Cancer-testis antigens in canine histiocytic sarcoma and other malignancies
Abstract
Cancer-testis antigens (CTAs) are a category of self proteins aberrantly expressed in diverse malignancies, mostly solid tumours, due to epigenetic de-repression. Normally expressed only in fetal or gametogenic tissues, CTAs are tantalizing immunotherapy targets, since autoimmunity risks appear minimal. Few prevalent CTAs have been identified in human hematologic cancers, and just two in their veterinary counterparts. We sought to discover new CTAs in canine hematologic cancers such as histiocytic sarcoma (HS) and lymphoma to foster immunotherapy development. To accomplish this, the ligandome binding the dog leukocyte antigen (DLA)-88*508:01 class I allele overexpressed in an HS line was searched by mass spectrometry to identify possible CTA-derived peptides, which could serve as CD8+ T-cell epitopes. Twenty-two peptides mapped to 5 human CTAs and 12 additional proteins with CTA characteristics. Expression of five promising candidates was then evaluated in tumour and normal tissue by quantitative and end-point RT-PCR. The ortholog of an established CTA, IGF2BP3, had unexpectedly high expression in peripheral blood mononuclear cells (PBMCs). Four other testis-enhanced proteins were also assessed. AKR1E2, SPECC1 and TPX2 were expressed variably in HS and T-cell lymphoma biopsies, but also at high levels in critical tissues, including kidney, brain and marrow, diminishing their utility. A more tissue-restricted candidate, NT5C1B, was detected in T-cell lymphomas, but also at low levels in some normal dog tissues. These results illustrate the feasibility of discovering canine CTAs by a reverse approach, proceeding from identification of MHC class I-presented peptides to a comparative RNA expression survey of tumours and normal tissues.
Abbreviations
-
- ACT
-
- adoptive cell therapy
-
- CAR
-
- chimeric antigen receptor
-
- CTA
-
- cancer-testis antigen
-
- CTL
-
- cytotoxic T lymphocyte
-
- DC
-
- dendritic cell
-
- DLA
-
- dog leukocyte antigen
-
- EEEA
-
- EMBL-EBI expression atlas
-
- EST
-
- expressed sequence tag
-
- HLA
-
- human leukocyte antigen
-
- HPA
-
- The Human Protein Atlas
-
- HS
-
- histiocytic sarcoma
-
- LC-MS/MS
-
- liquid chromatography-tandem mass spectrometry
-
- LLD
-
- lower limit of detection
-
- mAb
-
- monoclonal antibody
-
- MFI
-
- mean fluorescence intensity
-
- ORR
-
- objective response rate
-
- PBMCs
-
- peripheral blood mononuclear cells
-
- PTCL
-
- peripheral T-cell lymphoma
-
- qPCR
-
- quantitative PCR
-
- RT-PCR
-
- reverse transcription-PCR
-
- TCR
-
- T-cell receptor
-
- TIL
-
- tumour-infiltrating lymphocyte
-
- TSA
-
- tumour-specific antigen
-
- X
-
- isoform
1 INTRODUCTION
Adaptive immunotherapy for cancer can have dramatic results when conventional treatments fail.2 Widespread implementation of such treatment can be promoted by explicit identification of tumour-specific antigens (TSAs). Uncharacterized antigen mixtures administered as active-specific immunotherapy (ie, vaccines containing tumour cells or their constituents) have mostly been ineffective, regardless of the modification, delivery system or adjuvant. While adoptive cell therapy (ACT) using ex vivo-expanded, autologous tumour-infiltrating lymphocytes (TILs) that recognize unknown antigens can result in durable complete responses in human malignant melanoma, TILs are not available in all tumours and patients, and are often ineffective in cancer types other than melanoma.3 To circumvent these limitations, genes encoding αβ T-cell receptors (TCRs) and chimeric antigen receptors (CARs) that target established TSAs can be introduced into normal T cells to re-direct their cytotoxicity against cancer cells, and upon transfer, these engineered T cells can eradicate chemoresistant tumours.4, 5
Not surprisingly, then, the discovery and value assessment of TSAs is an important aspect in advancing adaptive immunotherapy development, whatever the form. Human cancer antigens have been prioritized according to nine criteria, including immunogenicity, role in oncogenicity, specificity, expression level and percentage of TSA-positive cells, and the number of patients with TSA-positive cancers.6 No TSA is ideal, of course, and valuations shift, depending on immunotherapeutic use. For example, vaccination with melanoma differentiation antigens such as gp100 and MART1 have been associated with measurable but very low objective response rates (ORRs; ~3%) and minimal adverse effects.7 Targeting gp100 and MART1 by TCR gene transfer improves the ORR, up to 30%, but autoimmune toxicity becomes severe, dimming the utility of this category of TSAs in ACT, a much more potent therapy.8 For the purposes of vaccination, attention has now largely turned to mutation-origin neoantigens, where off-tumour expression and tolerance are neglible.9, 10 However, the mostly private nature of mutation-origin neoantigens makes them currently unsuitable for ACT. For TCR and CAR development, which is a time- and resource-intensive process, antigens shared between patients are needed.
