Tumour necrosis factor-related apoptosis-inducing ligand induces apoptosis in canine hemangiosarcoma cells in vitro
Funding information Japan Society for the Promotion of Science, Grant/Award Number: 17K08101
Abstract
Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) is an apoptosis-inducing cytokine that shows potential therapeutic value for human neoplasms, and is effective in some canine tumours; however, its potential for killing canine hemangiosarcoma (HSA) cells is unknown. Thus, we evaluated the proapoptotic effect of TRAIL in nine canine HSA cell lines. Cells (JuA1, JuB2, JuB2-1, JuB4, Re11, Re12, Re21, Ud2 and Ud6) were cultured with three recombinant human TRAILs (rhTRAILs): TRAIL-TEC derived from Escherichia coli, TRAIL-TL derived from mammalian cells and isoleucine zipper recombinant human TRAIL (izTRAIL) containing an isoleucine-zippered structure that facilitates trimerization. TRAIL-TEC did not decrease the cell viability in any of the cell lines tested, whereas the other two rhTRAILs effectively decreased the viability of all cell lines as assessed by the WST-1 assay. In canine HSA cells, izTRAIL induced apoptosis more effectively than TRAIL-TL. In JuB4, Re12, and Ud6 cells, izTRAIL increased the activation of caspase-3 and caspase-8 and caused poly (ADP-ribose) polymerase degradation. Moreover, izTRAIL treatment increased the proportion of Annexin V+/ Propidium iodide (PI)− apoptotic cells and nuclear fragmentation in izTRAIL-sensitive cells. These results show that rhTRAIL can induce apoptosis in canine HSA cells, but the sensitivity of TRAIL was different depending on the cell lines. Therefore, TRAIL could be an effective therapeutic agent against canine HSA, but the specific mechanism of resistance should be determined to clarify under what conditions this treatment would be most effective.
1 INTRODUCTION
Canine hemangiosarcoma (HSA) is a malignant tumour that mainly occurs in the spleen, subcutaneous tissue and in the right atrium of the heart.1-3 HSAs are very aggressive tumours, demonstrating metastasis and invasive growth, contributing to a very poor prognosis, especially in case of visceral HSA.1, 3-5 Previous studies have demonstrated an association of several factors with the malignant behaviour and tumorigenesis of canine HSA, such as enhanced expression of vascular endothelial growth factor and basic fibroblast growth factor, mutations of several oncogenes and activation of various signalling pathways.6-10
Despite continuous progress in elucidating the oncogenic mechanism of canine HSA along with identification of potential therapeutic targets, there is still no effective therapeutic strategy. The survival time following splenectomy for dogs with splenic HSA is extremely short; even when splenectomy and chemotherapy are used in combination, the survival time is only about 6 months.2, 4, 11, 12 One of the main contributions to this poor prognosis in the high rate of metastasis for canine HSA; thus, systemic therapy targeting the metastatic lesions as well as the original tumour should be developed. Recently, many new concept small-molecule compounds such as tyrosine kinase inhibitors or anti-tumour cytokines have been applied to treatment of systemic cancers in both human and veterinary medicine.13-17
One such promising molecule is tumour necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), also called Apo2 ligand (Apo2L), which is a representative anti-tumour cytokine belonging to the TNF protein family.18, 19 TRAIL is transcribed in many normal tissues, including the spleen, thymus, prostate, lung and tonsillar T-cells, as well as in several lymphoma cells such as Raji and K299 cells.18 TRAIL induces apoptosis in various human tumour cell lines with no cytotoxic effect on normal cells.20, 21 Therefore, in human medicine, TRAIL is anticipated to be an effective new therapy for neoplastic diseases without exerting toxicity to the entire body.22 Besides simply administering TRAIL, another treatment strategy is to activate its signalling pathway, which can be achieved with anti-TRAIL receptor agonist or the small-molecule compound (TRAIL-inducing compound 10 [TIC10]) to induce intrinsic TRAIL production in vivo.18, 23 Therefore, various strategies for activation of the TRAIL pathway can be selected. In veterinary medicine, the effect of TRAIL has been evaluated in canine mast cell tumours, canine osteosarcoma cell lines, lymphoma cell lines and mammary cell lines;24-27 however, the mechanism of action of TRAIL in canine tumour cells has not been elucidated. Moreover, TRAIL resistance can be acquired in several types of human tumour cells, and the underlying mechanism has been clarified to some extent using various types of cell lines, whereas less is known about this mechanism in canine cancers.26, 28 Moreover, the effects of TRAIL on HSAs have not yet been reported for human or veterinary medicine. Therefore, the aim of this study was to clarify the cytotoxic effects of TRAIL in canine HSA to provide a scientific foundation for the potential effectiveness of TRAIL in the treatment of canine tumours. We explored the effects of TRAIL in various HSA cell lines to provide basic information on the TRAIL sensitivity of canine HSA cells. This information contributes to future studies about the mechanism of acquiring TRAIL resistance or the in vivo induction of TRAIL by small molecules, which studies are starting points for the development of new treatments of canine tumours.
