Longitudinal transcriptomic and genetic landscape of radiotherapy response in canine melanoma
Funding information Nouscom srl
Abstract
Canine malignant melanoma (MM) is a highly aggressive tumour with a low survival rate and represents an ideal spontaneous model for the human counterpart. Considerable progress has been recently obtained, but the therapeutic success for canine melanoma is still challenging. Little is known about the mechanisms beyond pathogenesis and melanoma development, and the molecular response to radiotherapy has never been explored before. A faster and deeper understanding of cancer mutational processes and developing mechanisms are now possible through next generation sequencing technologies. In this study, we matched whole exome and transcriptome sequencing in four dogs affected by MM at diagnosis and at disease progression to identify possible genetic mechanisms associated with therapy failure. According to previous studies, a genetic similarity between canine MM and its human counterpart was observed. Several somatic mutations were functionally related to MAPK, PI3K/AKT and p53 signalling pathways, but located in genes other than BRAF, RAS and KIT. At disease progression, several mutations were related to therapy effects. Natural killer cell-mediated cytotoxicity and several immune-system-related pathways resulted activated opening a new scenario on the microenvironment in this tumour. In conclusion, this study suggests a potential role of the immune system associated to radiotherapy in canine melanoma, but a larger sample size associated with functional studies are needed.
1 INTRODUCTION
Malignant melanoma (MM) is one of the deadliest forms of cancer being characterized by a high degree of local invasiveness and high metastatic propensity. In dogs, MM frequently involves the oral cavity, followed by mucosal junctions, feet (including digits) and haired skin. Regardless of the anatomical location, complete surgical resection associated with regional lymphadenectomy and/or radiotherapy is the preferred treatment modality. Systemic chemotherapy has yielded controversial and quite disappointing results and recently, attention has been focused on the role of the immune system resulting in the development of two immunotherapeutic approaches, namely a xenogeneic DNA vaccine against human tyrosinase (Oncept, Merial), and a CSPG4-immunotargeting vaccine.1
Despite a significant improvement in treatment during the last years, canine MM continues to represent a major clinical challenge. Consequently, there is a need for further research on disease aetiology and pathogenesis, possibly leading to the identification of biomarkers and drug targets. Although incompletely outlined, the genetic profile of canine MM has revealed similarities with the human counterpart. The mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K) pathways were found frequently activated in dogs2-5; conversely, activating mutations in BRAF, NRAS and KIT were scarcely identified.4-6 In addition, Poorman et al7 described amplification of MYC and KIT loci, deletion of CDKN2A locus and reported distinctive copy number abnormalities in canine chromosome 30, encoding MAPK pathway genes, such as SPRED1 and TRPM7. Whole exome sequencing (WES) has been extensively applied in human medicine for several diseases8 and recent studies have identified potential therapeutic targetable mutations in MM.9 In dogs, investigations to identify global somatic mutations by next generation sequencing (NGS) were previously conducted in lymphoma10 and in rare congenital,11 retinal12 and neurodegenerative13 disorders. Recently, two extensive NGS studies have been conducted in canine MM to map the genomic landscape of this tumour but progression after therapy were not described.14, 15
In the current study, WES was performed in dogs with treatment-naive MM at diagnosis and at disease progression to highlight the genetic mechanisms beyond progression. In addition, whole-transcriptome sequencing (RNA-seq) was performed on the same samples. The main objectives were (a) to characterize the mutational signature via the identification of somatic short and structural variants of canine MM both at diagnosis and at disease progression, (b) to combine gene mutations with gene expression alterations by functional analysis and finally (c) to compare gene expression profiles at diagnosis and disease progression.
