.94; X-ray tube potential, 120 kVp; and X-ray tube current, variable milliamperage determined by x-, y- and z-axis dose modulation. An intravenous iodine-based contrast medium (Omnipaque 300, Daiichi Sankyo, Tokyo, Japan, 300 mg I/mL) was administered using a power injector at a dose of 450 mg/kg into a cephalic catheter. After unenhanced scanning, post-contrast images were acquired at 30 to 60 seconds after the initiation of contrast medium injection, before the contrast medium flowed into the bladder.
2.3 Grouping and staging
Primary TCC localization and the presence of lymphadenomegaly and metastasis in each dog were determined by reviewing both archived abdominal ultrasonography and whole-body CT images. CT scans were reconstructed using a soft-tissue algorithm (window level, 10; window width, 300) for the urinary tract and LNs, a bone algorithm (window level, 300; window width, 1500) for the bones, and a pulmonary algorithm (window level, −500; window width, 1500) for the lung. All images were re-evaluated by a single author (R. I.) who was unaware of the patients' information and outcome. Subsequently, the dogs were divided into three groups according to primary TCC localization using each of CT and ultrasonography images: tumour affecting the bladder only (Bladder group), tumour affecting the urethra with or without prostate involvement but not extending to the bladder (Urethra group) and tumour extending over the bladder and urethra (Bladder and Urethra group; Figure 1). Additionally, tumours affecting the bladder were categorized as apical, mid-body or trigone. Prostatic involvement was confirmed using either fine-needle aspiration from the prostate lesion or using a combination of transurethral catheter biopsy at the prostatic urethra and imaging findings such as enlargement, mineralization, irregular shape or heterogeneous appearance.32, 33
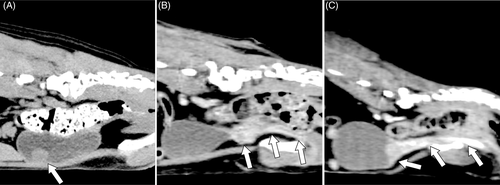
Iliosacral and sternal lymphadenomegaly were defined on the basis of previous CT findings. The dogs were considered positive for iliosacral lymphadenomegaly if they met any of the following criteria: the ratio of the maximum dimension of the medial iliac LN to the aorta (measured on a transverse view at the level of the L5-L6 intervertebral disc space) ≥ 1.3, the ratio of the maximum dimension of the internal iliac LN to the aorta ≥1.0, or the ratio of the maximum dimension of the sacral LN to the aorta ≥1.0.28 The dogs were considered positive for sternal lymphadenomegaly if they met both of the following criteria: the ratio of the short-axis dimension of the sternal LN to the thickness of the second sternebra ≥1.0 and pre-contrast attenuation of the sternal LN ≥37.5 Hounsfield units.20 Some of the LNs were sampled through fine-needle aspiration and subjected to cytological examination according to the clinicians' discretion.
All bone metastases were confirmed cytologically through CT-guided fine-needle aspiration of the lesions showing bone lysis and adjunct soft-tissue swelling on whole-body CT. Metastasis to the lung was considered positive when one or more nodules were found in the pulmonary field area on CT. Soft-tissue attenuating nodules more than 3 mm in diameter, as well as nodules that had a ground glass appearance or were poorly demarcated, were included.26, 34
2.4 Statistical analysis
Continuous variables were checked for normality of distribution by visually inspecting their histograms and by using the Shapiro-Wilk test; these variables were identified as being not normally distributed. Therefore, these values were expressed as the median and range. Categorical variables were expressed as frequencies and percentages. The statistical difference between groups was compared using the Kruskal-Wallis test for continuous variables, and Fisher's exact test for categorical variables. Cohen's kappa analysis was used to determine the agreement in the classification of primary tumour localization between CT and ultrasonography findings. ST was calculated from the date of treatment initiation to the date of death because of any cause. Dogs were censored from survival analysis at the last day of contact, if they were still alive. Kaplan-Meier estimations were used to create ST curves for categorical variables and for continuous variables categorized using median values. Univariate log-rank analysis between each variable and outcome was conducted to determine a potential prognostic factor. Using multivariable Cox's proportional hazard regression analysis with a stepwise model-selection method, the influence of confounding factors identified in the previous univariate model was removed. The explanatory variables were included in the multivariable models on the basis of their statistical significance level in the univariate analysis (P < .10) and multicollinearity tests. Multicollinearity between variables was evaluated using Spearman's rank correlation coefficient, with associations ≥0.7 considered as evidence of collinearity. In cases of comparison between three groups, a post-hoc test was used to compare between every two groups, whose significance level was adjusted using the Steel-Dwass method against the Kruskal-Wallis test and the Holm method against Fisher's exact test or log-rank analysis.