2 MATERIALS AND METHODS
2.1 Cells and culture conditions
Canine HSA cell lines established in our laboratory from the liver (JuA1, JuB2, JuB2-1 and JuB4), right atrium of the heart (Re11, Re12 and Re21), and spleen (Ud2 and Ud6) were used in this study.10, 29 Madin-Darby canine kidney (MDCK, resistant to TRAIL) and HeLa cells (sensitive to TRAIL) were used as negative and positive control, respectively (both from JCRB Cell Bank, Osaka, Japan).21, 30 Canine aortic endothelial cells (CnAOECs; Cell Applications Inc., San Diego, California) were used as a normal canine vascular endothelial cell line to evaluate the cytotoxicity of TRAIL against normal cells. The HSA cell lines, MDCK cells and HeLa cells were maintained in high-glucose Dulbeccos modified Eagle medium (Wako Pure Chemicals, Osaka, Japan) with 10% heat-inactivated fetal bovine serum (FBS), 100 units/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL amphotericin B (Penicillin-Streptomycin-Amphotericin B Suspension, Wako Pure Chemicals), and 100 μg/mL kanamycin (Wako Pure Chemicals) (10% FBS/D-MEM). CnAOEC cells were maintained in Canine Endothelial Cell Growth Medium (Cell Applications Inc.). All cells were incubated at 100% humidity, 37°C, with 20% O2 and 5% CO2.
2.2 TRAILs
Cytotoxicity and ability of apoptosis induction of three different TRAILs were assessed in this study: recombinant human TRAIL/TNFSF10 375-TEC (TRAIL-TEC, R&D Systems, Minneapolis, Minnesota), recombinant human TRAIL/TNFSF10 375-TL (TRAIL-TL, R&D Systems), and isoleucine zipper recombinant human TRAIL (izTRAIL, AdipoGen Life Sciences Inc., San Diego, California). TRAIL-TEC (amino acids 114Thr-281Gly) was expressed in Escherichia coli, TRAIL-TL (amino acids 95Thr-281Gly) was expressed in the NSO mouse myeloma cell line, and izTRAIL (amino acids 95Thr-281Gly) was expressed in E. coli.
2.3 Cell viability assay
Cell viability assays were performed with the premix WST-1 cell proliferation assay system (TaKaRa, Otsu, Japan). HSA and HeLa cells were seeded in 96-well plates at a density of 1.0 × 104 cells per well. MDCK cells were seeded at a density of 2.5 × 103 cells per well because of their relatively fast proliferation rate. After 12 hours, the cells were treated with 10% FBS/D-MEM containing 0, 0.01, 0.1, 1.0, 10 or 100 ng/mL of TRAIL-TEC, TRAIL-TL, or izTRAIL for 24, 48 and 72 hours. After the treatment, the cells were incubated with 10 μL of WST-1 reagent per well for 1 hour. Cell viability was then quantified by iMark microplate reader (Bio-Rad Laboratories, Hercules, California) as the relative absorbance value obtained in the treated wells compared with those of control (0 ng/mL TRAIL) wells. The half-maximal inhibitory concentration (IC50) of each TRAIL was calculated by Image J 1.51 K (National Institutes of Health, Maryland ) based on the results of the viability assay.