2 MATERIALS AND METHODS
2.1 Animal recruitment and sample acquisition
Client-owned dogs affected by MM undergoing a complete staging work-up and receiving treatment were eligible for recruitment. At first presentation (T0), all dogs had to undergo a surgical biopsy to confirm the diagnosis (Supporting Information Table S1). Furthermore, all dogs had to be followed-up and undergo a second surgical biopsy at disease progression (T1). All applicable international and national guidelines for the care and use of animals were followed and a signed owner consent was always obtained before inclusion. Tissue sampling procedures were kept consistent for all the samples and were obtained by the same hospital personnel.
2.2 DNA and RNA isolation
The neoplastic tissues collected at T0 and T1 were conserved in tubes with 2.5 mL of RNAlater (Applied Biosystems, Monza, Italy) until further processing. The total DNA and RNA were isolated and purified by the AllPrep DNA/RNA Mini kit (Qiagen, Milan, Italy). Total RNA concentration was determined using the NanoDrop ND-1000 UV-Vis spectrophotometer (NanoDrop Technologies Inc., Wilmington, Delaware), and its integrity was measured by the 2100 Bioanalyzer (Agilent Technologies, Santa Clara, California). RNA samples with an RNA integrity number (RIN) ≥8 were considered for the RNA-seq library preparation. The isolated RNA was stored at −80°C until further use.
2.3 WES library preparation and sequencing
Genomic DNA (gDNA) was isolated to prepare libraries for WES (n = 8). For each dog, gDNA from non-neoplastic mucosa was also extracted and employed as matched-control for subsequent analyses. The exome of the 12 samples (4 T0 MM, 4 T1 MM and 4 control mucosa) was captured and then sequenced by Illumina HiSeq2500 (125 PE) with target coverage of 150×. Briefly, gDNA was tested for concentration and purity and then subjected to fragmentation. Illumina-compatible whole exome libraries were prepared using the SPRIworks HT Reagent Kit (Beckman Coulter, Inc., Indianapolis, Indiana, Cat. No. B06938) and the resulting libraries were subjected to exome enrichment using the SureSelectXT Canine All Exon Kit (Agilent, Cat. No. 5190-5452), following the manufacturer's instructions. Final concentration and size distribution were tested with Agilent Bioanalyzer 2100.
2.4 RNA-seq library preparation and sequencing
Strand-specific RNA-seq libraries were prepared using the NEBNext Ultra RNA Library Prep Kit for Illumina (New England BioLabs, Hitchin, UK). In brief, using 0.3 μg of total RNA as input, poly(A) mRNA was enriched using the NEB magnetic mRNA isolation module, then fragmented and reverse-transcribed to generate cDNA. An Illumina-specific adaptor was sequentially ligated to the 3′ end of the cDNA fragments, purified using the AMPure XP beads (Beckman Coulter, Brea, California), and finally PCR-amplified (14 cycles) using an appropriate indexing primer that allows further samples multiplexing. The PCR-amplified libraries were purified with the AMPure XP beads (Beckman Coulter, Brea, California) and then assessed for their quality and fragments distribution using the 2100 Bioanalyzer DNA 1000 assay (Agilent Technologies). Libraries were quantified by Qubit Fluorometer (Life Technologies, Carlsbad, California) and qPCR-based NEBNext library quantification Kit (New England BioLabs, Hitchin, UK). For each sample, two separated libraries were prepared and the second library was always included as technical replicate. Finally, equimolar amounts of each four index-tagged libraries were multiplexed together in one pool (8 samples, 16 single libraries) and then sequenced by an Illumina HiSeq2500 for 50 sequencing cycles.
2.5 Whole exome sequencing data analysis
Somatic mutations discovery across all samples was performed following the Genome Analysis Toolkit16 v.3.7 Best Practices (https://software.broadinstitute.org/gatk/best-practices/). Single nucleotide variants (SNVs) and small insertion and deletions (indels) were identified with Mutect217 and Varscan18 v.2.4.1. Loss of heterozygosity (LOH) was further investigated by Varscan. SNVs, indels and LOH loci were considered as the union of Mutect2 and Varscan filtered somatic mutations. To extract biological information from somatic variants exclusively present in T1, functional analysis was performed using Cytoscape plugin ClueGO.19 Further details are provided in Supporting Information Material and Methods. Copy number aberration (CNA) analysis was performed on WES data using the NEXUS Copy Number v.9.0 software (Biodiscovery Inc., El Segundo, California). Additional details are reported in Supporting Information Material and Methods. Raw Illumina reads are deposited in the GenBank's Sequence Read Archive (SRA) repository under the accession ID SRP151732.