Statistical significance was defined as P < .05. All statistical analyses were performed using the EZR software, which is a graphical user interface for R, and is a modified version of R commander.35
3 RESULTS
3.1 Cases
Out of 107 dogs that were histopathologically diagnosed with urinary TCC at the Gifu University Animal Medical Centre between April 2010 and September 2017, 65 dogs met the inclusion criteria. All dogs were diagnosed through ultrasound-guided transurethral catheter biopsy. The breeds included were as follows: 12 Miniature Dachshunds, 7 mixed-breed dogs, 5 Beagles, 5 Toy Poodles, 4 Shetland Sheepdogs, 4 Pembroke Welsh Corgis, 3 Jack Russel Terriers, 3 Shiba Inus, 2 Bernese Mountain Dogs, 2 Labrador Retrievers, 2 Pomeranians and 2 West Highland White Terriers. The remaining 14 dogs were of other breeds. Compared with the Gifu University Animal Medical Centre population for the same period, the odds ratios were 5.9 (95% confidence interval [CI], 2.6-12.1; P < .001) for Shetland Sheepdogs and 4.1 (95% CI, 1.9-8.1; P < .001) for Beagles. Other breeds were not significantly over- or under-represented. The dogs included 15 (23.1%) intact males, 18 (27.7%) castrated males, 14 (21.5%) intact females and 18 (27.7%) spayed females. The median age of the dogs was 11.0 years (range, 7.2-16.1 years), and their median weight was 8.6 kg (range, 1.9-49.9 kg).
At diagnosis, 60 (92.3%) dogs had clinical signs: 46 (70.8%) had lower urinary tract symptoms (such as haematuria, stranguria and pollakiuria), 5 (7.7%) had skeletal pain (such as lameness and pain response on palpation), and 9 (13.8%) showed both of these. According to the primary TCC localization on CT, the dogs were divided into the Bladder (n = 16), Urethra (n = 26) and Bladder and Urethra groups (n = 23). All dogs with apical or mid-body involvement were classified into the Bladder group; in contrast, all dogs with trigone involvement were classified into the Bladder and Urethra group. Out of 33 male dogs, all 29 dogs in the Urethra group and the Bladder and Urethra group had prostate involvement. Prostatic involvement was confirmed through fine-needle aspiration in 5 dogs and through transurethral catheter biopsy at the prostatic urethra in 24 dogs.
The CT and ultrasonography findings showed almost perfect agreement in the classification of primary tumour localization (κ, 0.819; 95% CI, 0.701-0.936; Table 1). However, out of the 26 dogs that were classified in the Urethra group on CT, tumours were not detected in 4 dogs on ultrasonography. Additionally, 3 out of these 26 dogs in the Urethra group on CT were classified as Bladder and Urethra group on ultrasonography.
| Ultrasonography classification | |||||
|---|---|---|---|---|---|
| Bladder | Urethra | Bladder and Urethra | Undetected | ||
| CT classification | Bladder | 15 | 0 | 1 | 0 |
| Urethra | 0 | 19 | 3 | 4 | |
| Bladder and Urethra | 0 | 0 | 23 | 0 | |
| Undetected | 0 | 0 | 0 | 0 | |
Lymphadenomegaly of the iliosacral LNs and sternal LNs was seen in 31 (47.7%) and 12 (18.5%) dogs, respectively. Of these enlarged LNs, 11 iliosacral LNs and 2 sternal LNs were sampled, and their cytological analysis confirmed that all samples were metastatic. Metastasis to the bone and lung was seen in 16 (24.6%) and 23 (35.4%) dogs, respectively. At diagnosis, 20 locations in the 16 dogs were sampled and confirmed to be cytologically metastatic to the bone: femur (6); lumbar vertebra (5); ilium (2); and rib, frontal bone, humerus, sacral vertebra, scapula, thoracic vertebra and tibia (1 each). Of these 16 dogs, 14 dogs had clinical signs of skeletal pain and 2 dogs did not. Moreover, during the re-evaluation of the whole-body CT images, 2 out of these 16 dogs were found to have additional suspicious lesions of bone metastasis in the scapula that were not sampled. All lung metastases were well-demarcated nodules (range, 3 mm-4.5 cm in diameter); ground glass appearance or poorly demarcated nodules were not found.