For cancers of non-viral origin, the most well-studied category of shared TSAs with limited-to-absent somatic tissue expression are cancer-testis antigens (CTAs), which include MAGE, the first identified tumour antigen.11 The CTA category comprises hundreds of diverse, mainly intracellular antigens that are expressed during fetal development, silenced in normal adult tissues, except germ cells, and re-expressed in various tumours through epigenetic alterations. In fact, the CTAs MAGE-A3 and NY-ESO-1 are the only TSAs of self origin in the top 10 of the National Cancer Institute's prioritized antigen list with no known non-gametogenic tissue expression.6, 12 The therapeutic usefulness of such CTAs has been compellingly demonstrated. Administration of T cells bearing a high-affinity TCR against a human leukocyte antigen (HLA) class I-restricted peptide from NY-ESO-1 resulted in ORRs in patients with synovial cell sarcoma and melanoma, without apparent toxicities.13 At present, 26 clinical trials of ACT against this CTA are actively recruiting human patients with various cancers.14
In dogs, immunotherapy is a desperately needed therapeutic option for malignant round cell tumours, such as disseminated histiocytic sarcoma (HS) and high-grade lymphoma, where both inherent and acquired chemoresistance ultimately dooms almost all afflicted patients. Accordingly, using HS as a model haematopoietic cancer, we sought to determine whether any proteins whose tissue expression patterns approximated human CTAs could be found. Specifically, liquid chromatography-tandem mass spectrometry was utilized to profile the non-mutant ligandome of a classical dog leukocyte antigen (DLA)-88 class I allele expressed in representative canine HS cell line, and peptides were mapped back to source proteins. The expression pattern of promising candidates in tumour specimens and normal tissues was subsequently assessed by quantitative and end-point reverse-transcription PCR to estimate the value of these TSAs as targets of cytotoxic T cells.
2 MATERIALS AND METHODS
2.1 Tissue acquisition and processing
Frozen canine tumour samples were purchased from the Pfizer-Canine Comparative Oncology & Genomics Consortium (Frederick, MD, USA; pulmonary adenocarcinomas; histiocytic sarcomas; fibrosarcomas; peripheral nerve sheath tumours) and the NCSU-CVM Clinical Studies Core Tissue Bank (malignant mesothelioma; gastric adenocarcinoma; hepatocellular carcinoma; cecal gastrointestinal stromal tumour). Frozen lymph node biopsy samples in TRIzol from dogs with high-grade peripheral T-cell lymphomas (PTCL; n = 13) and B-cell lymphomas (n = 3; two diffuse large B-cell lymphomas, one late marginal zone lymphoma) were kindly provided by Dr. Steve Suter. Lymphoma subtypes were established by histologic analysis of all lymph node samples by a board-certified pathologist, Dr. Luke Borst. An oral malignant melanoma sample in RNAlater (ThermoFisher Scientific, Waltham, Massachusetts) was a gift of Dr. Marlene Hauck. Normal tissues were collected immediately post-mortem from healthy young adult Beagles (n = 2; lymph nodes only) and hound-mix dogs (n = 3; diverse tissues) that were maintained and euthanized by NCSU-CVM Laboratory Animal Resources for reasons unrelated to this study. Normal testis was also acquired as discarded tissue after routine elective castration of a healthy young adult mixed-breed dog at NCSU. Samples were placed in fivefold excess RNAlater in individual cryovials overnight at 4°C, and then stored at −20°C. Peripheral blood mononuclear cells (PBMCs) from normal and leukemic dogs were isolated from leftover EDTA-anticoagulated blood samples in the NCSU Clinical Pathology Laboratory, after complete blood count analyses had been performed.
2.2 Culturing and treatment of cell lines
The canine HS cell line DH82 (CRL-10389) and the T-cell lymphoma line OSW (PTA-9116) were obtained from the ATCC, and were grown in Dulbecco's Modified Eagle's Medium containing 15% FBS, penicillin (100 IU mL−1)/streptomycin (100 μg mL−1) (P/S) and RPMI 1640 containing 20% FBS, P/S and 2 mM L-glutamine, respectively. Isolated PBMCs were cultured in either R-10 or RPMI 1640 that contained 1% autologous dog serum, P/S and l-glutamine. The cell clones 9-15 (DH82 cells stably expressing DLA-88*508:01-FLAG) and BARC3 (RMA-S cells stably expressing DLA-88*508:01-FLAG) had been previously generated and were cultured as described.15, 16 In some experiments, cell lines and PBMCs were cultured in medium containing 10-20 μM 5-aza-2′-deoxycytidine (5-aza-dC; Sigma-Aldrich, St. Louis, Missouri), diluted from a 200 mM stock solution prepared in DMSO and stored at −80°C. The medium and 5-aza-dC were replaced every 12 hours for the 72-hour incubation period. All cells were grown under standard incubator conditions (37°C; humidified 5% CO2 atmosphere), unless otherwise stated.
2.3 Isolation and sequencing of DLA-88*508:01-bound peptides
The mass spectral data used here are from a previously reported study15 and additional peptide isolation experiments. Large-scale culture and weekly harvests of the 9-15 clone, affinity isolation of peptide-DLA-88*508:01-FLAG from 9–15 lysates, acid elution of bound peptides and additional peptide clean-up and concentration were performed as described.15 Eluates were analysed by LC-MS/MS, using a nanoACQUITY UPLC system (Waters) and Thermo Q Exactive HF-X mass spectrometer (Thermo Fisher Scientific). The software program Mascot 2.5.1 (Matrix Science; matrixscience.com) was used to search mass spectral data against a modified Canis lupus familiaris database, and peptide and proteins were identified using the program Scaffold (Proteome Software; proteomesoftware.com).
2.4 Generation and phenotypic evaluation of blood-derived dendritic cells (DCs)
Dendritic cells were prepared as previously described.17 Anticoagulated venous blood (~10 mL) was aseptically collected from a healthy Golden Retriever under a protocol approved by the NCSU Institutional Animal Care and Use Committee. PBMCs were isolated by centrifugation (400g × 30 minutes at room temperature) over a Ficoll-Paque PLUS 1.077 density gradient (GE Healthcare, Uppsala, Sweden). The harvested interface was washed twice with PBS, resuspended in RPMI 1640 containing 10% FBS, P/S and 2 mM l-glutamine (R-10 medium), and plated at 1.2 × 107 cells per well in a 6-well plate. Non-adherent cells (~70% of input) were removed after 2 hours by gentle pipetting with PBS, and R-10 containing recombinant canine IL-4 (50 ng mL−1; Kingfisher Biotech, St. Paul, Minnesota) and GM-CSF (33 ng mL−1; R&D Systems, Minneapolis, Minnesota) was added (2 mL per well). Fresh medium and cytokines were added on days 3 and 6. DCs were harvested for use on day 7. To assess surface expression of CD11c, 105 cells per well in a 96-well plate were washed twice with FACS buffer (PBS containing 2% FBS, 2 mM EDTA and 0.02% sodium azide) and then incubated with an anti-canine CD11c monoclonal antibody (mAb; clone CA11.6A1, Novus Biologicals, Littleton, Colorado) at a 1:6 v:v ratio for 30 minutes at 4°C. As a negative control to establish background fluorescence, the primary mAb was omitted. After two washings with FACS buffer, cells were stained with a phycoerythrin-conjugated goat anti-mouse IgG Ab (Bio-Rad, Hercules, California) for 30 minutes at 4°C, washed again, and analysed immediately on a Cytoflex flow cytometer (Beckman Coulter, Indianapolis, Indiana). List mode data was analysed with CytExpert software. Viable cells were discriminated using forward and side scatter gating. To obtain photomicrographs, an air-dried spread of day 7 DCs concentrated by centrifugation (400g × 6 minutes) were stained with Wright-Giemsa on an Aerospray Pro Stainer, and photographed with a Nikon DS-Fi2 camera through an Olympus BX41 microscope.