2.6 RNA-seq data analysis
All RNA-seq analyses were performed using conventional RNA-seq analysis tools. Detailed information is provided in Supporting Information Material and Methods. The sequencing data (FASTQ files) associated with this study are deposited in the Gene Bank's SRA repository under the accession ID SRP150063.
3 RESULTS
3.1 Clinico-pathological features
Four dogs were enrolled: one mixed breed, one Golden retriever, one Labrador retriever and one English setter. There were three intact males and one spayed female. Age ranged between 8 and 13 years, and weight ranged between 20 and 38 kg. Three dogs had an oral MM and one dog had a nail bed MM. Three MMs were staged T2N0M0 and one T3N1M0. All the tumours were positive to Melan A and PNL2, and Ki67 index was consistently higher than 19.5%. A detailed description of morphological and immunophenotypical features are shown in Table S2. All dogs were irradiated with five bi-weekly fractions of 6 Gy each, up to a total dose of 30 Gy; three dogs received adjuvant chemotherapy and one dog was treated with immunotherapy. At the time of disease progression, the tumour was biopsied again after 139, 175, 204 and 231 days from diagnosis, respectively. At the end of the study, all dogs had died because of local progression (n = 2) after 139 and 270 days, distant metastasis to skin (n = 1) after 188 days, or lungs and liver (n = 1) after 221 days from diagnosis.
3.2 CNA analysis identifies a similar pattern of chromosomal aberrations in MMs at first presentation and disease progression
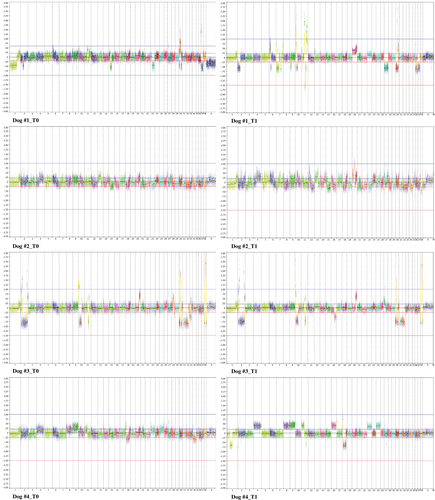
Focal and broad chromosomal aberrations totalling from 10 to 100 Mb were identified in all melanomas (Figure 1). Noteworthy, amplifications prevailed over deletions, and extension and frequency of CNAs were increased at T1. However, the comparison of CNA frequency by GISTIC revealed no significant regions distinguishing firstly occurring and progressed tumours. Therefore, we compared CNAs at T0 and T1 separately for each dog (Figure 2). On a general level, similar evolutionary CNA patterns were recognized in all the tumour pairs, although the patient relative number of primary- or progression-specific aberrations varied substantially. Dog #1 showed exclusively at T1 a broad amplification on Canis familiaris autosome (CFA) 20 and a whole chromosome deletion on CFA 27, comprising ATF1, PDE3A, WNK1 and SOX5. Dog #2 and #3 exhibited overlapping patterns both at T0 and T1, with the exception of several focal gains on CFA 20 in dog #2 and a loss on the terminal portion of CFA 16 in dog #3, encompassing ING2 and WWC2 genes. These aberrations were detected only at T1. The observed amplifications on CFA 20 overlapped in correspondence of RAF1, FOXP1 and MITF. Notably, several CNAs, namely whole chromosome amplifications on CFA 4, 8, 9, 16, 23 and 25, a gain on CFA 11, and a loss on CFA 1 encompassing EPM2A and THBS2 were exclusively identified in Dog #4 at T1.