3.2 Follow-up
All dogs received COX inhibitors: 45 (69.2%) received firocoxib, 18 (27.7%) received piroxicam and 2 (3.1%) received carprofen. Fifteen (23.1%) dogs received chemotherapy: 14 (21.6%) received mitoxantrone at a median dose of 4.5 mg/m2 (range, 4.0-5.0 mg/m2, IV, q3 weeks) and 1 (1.5%) received toceranib (2.8 mg/kg, PO, EOD). Some of the dogs with bone metastasis received radiotherapy to the osteolytic lesions (n = 8 [50.0%]; median dose per fraction, 7 Gy [range, 6.3-8.5 Gy]; median fraction number, 2.5 [range, 1-6]; irradiation interval, weekly; median total dose, 17.5 Gy [range, 6.3-42 Gy]) or zoledronic acid (n = 1 [6.3%]; 0.1 mg/kg, IV, q4 weeks).
Among the 23 dogs that were considered to have lung metastasis on CT, 15 (65.2%) dogs died without undergoing any imaging follow-up. The remaining 8 (34.8%) dogs were all confirmed to have progression at a median time of 38.5 days (range, 11-171 days) after the date of diagnosis by using radiography or CT.
The median ST for all dogs in this study was 196 days (95% CI, 108-266 days; range, 1-1174 days). Among the 57 (87.7%) dogs that died during the study period, the cause of death was available in 45 (69.2%); the causes were related to primary TCC (n = 12 [18.5%]), related to TCC except for the primary tumour (n = 27 [41.5%]), and unrelated to TCC (n = 6 [9.2%]; congestive heart failure [n = 3], acute pancreatitis [n = 1], gastric dilatation and volvulus [n = 1] and heat stroke [n = 1]). The remaining 8 (12.3%) dogs were still alive at a median of 557 days (range, 257-846 days). No patients were lost to follow-up.
3.3 Variables related to ST
The results of the univariate log-rank analyses between each variable and ST to assess a potential prognostic factor are summarized in Table 2. Sex, clinical signs at diagnosis, primary TCC localization, prostate involvement, lymphadenomegaly of the iliosacral LNs and sternal LNs, metastasis to the bone and lung and receipt of chemotherapy resulted in significantly different STs.
| Variable | Category | n (%) | Median ST (days) | 95% CI (days) | P- value |
|---|---|---|---|---|---|
| Sex | Intact male | 15 (23.1%) | 65 | 22-123 | .033 |
| Castrated male | 18 (27.7%) | 183.5 | 88-353 | ||
| Intact female | 14 (21.5%) | 340.5 | 77-502 | ||
| Spayed female | 18 (27.7%) | 293.5 | 77-434 | ||
| Age | ≤11.0 years | 33 (50.8%) | 137 | 77-247 | .359 |
| >11.0 years | 32 (49.2%) | 248 | 77-382 | ||
| Body weight | ≤8.6 kg | 32 (49.2%) | 202.5 | 88-353 | .554 |
| >8.6 kg | 33 (50.8%) | 124 | 76-284 | ||
| Clinical signs | None | 5 (7.7%) | 420 | 17-NA | <.001 |
| Lower urinary tract symptoma | 46 (70.8%) | 242 | 158-316 | ||
| Skeletal painb | 5 (7.7%) | 22 | 1-NA | ||
| Both of these | 9 (13.8%) | 76 | 20-137 | ||
| Azotemia | No | 59 (90.8%) | 198 | 120-284 | .186 |
| Yes | 6 (9.2%) | 83.5 | 7-NA | ||
| Tumour localization | Bladder | 16 (24.6%) | 420 | 163-1151 | .001 |
| Urethra | 26 (40.0%) | 121.5 | 65-203 | ||
| Bladder and urethra | 23 (35.4%) | 158 | 66-259 | ||
| Prostate involvement | No | 4 (12.1%) | 316 | 163-NA | .029 |
| Yes | 29 (87.9%) | 88 | 60-171 | ||
| Iliosacral lymphadenomegaly | No | 34 (52.3%) | 299 | 171-420 | .006 |
| Yes | 31 (47.7%) | 88 | 56-137 | ||
| Sternal lymphadenomegaly | No | 53 (81.5%) | 259 | 137-353 | <.001 |
| Yes | 12 (18.5%) | 62 | 17-120 | ||
| Bone metastasis | No | 49 (75.4%) | 266 | 196-406 | <.001 |
| Yes | 16 (24.6%) | 65.5 | 20-77 | ||
| Lung metastasis | No | 42 (64.6%) | 316 | 196-420 | <.001 |
| Yes | 23 (35.4%) | 77 | 56-137 | ||
| Treatment | COX inhibitors alone | 50 (76.9%) | 160.5 | 76-237 | .014 |
| COX inhibitors + chemotherapy | 15 (23.1%) | 420 | 108-1151 |
- Abbreviations: CI, confidence interval; COX, cyclooxygenase; NA, not available; ST, survival time.