We further evaluated the effect of izTRAIL (which had the most potent cytotoxic effect) on CnAOEC viability. CnAOECs were seeded in 96-well plates at a density of 1.0 × 104 cells per well. After 12 hours, the cells were treated with Canine Endothelial Cell Growth Medium containing 0, 0.01, 0.1, 1.0, 10 and 100 ng/mL of izTRAIL for 24, 48 and 72 hours. Measurement of cell viability and calculation of IC50 valued were carried out in the same manner as described above.
2.4 Flow cytometric analysis of apoptosis
JuB4 and Ud6 cells were identified to be the most sensitive cell lines to TRAIL for each lineage, and were thus selected for further analysis of the induction of apoptosis. Since Re12 and Re21 cells showed similar susceptibility to TRAIL, only Re12 was used for further analysis. JuB4, Re12 and Ud6 cells were treated with 100 ng/mL izTRAIL, and then apoptosis was detected with flow cytometry, after 4, 8, 12, 24 and 36 hours, corresponding to the time periods before cell viability significantly decreased. The cells were collected with 0.25% Trypsin-1 mmoL/L EDTA•4Na solution (T/E solution, Wako Pure Chemicals) and washed with Dulbeccos phosphate-buffered saline (D-PBS, Wako Pure Chemicals), and then the cells were stained with Annexin V/propidium iodide (PI) (Alexa Fluor 488 Annexin V/Dead cell Apoptosis Kit, ThermoFisher Scientific, Waltham, Massachusetts). The cells were counted by FACS Calibur (BD Biosciences, Franklin Lakes, New Jersey) and analysed by BD CellQuest Pro ver. 5.1.1 (BD Biosciences).
For analysis of the cell cycle, JuB4, Re12 and Ud6 were treated with 100 ng/mL izTRAIL for 48 hours (the point at which cell viability significantly decreased), the supernatant and cells were collected with T/E solution, and washed with D-PBS. The collected cells were then incubated with PI (PI/RNase staining solution, Cell Signalling Technology, Danvers, Massachusetts). Stained cells were counted by BD FACSCantoII(BD Biosciences) and analysed by BD FACSDiva 6.1 software (BD Biosciences).
2.5 Observations of nuclear fragmentation
JuB4, Re12, Ud6 and MDCK cells were cultured at 2.0 × 105 cells per well in a chamber slide for 12 hours for observation of the effect of TRAIL on nuclear fragmentation using fluorescence microscopy. The cells were treated with 100 ng/mL izTRAIL for 36 hours, and then washed with D-PBS, and fixed in 4% paraformaldehyde (Wako Pure Chemicals) for 30 minutes. The slides were washed with D-PBS, and mounted with ProLong Diamond antifade Mountant with 4′,6-diamidino-2-phenylindole (DAPI) nuclear stain (ThermoFisher Scientific), and the presence of DNA fragmentation was assessed using fluorescence microscopy (IX73, Olympus, Tokyo, Japan).
2.6 Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
To detect the effects of TRAIL on apoptosis-related proteins, JuB4, Re12, Ud6 and MDCK cells were collected by a cell scraper at 1, 3, 6, 12 and 24 hours after treatment with 100 ng/mL izTRAIL. The cells were then lysed using RIPA buffer (ThermoFisher Scientific) containing proteinase inhibitor cocktail and EDTA (ThermoFisher Scientific). The concentration of the extracted protein was measured by the DC Protein assay kit (Bio-Rad), and standardized by serially diluted bovine serum albumin (Thermo Fisher Scientific).