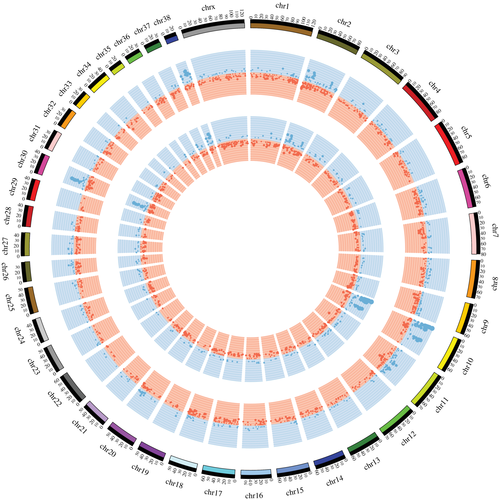
Exploring all the MMs together, recurrent gains were found on CFA 10, 27, 30 and 38 (G > 4.8 and Q < 1.945e−02), whereas CFA 2, 11, 30, 31 and 38 presented recurrent losses (G > 2.4 and Q < 3.877e−02) (Table S3). The most frequent copy number gains were located on CFA 6, 8, 9, 17 and 25 (Table S4) and occurred in all tumours. The most recurrent copy number loss (50% samples at T0, and 75% at T1) involved CFA 11 and was a ~600 kb region located at 41055923 41652538 (Table S4) encompassing CDKN2A locus. A focal amplification located on CFA 10 and corresponding to MDM2 locus (50% samples at T0 and 50% at T1) was also identified. Interestingly, two dogs showed a peculiar aberration on CFA 30, displaying a sigmoidal pattern of copy number loss (spanning 0.5-12 Mb) followed by a gain (spanning 9.5-19 Mb). The amplification contained 12 gene loci, including SLC27A2, GABPB1, USP8, TRPM7, SPPL2A and CYP19A1, whereas SPRED1 and FAM98B were deleted.
3.3 WES shows an additional burden of mutations in MMs at disease progression
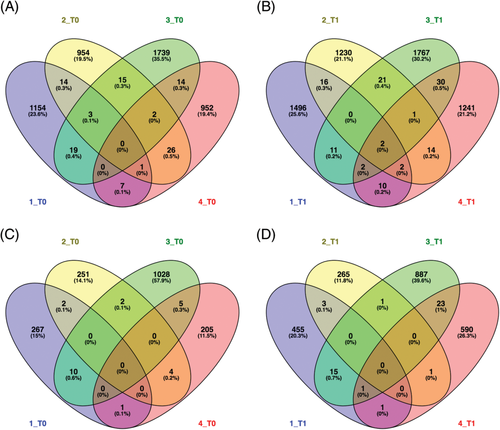
A total of 6807 somatic mutations were found in melanomas at T0: 4036 SNVs, 971 indels and 1800 LOH calls, respectively (Table S5). However, only 1 non-synonymous SNV, 4 non-coding SNVs and 1 frameshift substitution were in common in at least 3 samples and none when considering all four (Figure 3A,C). The median tumour mutational burden (TMB) was 1.8 mutations/Mb (range 0.3-3.4). At T1, 8246 somatic mutations were identified: 4866 SNVs, 1094 indels and 2286 LOH calls, respectively. Two non-coding SNVs were shared by all tumours, while 5 non-coding SNVs and 1 LOH call were in common in 3 dogs (Figure 3B,D). The median TMB was 4.9 mutations/Mb (range 4.5-5.8). The sum of different SNVs obtained at T0 and T1 was 8902, and the ratio of transitions (5779) to transversions (3123) was 1.85. Also, a significant higher rate of C>T/G>A transitions (18.9%; Fisher's exact test, P < 0.01) was calculated. Other frequent patterns comprised T>C/A>G transitions (13.8%) and C>A/G>T transversions (5.2%; Figure 4B and Table S6). Furthermore, MMs revealed an enrichment of signature 1B resembling human melanoma.20 Interestingly, no significant signature changes related to localization (oral vs digital MMs), chemotherapy treatment (none vs temozolomide vs cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP)) and evolution (T0 vs T1) were observed.