2.5 Isolation of RNA and preparation of cDNA
Thawed tissue samples were homogenized in TRI Reagent (Zymo Research, Irvine, California) using a mini-bead beater (Biospec Products, Bartlesville, Oklahoma). For cell samples, 1-5 × 106 cells were processed in 500 μL TRI Reagent. Total RNA was extracted using the Direct-zol RNA MiniPrep Kit (Zymo Research) according to the manufacturer's instructions, re-suspended in nuclease-free H2O and stored at -80°C. RNA concentrations >100 ng/μL, with 260 nm/280 nm and 260 nm/230 nm absorbance ratios >1.8 and > 1.0, respectively, as determined by NanoDrop 2000 spectrophometer, were judged to be the minimum quality for analysis. Prior to cDNA synthesis, samples were DNase-treated using a Turbo DNA-free Kit (ThermoFisher Scientific). DNase-free RNA (2.5 μg) was reverse transcribed using an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, California), performed according to the manufacturer's instructions, on a Mastercycler Pro thermocycler (Eppendorf, Hauppauge, New York), using the following reaction times: priming: 5 minutes at 25°C; reverse transcription: 30 minutes at 42°C; reverse transcriptase (RT) inactivation: 5 minutes at 85°C. Control samples were prepared in parallel by omitting RT from the reaction mixtures. The cDNA was cooled to 4°C and stored at −20°C.
2.6 PCR amplification of normal and tumour tissue cDNA
Oligonucleotide primers (Supporting Information Table S1) for PCR amplification were designed with NCBI/Primer-BLAST, and synthesized by Invitrogen (Carlsbad, California) or Integrated DNA Technologies (Coralville, Iowa). Quantitative gene expression was performed on a LightCycler 480 System (Roche, Indianapolis, Indiana) using iQ SYBR Green (Bio-Rad) and the cycling conditions listed in Table S2 (Program 1). Reaction efficiency curves were constructed using dilutions of cDNA from gene-positive samples (ie, DH82 cells and/or testis), and for all genes, efficiency values exceeded 1.85. The ribosomal proteins L8 and L32 (RPL8; RPL32) genes served as reference genes, using previously published primer pairs.18 Because both yielded similar quantitative data in multiple qPCR analyses, RPL32 was omitted from later experiments. Thawed cDNA samples were diluted 1:10 in nuclease-free H2O prior to qPCR. Reactions were run in duplicate wells, and included a no-RT control (for each tissue) and a no-template control (for each gene). Testis cDNA served as a positive control in all experiments. Amplification of the intended genes was verifed by Sanger sequencing of reaction products (not shown). Data was analysed using the LightCycler 480 software, v. 1.51.62. Cq values >35.00 were considered non-detectable. Expression levels of each gene relative to the reference gene was calculated by the 2-ΔΔCT method (2Cq(reference)-Cq(target)), using Microsoft Excel, and the data was displayed graphically with Prism 5.0 (GraphPad Software, La Jolla, California).
Endpoint RT-PCR amplification was performed with a Hotstar HiFidelity Polymerase Kit with Q solution (Qiagen, Germantown, Maryland) in 25 μL reaction volumes with 1 μL cDNA and 1 μM primers, using a Mastercycler Pro running cycling program 2 (Table S2). In all experiments, water was substituted for cDNA template as a negative control. Reaction products (5 μL) were electrophoresed on a 2% agarose gel containing GelRed Nucleic Acid Stain (Biotium, Hayward, California) at 130 V for 30 minutes, and visualized and photographed using an Epi Chemi II Darkroom (Ultra-Violet Products, Upland, California). A 100 bp (Novagen Perfect DNA 100 bp, Millipore/Sigma, Burlington, Massachusetts) or mid-range (Fisher exACTGene; ThermoFisher Scientific) ladder was included on each gel as a molecular weight reference. Gel pictures in the figures were cropped and re-sized to provide a uniform appearance, but no other image manipulation was performed. Verification of the integrity of each tissue cDNA was performed by obtaining a single bright band of the correct length following RT-PCR amplification of RPL8, employing the same primer pair as in the qPCR assays (not shown).
2.7 Cloning and sequencing of NT5C1B
The RT-PCR products from DH82 cells, testis and brain were gel-extracted, isolated using the Zymoclean Gel DNA Recovery Kit (Zymogen), and TA-cloned into the pGEM-T Easy Vector (Promega, Madison, Wisconsin). Plasmid DNA isolated from transformed E. coli clones (G10 Chemical Competent Cells, Genesee Scientific, San Diego, Callifornia) were screened by EcoRI restriction digest. Positive plasmids were sequenced using standard T7 and SP6 primers by the NCSU Genomics Science Laboratory. Sequences were aligned to the canine NT5C1B gene with Geneious software (Biomatters, Auckland, New Zealand).