The equalized protein samples were mixed with 4× Laemmli buffer (Bio-Rad Laboratories) supplemented with 5% β-mercaptoethanol (Sigma-Aldrich, St. Louis, Missouri) and incubated at 95°C for 10 minutes. Proteins (15 μg/lane) were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (4%-12.5% Mini-PROTEAN TGX Precast Protein Gels, Bio-Rad) and transferred onto a polyvinylidene difluoride membrane (Amersham Hybond P0.45 PVDF, GE Healthcare Bioscience, Pittsburgh, Pennsylvania) at 60 V for 1.5 hours at room temperature (RT).
2.7 Western blotting
The membranes, with transferred proteins, were immersed in 5% blocking reagent (ThermoFisher Scientific) for 1 hour at RT, followed by incubation with primary goat polyclonal anti-caspase-8 (1:800, T-16, Santa Cruz Biotechnology, Dallas, Texas), rabbit polyclonal anti-caspase-3 (1:1000, Cell Signalling Technology), rabbit polyclonal anti-Poly (ADP-ribose) polymerase (PARP, 1:1000, ThermoFisher Scientific), or β-actin (1:1000, Cell Signalling Technology) for 1 hour at RT. The primary antibodies were used after confirming reaction to proteins of the same size as indicated in the previous study for both the HeLa cells and canine HSA cells.30-32
The membranes were washed with TBS-T (0.05 M Tris, 0.138 M NaCl, 0.0027 M KCl, and 0.05% Tween 20; Sigma-Aldrich) and incubated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody (anti-rabbit, 1:5000, Cell Signalling Technology or anti-goat, 1:2000, Abcam, Cambridge, UK) for 1 hour at RT. The immunoblot was visualized using Lumina Forte Western HRP Substrate (Merck Millipore, Darmstadt, Germany). The target protein bands were visualized by C-DiGit Blot scanner (LI-COR Biotechnology, Lincoln, Nebraska), and Image Studio Digits ver4.0 (LI-COR Biotechnology) and were verified semi-quantitatively relative to the expression of β-actin.
2.8 Inhibition of caspase-8 or caspase-3
To determine the involvement of the caspase cascade in TRAIL-induced apoptosis, all HSA cell lines seeded in 96-well plates were treated with 0, 2.5, 10 and 40 μM of a caspase-8 inhibitor (Z-IETD-FMK, MBL, Nagoya, Japan) or caspase-3 inhibitor (Z-DEVD-FMK, MBL) for 2 hours. Both caspase inhibitors were dissolved in dimethyl sulfoxide. Subsequently, the cells were incubated with 100 ng/mL izTRAIL for 24 and 48 hours, and the WST-1 assay was performed as described above. JuB4, Re12 and Ud6 cells were treated with caspase-8 and -3 inhibitors before the addition of izTRAIL, the attached and suspended cells were collected and stained with PI/RNase staining solution (Cell Signalling Technology), and the cell cycle analysis was performed by flow cytometry.
2.9 Statistical analysis
All experiments were performed more than three times independently. Cell viability assay results were statistically evaluated by one-way analysis of variance and Dunnets post-hoc test. The percentage of Annexin V-positive and PI-negative (AnV+/PI−) cells (ie, apoptotic cells) or in the sub-G1 phase were compared between groups by Students t-test. These tests were performed by BellCurve for Excel (Social Survey Research Information Co., Ltd, Tokyo, Japan). All values are expressed as mean ± SD. Results were considered significant when P < 0.05.
3 RESULTS
3.1 Cell viability and IC50 of TRAILs
Despite slight changes in the relative absorbance values of canine HSA cells, MDCK cells and HeLa cells at some time points after treatment with up to 100 ng/mL TRAIL-TEC, there was no significant decrease in cell viability up to 72 hours (Figure 1).