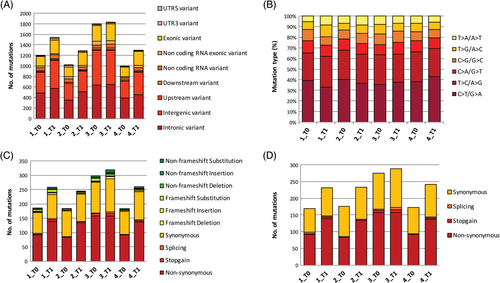
An average of 16.9% (10.9-25.9%) mutations were found in common at T0 and T1 and only 20.7% (15-27.6%) were exonic with predicted protein sequence effects (Table S7 and Figure S1). The allele frequencies of the coding mutations were increased in three dogs at T1 (#1, #2 and #4), being statistically significant in Dog #1 and #4 (Wilcoxon-signed rank test: P = 0.004 and P = 2.045e−12, respectively). Focusing on somatic coding mutations exclusively present in melanomas at T1, Dog #1 had the highest percentage (51.4%) of private mutations. Cross-analysis of these mutations evidenced genes involved in cell development, RNA processing, catabolic processes and responses to external stimuli. Also, mutations in genes associated to melanoma development were retrieved: the list of private and shared mutations between T0 and T1 is reported in Table S8.
A total of 101 and 109 somatic mutations (SNVs and indels) shared by at least two dogs were calculated at T0 and T1, respectively (Table S9). At T0 and T1, 17 and 18 somatic mutations were identified in the exons, respectively. Distribution and consequences of coding mutations according to ANNOVAR and variant effect predictor are depicted in Figure 4A,C,D. Interestingly, 14 somatic mutations present both at T0 and T1 were shared by at least two dogs, including TMEM256-PLSCR3, DCAF10, LAMA3, GALC, SMARCAL1, TSPYL1, SOX17, MCM5, and CYP2A13. The frameshift substitutions harboured on TMEM256-PLSCR3 (chr5:32330460:A>-C) and on SOX17 (chr29:5979449:G > -TT) and the non-synonymous SNV on GALC (chr8:59317742:A>C) were the sole coding mutations. A total of 94 mutations were found in at least 50% of samples at T1 and never detected at T0. Exonic mutations were seven non-synonymous SNVs, one non-frameshift substitution on eEF1A1 (chr3:60899961:G>A) and one non-frameshift deletion on TRPM1 (chr3:37948404:CCC>-).
A functional analysis was also performed on the 4334 somatic variants exclusively present at T1. A total of 2339 mutated genes (MGs) were obtained when variants were collapsed to genes. Using ClueGO, 57 pathways and gene ontology terms were significantly enriched (Table S10). Pathways related to drug and hormone metabolism as well as PI3K/AKT signalling pathway were overrepresented. Noteworthy, excluding Dog #3 that was treated with CHOP, no significant differences related to treatment were retrieved. Finally, considering the low number of differentially expressed genes (DEGs) identified in the differential expression analysis, no overlapping emerged comparing DEGs and MGs.
When integrating somatic mutations with gene expression data, an average of 70% of mutations was expressed. Only 11 and 9 somatic mutations were supported by RNA-seq reads in at least 2 dogs at T0 and T1, respectively. When comparing expressed mutations between T0 and T1, only a small number was in common (26.5%). Genes belonging to keratin family and keratin-associated proteins were related to the anatomical site, rather than being associated to the disease itself.21 The coding mutation located on GALC gene was covered by RNA-seq reads in six MMs. To investigate the presence of RNA-seq reads that supported genes with somatic mutations, SNVs and indels were collapsed to genes. Particularly attractive was AHNAK being mutated in six samples.