- a For example, haematuria, stranguria and pollakiuria.
- b For example, lameness and pain response on palpation.
On the basis of the P-values in the previous univariate analysis and multicollinearity test, sex, primary TCC localization, prostate involvement, lymphadenomegaly of the iliosacral LNs and sternal LNs, metastasis to the bone and lung and receipt of chemotherapy were extracted for multivariable analysis as the explanatory variables. Because of the positive correlation between clinical signs and bone metastasis (r = 0.766; P < .001), the latter variable was extracted for analysis. Using Cox's proportional hazard regression analysis, primary TCC localization, bone metastasis and sternal lymphadenomegaly were identified as significant variables individually associated with ST (Table 3).
| Variable | Hazard ratio | 95% confidence interval | P value |
|---|---|---|---|
| Tumour localization | 1.90 | 1.04-3.47 | .037 |
| Bone metastasis | 2.76 | 1.23-6.17 | .013 |
| Sternal lymphadenomegaly | 3.56 | 1.50-8.50 | .004 |
3.4 Characteristic comparisons between TCC localizations
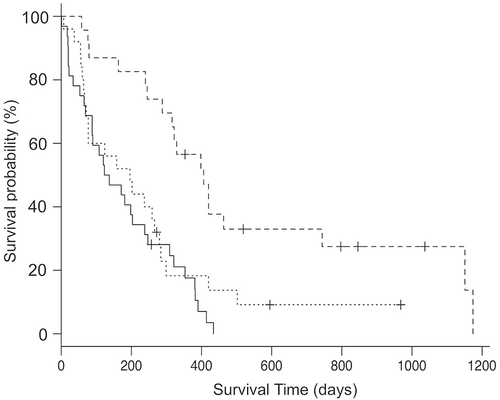
The features between the three groups were compared (Table 4). At diagnosis, metastases to the bone and lung were more frequent in the Urethra group than in the Bladder group; the Bladder and Urethra group also showed more frequent lung metastasis than did the Bladder group. The median ST in the Bladder group was 420 days (95% CI, 163-1151 days), which was significantly longer than the 121.5 days (95% CI, 65-203 days) in the Urethra group (P < .001) and 158 days (95% CI, 66-259 days) in the Bladder and Urethra group (P = .009; Figure 2). To avoid the influence of prostate involvement on ST, the median ST without male dogs was compared between the groups. The median ST in the female dogs alone in the Urethra group was 247 days (95% CI, 1-382 days), which was shorter than the 420 days (95% CI, 76-1151 days) in the Bladder group (P = .031). Two of 17 (11.8%) dogs in the Urethra group died because of the primary lesion; this number was significantly lower than the number in the Bladder group (5/8 [62.5%]). None of the variables was statistically different between the Urethra group and the Bladder and Urethra group.
| Variable | Category | Bladder (n = 16) | Urethra (n = 26) | Bladder and Urethra (n = 23) | P-value |
|---|---|---|---|---|---|
| Bone metastasis | No | 15 (93.7%) | 15 (57.7%) | 19 (82.6%) | .045a |
| Yes | 1 (6.3%) | 11 (42.3%) | 4 (17.4%) | ||
| Lung metastasis | No | 15 (93.7%) | 14 (53.8%) | 13 (56.5%) | .022a, 0.027b |
| Yes | 1 (6.3%) | 12 (46.2%) | 10 (43.5%) | ||
| Death related to primary TCC (n = 39)c | Yes | 5 (62.5%) | 2 (11.8%) | 5 (35.7%) | .042a |
| No | 3 (37.5%) | 15 (88.2%) | 9 (64.3%) |
- a Significant difference between the Bladder group and Urethra group.