2.8 Assessment of CTA-derived peptide binding to DLA-88*508:01
Peptides were synthesized by Peptide 2.0 (Chantilly, Virginia), reconstituted in DMSO and stored as stock solutions at −80°C. A peptide-MHC class I surface stabilization assay was performed as previously described.15, 16 Briefly, BARC3 cells (105 per 100 μL) were cultured in R-10 containing 100 μM peptide overnight at 27°C, and then incubated for an additional 5 hours at 37°C prior to staining with a primary murine anti-MHC class I mAb (H58A; Monoclonal Antibody Center, Washington State University, Pullman, Washington), followed by washing and secondary incubation with an Alexa Flour 647-labelled goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, Pennsylvania). Peptide-independent MHC class I staining of BARC3 cells at 27 and 37°C was performed to validate assay performance. The canine self peptide K-11 (RFLDKDGFIDK) served as a positive binding control.15 Data was acquired on a modified FACScan flow cytometer (Becton Dickinson, Franklin Lakes, New Jersey) and analysed with FlowJo software (FlowJo LLC, Ashland, Oregon). A semi-quantitative binding score was computed from the mean fluorescence intensity (MFI), using the formula: (MFI X@37C - MFIno pep@37C)/(MFIno pep@27C - MFIno pep@37C), where X represents the tested peptide. The motif-negative viral peptide NP396-204 (FQPQNGQFI; GenScript, Piscataway, New Jersey), an established non-binder, was used to compute the assay's lower limit of detection, defined as the mean NP396-204 binding score plus two standard deviations.
3 RESULTS
3.1 Proteins with enhanced testis expression identified in an HS cell line by LC-MS/MS
To investigate the possible presentation of HS-associated CTA peptides by the canine classical MHC class I molecule, DLA-88, we used a cell clone derived from DH82 cells stably transfected with a FLAG epitope-tagged DLA-88*508:01 heavy chain gene.15 DLA-88*508:01 was used as a model allele because it is carried by ~40% of Golden Retrievers,19 a breed with an increased risk of HS, lymphoma and other malignancies.20 Peptides eluted from DLA-88*508:01 complexes isolated by FLAG immunoprecipitation from 9–15 cell lysates were identified by LC–MS/MS.15 From five independent analyses, we found peptides derived from 17 self proteins that appeared likely to have increased expression in canine testis relative to somatic tissues, which could qualify one or more of them as legitimate CTAs.
3.2 Human CTA orthologs found in canine HS specimens
Six peptides representing five predicted canine proteins with orthologs listed in a public database of human CTAs21 were recovered (Table 1). Within this group, IGF2BP3 (Insulin-Like Growth Factor 2 MRNA Binding Protein 3; also known as KH Domain-Containing Protein Overexpressed In Cancer [KOC] or IGF2 MRNA-Binding Protein 3 [IMP-3]), originally discovered in a melanoma cell line,25 appeared to be the most promising member. Human IGF2BP3 expression is largely restricted to the fetal tissues, ovary, testis, brain and placenta and to tumours of diverse origin, including lymphomas (data from The Human Protein Atlas [HPA], www.proteinatlas.org, v.16.126). IGF2BP3 may have immunotherapeutic value (Table 2).
| Gene name | CT #a | NCBI RefSeq # | Chr #b | DLA-88*508:01motif-matchedc | Non-motifd | |||
|---|---|---|---|---|---|---|---|---|
| S | W | N | N/T | |||||
| CTNNA2 | 114 | XP_005630556.2 | 17 | 1 | ||||
| IGF2BP3e | 98 | XP_539474.2 | 14 | 1f | 1 | |||
| IGSF11 | 119 | XP_005639577.1 | 33 | 1 | ||||
| RQCD1 | 129 | XP_005640704 | 37 | 1 | ||||
| SPAG6g | 141 | XP_535163 | 2 | 1 | ||||
| AKR1E2 | XP_005617237 | 2 | 1 | 1 | ||||
| ATXN2L | XP_859544 | 6 | 1 | |||||
| C30H15orf39 | XP_013965118.1 | 30 | 1 | |||||
| CASC1 | XP_013963976 | 27 | 1 | |||||
| DLGAP2 | XP_013966496 | 37 | 1 | |||||
| DLGAP3 | XP_013974723 | 15 | 1 | |||||
| DPYSL5 | XP_540119.2 | 17 | 2 | |||||
| KIF5C | XP_533351.3 | 19 | 1 | |||||
| NT5C1B | XP_003639621 | 17 | 1 | |||||
| SPECC1 | XP_013969110 | 5 | 1 | |||||
| TPX2 | XP_005634968 | 24 | 3 | |||||
| ZNF570 | XP_013971091 | 1 | 1 | |||||
- a Number assigned to the orthologous human CTA in the CTDatabase (http://www.cta.lncc.br/index.php).21
- b Number of the canine chromosome where the gene is located.
- c As assessed by peptide–MHC surface stabilization assay. S, strong (≥10-fold over the lower limit of detection [LLD]); W, weak (>LLD but <10-fold); N, negative (≤LLD); N/T, not tested. Number indicates the number of retrieved peptides from that gene product.
- d Peptide(s) did not match the DLA-88*508:01 motif15 and were not assessed for MHC class I binding.
- e Boldfaced genes were evaluated for mRNA expression in canine tumours and normal adult tissues.
- f The IGF2BP3 peptide initially recovered, LLVPTQFVGAIIGK, is an improbable binder of DLA-88*508:01, which strongly prefers 9- to 12-mers. An LC–MS/MS analysis performed subsequent to the qPCR survey yielded a DLA-88*508:01 motif-matched nonamer peptide (IMKKIRESY) from IGF2BP3, but given the unlikely utility of this CTA, class I binding was not verified experimentally.