3.4 Gene expression profiling reveals immune signatures differentiating MMs at first presentation vs disease progression
A summary of the sequencing output, quality scores and mapping percentage is reported in Table S11. By comparing the transcriptomes of T1 and T0 samples a total of 25 and 29 protein-coding genes were found differently regulated in the biological and technical replicates, respectively (Table S12). To define a list of non-biased DEGs, only those genes that overlapped (n = 18) between the two independent analyses (biological and technical replicates) were considered as the final DEGs list (Table S12). Of the 18 DEGs, 15 genes were down-regulated at T1, with ASPRV1, MSTN and SPINK7 being the most down-regulated genes, while the other 3 genes were up-regulated (Table S12). Interestingly, by GSEA, immune-system-related pathways were mainly enriched in T1, including genes associated with natural killer cell-mediated cytotoxicity, chemokines and T-cell receptors. A complete list of all enriched GO terms, KEGG pathways and Cancer Hallmarks are reported in Table S13.
4 DISCUSSION
Understanding the relationship between treatment and dynamics of mutations in naïve and progressed cancers has dramatically changed the therapeutic perspectives in cancer. When considering mutational profiles, treatment-naïve melanomas showed similar signatures to human melanoma and TMB reflected the complexity.22, 23 In addition, the SNVs spectrum was similar to the mutational pattern described by Hendricks et al. At T1, the signatures remained unchanged, whereas TMB was considerably increased. By definition, TMB measures the number of mutations carried by tumour cells and in our experiment this result is supported by a possible clonal evolution after treatment because of the selective pressure. Recent studies have also reported that tumours with a high number of mutations have a higher likelihood to respond to immunotherapy, but this is still unexplored in dogs.
Unfortunately, no CNAs separating MM at T0 and T1 were identified by GISTIC, most likely because of the low number of cases. However, interesting data were obtained analysing dogs individually. Two dogs (#1 and #2) revealed overlapping amplifications encompassing RAF1, FOXP1 and MITF gene loci. Notably, these amplifications matched at T1 leading to up-regulation. RAF1 is a proto-oncogene and belongs to the same protein family as BRAF.24 FOXP1 is a transcription factor and its regulation causes PI3K/AKT signalling pathway activation in tumours.25 MITF is recognized as a fundamental driver of melanoma progression and is amplified in 10% to 20% of melanoma metastases.26 The loss on CFA 16 was exclusive for Dog #3 and caused down-expression of ING2 and WWC2. The CNAs pattern of the Dog #4 at T1 was particularly different compared to T0 and a deletion on CFA 1 covering EPM2A and THBS2 was correlated to a reduction of expression. In two dogs, a sigmoidal pattern aberration on CFA 30 previously described7 was identified at T0 and also retained at T1. The amplification comprised important genes, such as SLC27A2, GABPB1, USP8, TRPM7 and SPPL2A. In the same genomic region, the loss of SPRED1 was also retrieved. Experimental data show that deletion of SPRED1 and amplification of TRPM7 are responsible to activate MAPK signalling pathway in human cancers.4, 7 By RNA-seq, TRPM7 expression was increased up to 20 times in melanomas presenting the amplification. A recurrent focal amplification on MDM2 locus was identified, and associated with an expression 19 times higher than MM with no aberrations. MDM2 is a p53 negative regulator promoting tumour formation by targeting several tumour suppressors.27, 28 This gene is transcriptionally regulated by p53, conversely overexpression because of amplification is reported in a variety of different tumours, including melanomas.14, 29 In our study p53 mutation was not retrieved, supporting the hypothesis of a mutual exclusivity of the two mechanisms. Finally, loss in CDKN2A locus was observed in two dogs both at T0 and T1, and another dog acquired the deletion at disease progression confirming similarities with previous data in dog.7, 29