- b Significant difference between the Bladder group and Bladder and Urethra group.
- c Among the 45 dogs that the cause of death was obtained, 6 dogs that died unrelated to TCC were excluded.
4 DISCUSSION
This retrospective study was designed to evaluate factors associated with ST and to compare characteristics between tumour localizations in dogs with urinary TCC that underwent whole-body CT at diagnosis. Because bladder TCC involves the urethra in 56% to 58.8% of dogs and the prostate in 29% to 54.5% of male dogs, few studies have distinguished the characteristics of urethral TCC from that of bladder TCC.8, 17 Our results revealed two major findings about the clinical behaviour and outcome of urethral TCC and new factors associated with ST. First, dogs with urethral TCC had higher metastasis rates at diagnosis and shorter ST than did dogs with bladder TCC. Second, primary TCC localization, sternal lymphadenomegaly and bone metastasis were identified as significant variables individually associated with ST. These results indicate the importance of evaluating both primary TCC localization and metastasis other than to the regional LNs and lung. Furthermore, all dogs in this study were evaluated using whole-body CT, suggesting that this diagnostic tool could be effective for obtaining precise information about the prognosis. CT may offer advantages to or complement the use of ultrasonography, especially in the detection of primary TCCs affecting only the urethra, as 4 out of 26 tumours were missed by ultrasonography in this study.
Significant differences in the metastasis rates at diagnosis and in ST were found for dogs between the Bladder and Urethra groups. A similar result was also obtained in the analysis that was conducted using female dogs alone to avoid the influence of prostate involvement, which was reported to be a negative prognostic factor in male dogs,8, 13 thus, indicating urethral TCC itself might be a negative prognostic factor regardless of prostate involvement. Previous studies have reported that metastasis rates to the lung and bone at diagnosis were 6% to 29% and 8% to 10%, respectively.1, 6, 8, 9, 17-19 Our data showed that 35.4% and 24.6% of dogs had metastasis to the lung and bone, respectively. In particular, metastasis to the lung and bone was identified in 46.2% and 42.3% of dogs in the Urethra group that was significantly higher than that in the Bladder group. The proportion of dogs diagnosed with urethral only TCC in our study is higher compared with other studies, and this may explain the overall higher metastatic rate found in our results. Twelve Miniature Dachshunds and 4 Pembroke Welsh Corgis, accounting for 33% of the dogs with urethral TCC, were included. All 12 Miniature Dachshunds and 4 Pembroke Welsh Corgis had either urethra or urethra and bladder involvement, which may suggest a predisposition for these breeds to develop TCC in these specific locations. A report documented that TCC localization to the bladder alone was more common in the higher-risk breeds and that to the urethra was more common in Dalmatian than in mixed-breed dogs.4 Further studies are needed to confirm these breed predilections for urethral TCC.
It should be noted that potential selective bias may have increased the inclusion of dogs with urethral TCC in our study population, such as selection of patients presenting with bone pain because of bone metastasis rather than lower urinary tract signs (which was only seen in patients with urethral TCC) or inclusion of patients whose tumours could only be detected through CT scan because of their intrapelvic location.
Twenty-seven out of 45 (60%) dogs in this study died of complications associated with the metastatic disease, which was higher than the 14% to 25% mortality reported in previous studies.4, 8 Considering that 15 out of 17 (88.2%) dogs in the Urethra group died of complications unrelated to primary TCC, this was also probably because of the difference in the study population. It is not known why dogs with urethral TCC had higher rates of distant metastasis at diagnosis and higher mortality related with metastatic disease than those with bladder TCC. One of the possible reasons is that urethral TCCs may be diagnosed later in the disease; and its possible factors are that dogs with urethral TCC may not have clinical signs of lower urinary tract symptoms as severe as other locations in an early stage of the disease, and that even if they exhibit the clinical signs, tumours arising from the intrapelvic urethra make it difficult to be detected by initial diagnostic imaging such as ultrasonography or radiography. In a previous report, a dog with a TCC that originated from the prostatic urethra was documented to have metastasis to the humerus without any lower urinary tract symptoms.36 Although this report did not refer to the reason why clinical signs typically associated with TCCs were not recognized, it described that the localization of the tumour invading the prostatic tissue made it undetectable on a rectal examination. The findings of this case report have supported our results that urethral TCC may lead to distant metastases without showing the lower urinary tract symptoms.