- g Sperm-associated antigens (SPAG) are also potential immunotherapeutic targets,22 and SPAG6 is greatly overexpressed in paediatric acute myeloid leukaemia.23 While we found a peptide capable of binding DLA-88*508:01, TVVDVGAIAHL, this particular CTA appears to be normally expressed in human brain, trachea, lung22 and in murine lymphocytes24 and hence, was not investigated further in the dog.
| Gene name | Reported association | This study's conclusions |
|---|---|---|
| IGF2BP3 | Immunogenic (B- and T-cell reactivity) in cancer patients27, 28, 43 | High expression in PBMCs and lung precludes use as antigenic target |
| HLA-restricted minimal epitopes identified29, 30, 44 | ||
| Peptide vaccine elicits CTL and prolongs survival in SCC31, 32 | ||
| AKR1E | Expressed in human carcinomas and melanomas (HPA) | High expression in kidney precludes use as antigenic target |
| SPECC1 | Fusion partner with PDGFRβ in myelomonocytic leukemia33 | High expression in brain and bone marrow precludes use as antigenic target |
| Pro-apoptotic gene in head and neck carcinoma lines34, 35 | ||
| TPX2 | Most malignancies contain some positive cells (HPA) | High expression in brain and bone marrow precludes use as antigenic target |
| HLA-restricted peptides pulsed on DCs elicit CTL36 | ||
| NT5C1B | Expressed in human carcinomas, melanomas and gliomas (HPA) | Minimal somatic tissue expression; potential use as immunotherapy target with 5-aza-dC |
- Abbreviations: CTL, cytotoxic T lymphocyte; DC, dendritic cell; HLA, human leukocyte antigen; HPA, Human Protein Atlas; PBMCs, peripheral blood mononuclear cells; PDGFRβ, platelet-derived growth factor receptor β; SCC, squamous cell carcinoma; 5-aza-dC, 5-aza-2′-deoxycytidine.
As a first step in evaluating the potential utility of this CTA, we used qPCR to investigate the relative expression of IGF2BP3 mRNA in selected normal tissues, which is unknown. Expressed sequence tags (ESTs) can be helpful in predicting expression,37 but no canine IGF2BP3 ESTs have been reported (ESTs were searched in the NLM/NCBI database using the BLASTN 2.7.1 program38). Figure S1A shows that, using primer pair 1, IGF2BP3 mRNA was found in DH82 cells, but was unexpectedly low in testis and high in PBMCs. The expression seemed discordant with human and murine datasets (available in the EMBL-EBI Expression Atlas [EEEA], (http://www.ebi.ac.uk/gxa)39 showing very restricted distribution of IGF2BP3 mRNA and protein, although two RT-PCR studies have reported “weak expression” in human leukocytes,40 and expression in normal adult mouse brain, heart, lung, ovary and several other tissues, without providing data.41 We sought to confirm or refute our initial findings using different primers (Table S1, pair 2), and obtained similar results (Figure S1B). IGF2BP3 mRNA was surprisingly high in canine lung. While message was also found in all eight HS biopsy specimens that we tested (not shown; range 0.17-1.1), the high expression in several indispensable somatic tissues suggested that IGF2BP3 might not be a useful TSA in dogs.
3.3 Additional proteins from DH82 cells with enhanced testis expression of mRNA
In addition to the 5 human CTAs, our LC-MS/MS datasets contained peptides from 12 additional predicted proteins (Table 1) whose orthologous human genes appear to have largely tissue-restricted (testis or testis/brain) expression, according to public gene expression databases: HPA, EEEA and the UniProt Knowledgebase, http://www.uniprot.org/uniprot/.42 Eight were represented by at least one peptide that matched the DLA-88*508:01 binding motif and thus were considered eligible targets of cytotoxic T lymphocytes (CTL) restricted by that allele. Of those, one was a homologue of a scantly-characterized open reading frame (C30H15orf39), and three (ATXN2L; CASC1; ZNF570) were considered likely to have mixed tissue expression based on human data, so these four were not evaluated. Peptides from the remaining four gene products (AKR1E2; SPECC1; TPX2; NT5C1B) were synthesized and tested for class I binding in a peptide-MHC surface stabilization assay.16 All bound DLA-88*508:01 to varying degrees (Table 1; Figure S1C). Given their potential immunogenicity for CD8+ T cells, as well as their reported associations with cancer (Table 2, center column), the expression patterns of the parent genes in malignant and normal tissue samples were assessed.
The enzyme AKR1E2 (aldo-keto reductase family 1 member E2; also known as Human Testis-Specific Protein [HTSP]) is a large superfamily member and purported testis-restricted protein.45, 46 Analysis of seven HS biopsies showed moderate expression in all samples, ranging between 1.8% and 6.4% of testis (Figure S1D). However, it is also evident AKR1E2 is expressed in a number of canine somatic tissues; in fact, renal expression was equivalent to testis. Human CTAs can be classified according to several overlapping schemes. One system designates CTAs as either (1) testis-restricted (testis only, ± placenta); (2) testis/brain-restricted and (3) testis-selective.47 Another approach segregates these genes based on RT-PCR profiling into (1) testis-restricted; (2) tissue-restricted (testis and ≤2 non-gametogenic tissues); (3) differentially-expressed (testis and ≤ 6 [of 13 standard] non-gametogenic tissues); and (4) ubiquitously expressed CTAs.48 By this latter metric, canine AKR1E2 falls into the last category, and hence, seems unpromising as a tumour-discriminating target for CTL responses.