Unexpectedly, a small number of mutations were in common between T0 and T1 and only 20% of them were exonic with predicted protein sequence effects. Two different hypotheses might explain these results. First, the majority of mutations were identified in intronic and intergenic regions, revealing a significant percentage of off-target sequences in the canine exome enrichment protocol.30 Second, a more pronounced selective pressure in the exonic regions of the tumour might cause a higher variation in non-coding regions.31 Interestingly, a clonal mutation selection during disease progression was demonstrated by the increased allele frequencies at T1. Among the genes involved, GALC showed a non-synonymous SNV that was confirmed by the absence of reads in RNA-seq. GALC encodes a lysosomal protein which hydrolyzes glycosphingolipids leading to the consequent production of bioactive lipids that negatively affect cancer progression.32, 33 SOX17 harboured a frameshift mutation that was not supported by RNA-seq reads. This transcription factor is considered an antagonist of the canonical Wnt/β catenin signalling in several tumours, and the down-regulation is correlated to progression in primary and metastatic human melanomas.34 Conversely, target melanoma oncogenes, such as BRAF, RAS and KIT did not harbour variants, thereby confirming previous findings.4-6
A non-frameshift deletion located on TRPM1 was identified in two dogs at T1 and the number of reads mapping TRPM1 was reduced compared to dogs not harbouring this mutation. Moreover, in Dog #1 the mutation was concurrent with MITF mutation, which represents a major TRPM1 transcriptional regulator. TRPM1 gene encodes for a calcium permeable cation channel involved in regulation of melanocytes physiology and melanoma development.35 Loss of TRPM1 expression has been correlated to melanoma aggressiveness36 and is now considered an independent prognostic marker of disease free and overall survival.37, 38 Finally, a non-synonymous SNV on eEF1A1 locus showing no reads supporting the variant allele was reported in two melanomas at T1. eEF1A1 has been proposed as a premature senescence marker39 and its expression was found to be decreased after radiotherapy regimen similar to the one used in these two dogs.40
Then, we investigated the transcriptome of melanomas at T0 and T1. A positive enrichment of several immune-system-related pathways, such as natural killer cell-mediated cytotoxicity, was found in progressed MM. Melanoma is an immunogenic tumour and its relationship with the host immune system is currently under investigation.41 Different studies have shown the capacity of melanoma to activate or modulate the adaptive immune response, resulting in a balance between survival and proliferation of tumour cells.41, 42 Interestingly, tumours that progressed after radiotherapy were able to selectively modulate the immune system in their favour. We also tested tumours by immunohistochemistry with several antibodies for lymphocytes, but no significant results were obtained (data not shown). However, our results open a new perspective on the role of the microenvironment in canine MM after radiotherapy.41, 43 Also, in MMs at progression a negative enrichment of several fatty acid metabolism pathways was identified. Even if never described in dog, this data is not surprising considering the ability of cancer cells to reprogram their metabolic pathways to satisfy both proliferation and survival demands.44-46 This ability should be considered in future targeted therapies.
In conclusion, the results from our study confirm the genetic similarity of canine MM with its human counterpart.2, 47, 48 Genetic molecular mechanisms beyond pathogenesis and progression are related to CNAs rather than point mutations and somatic mutations are located in genes other than BRAF, RAS and KIT. Nevertheless MAPK, PI3K/AKT and p53 signalling pathways are affected revealing intriguing data to test target therapy in this tumour. Several allelic mutations related to therapy were observed but a larger number of cases and functional studies are needed in future to describe their role. Finally, RNA-seq analysis revealed the importance of adaptive immune system regulation and the TMB increase in T1 opened the opportunity to further investigate the potential of immunotherapy in canine melanoma.
ACKNOWLEDGEMENTS
This study was supported by Nouscom srl.
CONFLICT OF INTEREST
The authors declare no conflict of interests.