Urinary TCCs are divided into papillary or non-papillary tumours and infiltrating or non-infiltrating tumours according to their growth pattern, and metastatic tumour is more common in anaplastic tumours, followed by the non-papillary and infiltrating variants.31 Because the tissue samples obtained were small and were confined to the mucous lesion, the transurethral catheter biopsy performed in this study was inadequate for further tumour classification. Therefore, further studies on pathological evaluation using other biopsy techniques in relation to each tumour's localization are needed to resolve this issue.
Primary TCC localization, sternal lymphadenomegaly and bone metastasis were identified as significant variables individually associated with ST. Survival has been reported to be associated with the TNM stage and tumour pathological grade.1, 3 However, the specific impact of sternal LNs and/or osseous metastases on outcome has not been described. In a previous study, 4 of 137 (3%) dogs were reported to have sternal LN metastasis at necropsy.4 A recent study also reported that sternal LN metastasis was likely to occur in tumours originating from the urogenital system, including urinary TCC.20 The present study showed that the median ST in dogs with sternal lymphadenomegaly was only 62 days, thus indicating the importance of evaluating the sternal LNs in addition to the pulmonary area at diagnosis. Afferent vessels to the sternal LNs receive drainage from a broad region including the thoracic and abdominal walls, ribs and abdominal and pelvic cavities.37 Therefore, the sternal LNs can be enlarged because of TCC invasion to these regions; this may suggest that sternal lymphadenomegaly reflects a progressive stage as do the other distant metastases. Furthermore, in this study, ST in dogs with bone metastasis was similar to that in dogs with lung metastasis or sternal lymphadenomegaly. Whole-body CT has recently been reported to be useful for detecting osteolytic lesions that suggest potentially osseous metastasis.19 In the present study, out of the 16 dogs in whom metastasis to the bone was cytologically confirmed, 2 dogs did not have clinical signs of skeletal pain or lameness. Whole-body CT could detect these asymptomatic metastases, which may explain its usefulness as a screening test at diagnosis because osseous metastasis was shown to be one of the poor predictors in our study.
This study has several limitations. First, although primary TCCs of the bladder and/or urethra in each dog were re-evaluated by a single observer and classified into three groups on the basis of both ultrasonography and CT findings, definite identification of the tumour development region was occasionally difficult because of the retrospective nature of the study, particularly in cases in which the tumour existed at the boundary between the bladder and urethra. To reduce the variability in primary tumour findings, TCC localization across dogs should be interpreted under constant conditions when using imaging modalities, such as body position, imaging plane and degree of bladder distension.17
Second, dogs included in this study were pathologically confirmed to have TCCs from the primary tumour and bone metastasis but not from most of the iliosacral and sternal LNs. In addition, it should be noted that the report describing the measurement method used to detect iliosacral lymphadenomegaly in this study did not evaluate the diagnostic accuracy for detecting metastasis to the LNs. Only 11 out of 31 enlarged iliosacral LNs and 2 out of 12 enlarged sternal LNs were sampled and confirmed to be metastatic on cytology. Therefore, it is unknown whether the other 20 iliosacral LNs and 10 sternal LNs that were not sampled were enlarged because of the metastasis or inflammation. However, interestingly, dogs showing LN abnormality on CT had significantly shorter STs than did dogs with normal LNs, suggesting the utility of the LN evaluation criteria adopted in this study. In particular, a study reported that the measurement method using the combination of size and attenuation achieved a specificity and positive predictive value of 100% in differentiating metastasis to the sternal LNs on CT.20 Therefore, sternal LN measurement would reflect the prognosis in dogs with TCC more accurately than would iliosacral LN measurement. Evaluation concerning ST or other outcomes in dogs with true metastasis-positive LNs confirmed pathologically needs to be considered in future analyses of the diagnostic accuracy on CT.
In conclusion, the results of the present study showed that dogs with urethral TCC had higher metastasis rates at diagnosis and shorter STs than did dogs with bladder TCC. Primary TCC localization, sternal lymphadenomegaly and bone metastasis were identified as significant variables individually associated with ST. Although abdominal ultrasonography can provide valuable information in the characterization of bladder TCCs, whole-body CT may allow for a thorough screening of the patient, leading to detection of prognostic findings such as intrapelvic urethral TCC location, lymph node metastasis and bone metastasis.
CONFLICT OF INTEREST
The authors declare no conflict of interest.