We next investigated SPECC1 (Sperm Antigen With Calponin Homology And Coiled-Coil Domains 1; also known as Cytokinesis and Spindle Organization B [CYTSB]), identified from a very strongly DLA-88*508:01-binding peptide. In the dog, a single EST, from testis, has been reported (GenBank CX988153.1). Northern blot analyses have shown negligible expression in normal human tissues, including the thymus, but high transcript levels in testis and some tumour cell lines.49 SPECC1 mRNA was found in DH82 cells (Figure 1A) and in 9 of 10 histiocytic biopsy samples (Figure 1B). Using previously established criteria of weak (>0.1% of testis), moderate (>1.0% of testis) and strong (>10% of testis) expression,50 moderate expression was seen in 5 of 10 (50%), and strong expression was observed in 4 of 10 (40%) specimens. SPECC1 message was also detected at moderate or higher levels in canine lung adenocarcinomas and various sarcomas (Figure S1E), but was negative (n = 1), weak (n = 4; 0.34, 0.42, 0.44, 0.52% of testis) or moderate (n = 4; 1.1, 1.1, 1.6, 8.0% of testis) in PTCL biopsies (not shown). By comparison, in those assays, no message was found in three of four normal lymph nodes included as controls; one node had weak SPECC1 expression in one of two replicate wells (the other was not detectable). An accompanying spleen sample was negative, as were PBMCs. Because dendritic cells (DCs) are the parent of most canine histiocytic malignancies, but normally present at very low frequencies in blood, we enriched PBMCs for DCs by 7-day incubation with GM-CSF and IL-4, yielding ~50% CD11c + cells with characteristic morphology (Figure S1F), but found no SPECC1 expression. Given these encouraging results, the distribution of SPECC1 in other normal somatic tissues was investigated further, revealing strong expression in the central nervous system (CNS), bone marrow and urinary bladder, and moderate expression in pituitary gland, small and large intestine, and skeletal muscle (Figure 1C, upper). The brain and marrow findings were confirmed in a partial assessment of a second dog's tissues (Figure 1C, lower).
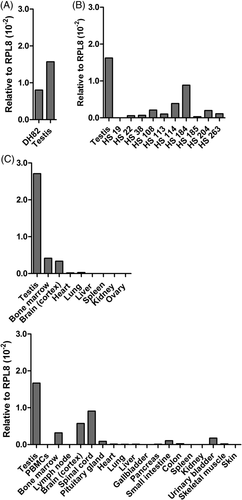
Two separate LC-MS/MS analyses yielded a total of three DLA-88*508:01-binding peptides (Table 1) derived from TPX2 (TPX2, Microtubule Nucleation Factor; also known as Differentially Expressed in Cancerous and Non-Cancerous Lung Cells [DIL] 2; or Targeting Protein for Xklp2). Originally found by mRNA differential display in human lung carcinoma lines, TPX2 was shown to be expressed in fetal, but not adult, lung tissues.51 Data from human atlases indicated mostly weak, mixed expression in normal tissues, with substantial enhancement in testis.
As a key regulator of Aurora-A kinase, which controls spindle assembly and function during mitosis,52, 53 TPX2 expression in the DH82 line might simply result from the fact that, at any given time, a substantial fraction of cultured cells are undergoing division, and similarly robust expression might not be observed in clinical HS specimens. Analysis of biopsies by qPCR, however, showed strong and moderate expression in eight and two samples, respectively (Figure 2A). Message levels in HS were generally much higher than in samples of various other solid tumour types, shown in Table S3. TPX2 mRNA was also found in 12 of 12 PTCL samples, with strong expression in half, and moderate expression in the remainder (Figure 2B), as well as in the T-cell lymphoma line, OSW (Figure 2C). To gauge the extent to which TPX2 expression might be restricted to testis and tumours in the dog, we again evaluated a panel of normal canine tissues. Blood-derived, DC-enriched cultures only yielded weak TPX2 expression (<1% of testis; not shown), and as seen in Figure 2D (dog #1), most somatic tissues, including lymph nodes, were negative or weakly positive. However, brain and bone marrow strongly expressed TPX2 (14.7 and 15.9% of testis, respectively). Similar findings were observed in selected tissues from additional dogs, although the brain was negative in dog #3. Interestingly, ESTs for TPX2 have only been derived from canine heart and thymic tissues.
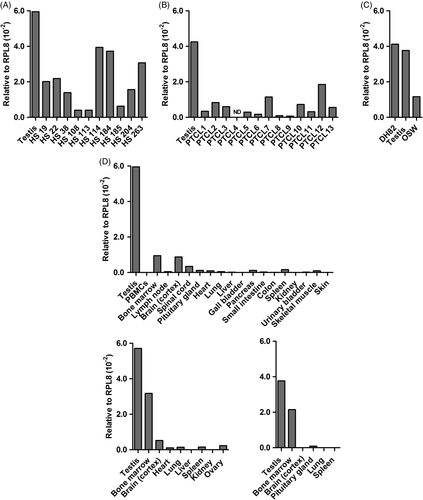
Finally, we evaluated NT5C1B (5’-Nucleotidase, Cytosolic IB). The human ortholog is also known as AIRP (autoimmune infertility-related protein). One report shows NT5C1B expression in diverse non-gametogenic murine and human tissues using RT-PCR,54 while other datasets from humans show testis-restricted mRNA expression.
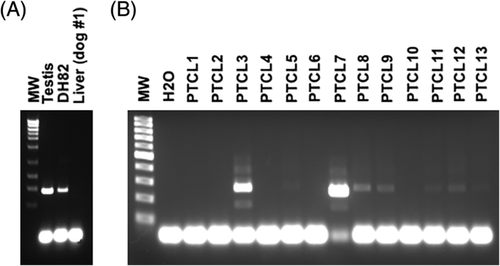
By qPCR with primer pair 1, NT5C1B expression was seen in canine testis, but not in somatic tissues, nor in the DH82 cell line or selected HS or PTCL biopsy samples. Cloning and sequencing of the qPCR amplimer confirmed the NT5C1B target. To potentially improve detection, we tested a second set of qPCR primers (pair 2). In this next round of analyses, 0 of 8 HS specimens (HS114 had a negligible signal, 0.02% of testis) and 1 of 12 PTCL specimens (PTCL7 showed weak expression, 0.2% of testis) were positive (Figure S2A,B). We also assayed for NT5C1B message in PBMCs of patients with overt leukemias (1 PTCL; 1 undifferentiated) and in B-cell lymphoma nodal biopsies (n = 3), but no signal was detected. In humans, expression by immunohistochemical staining is observed in melanomas, gliomas and diverse carcinomas (highest expression, gastric and pancreatic; most frequently positive, colorectal [data from HPA]), but there was essentially no message in a small panel of canine solid tumours (Table S3). We also saw no NT5C1B mRNA in selected somatic tissues from three normal dogs (Figure S2C), except for negligible expression in a single brain sample (0.03% of testis) and in one kidney sample (1 well was 34 cycles, the other >35 [not detectable]). As with primer pair 1, no expression was detected in DH82 cDNA (Figure S2A), despite the fact that an NTC51B-derived peptide had been retrieved from these cells in two separate LC-MS/MS analyses. A possible explanation is the well-known discordance between mRNA and protein expression: high protein concentrations can result from high translation rates and protein stability, despite minimal transcription.55 In that case, our qPCR may not have been sufficiently sensitive for NT5C1B detection; however, a robust signal was always returned from testis. Nonetheless, we re-probed samples for NTC51B mRNA by endpoint RT-PCR, using two different primer sets (pairs 3, 4; Table S1). Now, expression was observed in testes and DH82, but not in liver (Figure 3A). The HS biopsy samples were then evaluated over multiple assays, yielding weak bands in most samples, but signals were inconsistent across reactions (not shown). HS114, the sample that generated a negligible signal by qPCR (Figure S2A) was most consistent, with six of nine PCRs producing a band. No NT5C1B was amplified from cDNA isolated from a PBMC-derived DC culture. NT5C1B message was detectable in 8 of 13 PTCL biopsies, with bright bands in 2 samples, and faint bands in the remaining 6 (Figure 3B). Encouragingly, no bands were observed when multiple somatic tissues from dog #1 were assayed (not shown). To verify these results, similar tissues from dog #2 and #3 were evaluated, and most were negative; however, in some repetitions, bands were intermittently seen in brain, lung and kidney (4 of 5 PCRs each) and bone marrow, pituitary gland, heart and spleen (2 of 5 PCRs each; Figure S2D shows a representative gel). Sequencing of the product from brain returned NTC51B, ruling out an off-target amplification as an alternate explanation for the observed band. Overall, the endpoint RT-PCR mirrored the qPCR data, showing that there is low NTC51B message expressed in canine HS and PTCL, as well as in some normal tissues of some dogs. In reviewing available canine EST data, there are 14 NT5C1B cDNA clones from testis, but also 2 clones from kidney, consistent with our findings showing transcripts in this organ. Interestingly, the latter two clones (GenBank DN331931.1; DN330130.1) are splice variants, both retaining a 136-bp fragment of the 3′ end of intron 6, followed by exons 7 and 9; exon 8 is skipped. In the five ESTs from testis that cover this region, as well as the sequence that we obtained, exon 8 is expressed. If consistent, this difference could be exploitable for immunotherapy: that is, should splice variants in tumours (and testis) yield peptides not encoded by NT5C1B transcripts in normal somatic tissues, then the corresponding CTL would pose no risk for autoimmunity. However, sequencing of the NT5C1B amplimer from brain confirmed expression of exon 8, ruling out this possibility.
4 DISCUSSION
In this study we sought to identify CTAs in canine haematopoietic malignancies (HS and lymphoma) as potential targets for T-cell immunotherapy. In our approach, the discovery process was positioned downstream, at the level of the MHC class I-presented peptide, to insure that any CTA that was found was not only present as protein but also potentially immunogenic: that is, processed and available for CD8+ T-cell recognition. The limitation of this method is that individual tumours could not be probed for CTA-derived peptides, because specific antibodies for DLA-88 immunoprecipitation are lacking. Hence, any positive findings in DH82 cells had to be extended to patient biopsy samples by RT-PCR analysis. Nonetheless, a powerful advantage of direct class I ligandome searching is that peptides from any high-value CTA can immediately be synthesized to isolate the cognate TCR, by T-cell cloning or peptide-MHC tetramer sorting, for ACT development.
We identified 22 peptides that mapped to 17 proteins with characteristics of CTAs. While a large number of CTAs have been identified in human tumours, few have been reported in veterinary oncology. Testis-enhanced TSAs were recently reported in canine mammary gland tumours.56 The CTAs MAGE and MAGE-2 have been found in feline, but not canine, lymphomas.57-60 The testis-enhanced TRAF-interacting protein (TRAIP) gene was shown to be overexpressed 2.5-fold in a canine T-cell lymphoma xenograft, when compared to normal lymph nodes.61 However, a pioneer proteomics study in canine lymphoma did not report any CTAs.62
To assess the potential therapeutic worth of CTA candidates identified by MS, three criteria, similar to those used to assign relative value to human antigens,6, 63 were considered: (1) a previous connection (ie, human or veterinary literature) tying the protein to cancer; (2) expression across multiple patient tumour samples; and (3) lack of normal tissue expression, with this last category carrying the greatest weight. Unfortunately, none of the CTAs that we found exhibit the pristine absent somatic tissue expression that typifies high-value human CTAs such as MAGE-A3 and NY-ESO-1 (Table 2, right column). This characteristic probably reflects the fact that the CTAs that we identified were all single-copy genes, distributed across autosomes (Table 1). In humans, a slight majority of CTAs originate on chromosome X, particularly those in multigene families, such as MAGE and SSX. Transcription of these X-linked CTAs in somatic tissues appears to be more tightly regulated than that of their non-X counterparts. Thus, of the 39 human CTAs that are highly testis-restricted (ie, no mRNA expression other than testis and placenta), 35 originate on the X-chromosome.47 Why no X-linked CTAs were recovered here is unknown, but may reflect the insensitivity of the mass spectrometric approach or peculiarities of the DH82 cell line (note that peptides from nine non-CTA genes residing on the X chromosome were recovered in our experiments, ruling out whole chromosome loss). Interestingly, Chen et al57 observed MAGE expression via immunohistochemistry in histiocytic proliferative disease biopsy specimens, but to date we have not identified MAGE peptides in DH82, even when a different DLA-88 allele, *034:01, has been immunoprecipitated (in three independent experiments).64 An additional explanation for this dearth is that the canine genome appears to lack definitive orthologs of many of the human X-linked CTAs, which are evolutionarily young genes, with many family members arising after the divergence of Laurasiatherian mammals.65, 66
Expression of some CTAs in transformed cells may represent an epiphenomenon of altered genomic methylation or acquisition of stem-like properties, but in general, CTAs are thought to contribute to oncogenesis,67 presumably to a degree sufficient to offset the increased immunogenicity and susceptibility to immunoediting (expression of CTAs can be associated with CD8+ T-cell signatures in tumors.68, 69) The role of many autosomal CTAs in oncogenesis is generally better established than for their X-linked counterparts, increasing their relative worth as therapeutic targets. Even if their less-well-restricted tissue expression rules out use in immunotherapy, inhibiting the function of these oncogenic proteins in hematologic malignancies by pharmacologic means may be an important, under-investigated treatment approach.70 Of course, some testis-enhanced, autosomal-encoded proteins may have functions in the few other somatic tissues where they are expressed that would preclude safe inhibition. For example, we observed high SPECC1 message in CNS tissue. When SPECC1 is knocked out in mice, decreased avoidance learning results,71 so pharmacologic inhibition could also have adverse higher-order behavioural effects, in addition to anti-tumour benefits. Cancer-testis antigens with similar expression patterns have been considered to belong to a distinct subgroup known as cancer-testis-brain antigens, which includes other gene products that we recovered: CTNNA2, DLGAP2, DLGAP3, DPYSL5 and KIF5C. It is now recognized, however, that some of these proteins are more appropriately considered brain differentiation antigens, with concurrent high expression in testis.50 Brain expression of TPX2 would also appear to rule out safe immune targeting; however, this protein could have other value in veterinary oncology. TPX2 is a quantifiable indicator of cell proliferation in mantle cell lymphoma that can be correlated with overall survival,72 and could have similar utility in gauging prognosis in lymphomas of dogs. Additionally, given its apparent absence in canine PBMCs, TPX2 conceivably could be used to measure minimal residual disease in canine lymphoma.
By no means should the findings reported here be considered definitive. A commonality of all CTA discovery studies is that quite different conclusions regarding the expression of gene candidates in normal and cancerous tissues are often reached. In qPCR analyses, this discordance can occur even when the same tissue panels are probed.73 Not surprisingly, there are some discrepancies between our findings and canine data deposited in the NCI OncoGenomics database74 regarding the tissue expression of the five gene products profiled here. Also, as we and others have shown, there are also variations in expression in tissues obtained from different individual animals. Moreover, in many instances, there is disagreement between CTA mRNA and protein expression. For instance, NY-ESO-1 mRNA has been detected in liver and pancreas, but protein expression has never been documented in these tissues.48 Thus, the next, higher-level evaluation of a promising canine CTA such as NT5C1B will require analysing protein expression with a validated anti-canine antibody. Additionally, different mRNA and protein isoforms of CTAs are possible, which could serve as another source of tissue-specific peptides. For example, there are five predicted isoforms (X) of NT5C1B. One (X2) results from an alternate transcription start site, and another (X3) has a 183-bp deletion (supported by EST BM537000.1). The remaining 5′ EST clones support isoforms X1, X4 and X5. The single 3’ EST supports X1, X2 and X3. Evaluating whether such isoforms are differentially expressed by tissue or tumour type would certainly be needed prior to ultimately assigning a value to NT5C1B.
Finally, in most CTA-positive tumours, only a fraction of malignant cells typically produce the protein, which means that not all cells will be susceptible to T-cell killing. Such heterogeneity may reflect the fact that, in some cancers, CTA re-expression is a characteristic of a stem cell subpopulation.75 This obstacle to immunotherapy could potentially be mitigated by treatments that simultaneously target multiple CTAs, such as a polyvalent vaccine. Also, the DNA demethylating agent 5-aza-dC has been shown to induce or enhance expression of human CTAs in tumour cell lines, which can persist for weeks following removal of the drug.76, 77 In preliminary experiments (Figure S2E,F), we observed that treatment with 5-aza-dC induced expression of NT5C1B in OSW cells, but not in normal PBMCs, suggesting that this agent could be a valuable adjuvant to an NT5C1B-targeted immunotherapy. As expected, 5-aza-dC effects were non-specific, and expression of other CTA mRNAs were concurrently increased, as measured by qPCR: IGF2BP3, 3.9-fold; AKR1E2, 2.2-fold; SPECC1, 1-fold; and TPX2, 2.5-fold. Expression of NT5C1B was also induced in DH82. Treatment with 5-aza-dC has been shown to sensitize human cancer cell lines to lysis by CD8+ T cells,78 enhance the homogeneity of CTA expression within tumors,79 and increase MHC class I expression at the cell surface.80, 81 For these reasons, it seems that co-treatment with 5-aza-dC or other demethylating agents could be a significant contributor to any successful immunotherapy that targets CTAs in dogs, analogous to combination therapies being evaluated for human cancer patients (reviewed in Chiappinelli et al82). Safe administration of 5-aza-dC to dogs with urothelial carcinomas has been described.83
5 CONCLUSION
This study shows that a reverse antigen discovery process - from peptide to protein to mRNA - can be a fruitful means for finding shared TSAs, such as CTAs. Expanding this approach by surveying the MHC class I ligandome of other cell lines representing common and currently incurable haematopoietic (lymphoma) and solid (osteosarcoma; hemangiosarcoma) cancers to foster ACT development seems warranted.
ACKNOWLEDGEMENTS
The authors thank Drs. Steve Suter and Marlene Hauck for tumour samples; Dr. Barbara Sherry for helpful discussions and guidance with qPCR analyses; and Laura DuBois and Drs. Arthur Moseley and Erik Soderblom at the Duke University Proteomics and Metabolomics Core Facility for the LC-MS/MS analyses. This work, performed at the NCSU College of Veterinary Medicine, Raleigh, NC 27606, USA, was supported in part by a grant from the Morris Animal Foundation (D15CA-015 to P.R.H.) and donations to the NCSU-CVM Veterinary Medical Foundation, and is dedicated to the memory of Murphy.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.