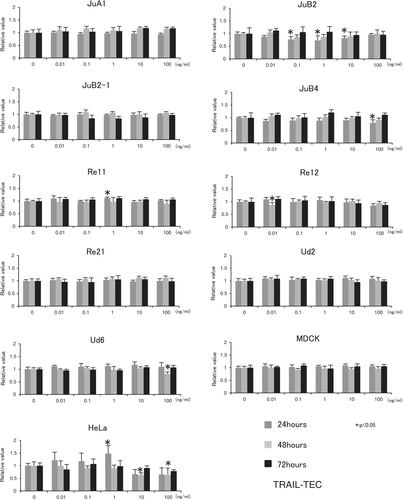
However, 100 ng/mL TRAIL-TL significantly decreased the cell viability of JuB2, JuB2-1, JuB4, Re12 and HeLa cells after 24, 48 and 72 hours. In Re21 cells, 1, 10 and 100 ng/mL of TRAIL-TL decreased the cell viability after 72 hours. However, no reproducible significant cytotoxic effect of TRAIL-TL was observed in the other cell lines up to 100 ng/mL at any time point (Figure 2).
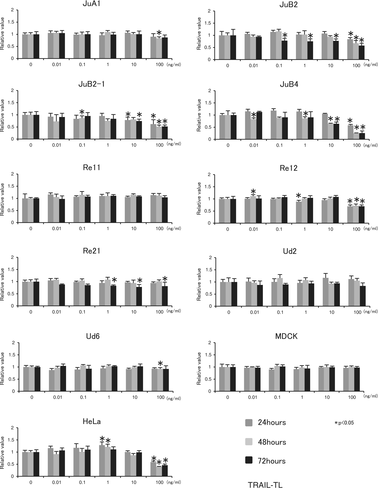
izTRAIL clearly decreased cell viability in all canine HSA cells except for Re11, and in HeLa cells in both time- and concentration-dependent manners (Figure 3). However, izTRAIL did not have a significant cytotoxic effect on MDCK or CnAOEC endothelial cells (Figure 3). The IC50 values of izTRAIL at 72 hours of treatment are summarized in Table 1; however, the IC50 values could not be estimated for TRAIL-TEC because of its weak overall cytotoxic effect. In TRIAL-TL, IC50 could only be calculated with JuB2-1 (90.92 ± 0.90) and JuB4 (13.09 ± 0.46). The IC50 values of izTRAIL to HeLa cells were similar. Overall, JuB4 cells were the most sensitive cell line to izTRAIL.

| Cells | izTRAIL (ng/mL) |
|---|---|
| JuA1 | 12.40 ± 8.11 |
| JuB2 | 3.40 ± 2.75 |
| JuB2-1 | 4.59 ± 5.56 |
| JuB4 | 1.22 ± 0.46 |
| Re11 | — |
| Re12 | 11.57 ± 6.75 |
| Re21 | 14.18 ± 3.53 |
| Ud2 | 9.21 ± 6.98 |
| Ud6 | 9.68 ± 5.00 |
| MDCK | — |
| HeLa | 12.65 ± 9.64 |
- Abbreviations: izTRAIL, isoleucine zipper recombinant human tumour necrosis factor-related apoptosis-inducing ligand; MDCK, Madin-Darby canine kidney.
- IC50 values of izTRAIL in canine hemangiosarcoma cell lines. Results are presented as mean ± SD of three independent experiments.
- —, cases in which the IC50 could not be calculated.
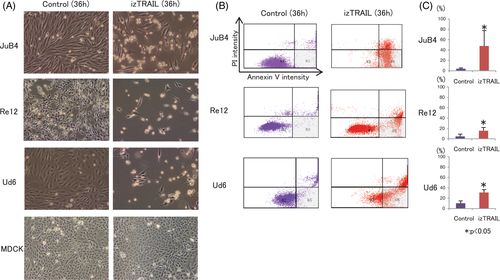
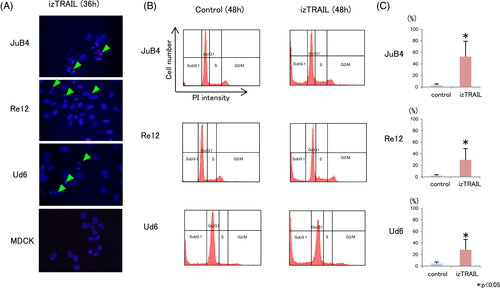
3.2 Effects of izTRAIL on cell morphology and apoptosis induction
Since izTRAIL had the most potent cytotoxic effect on cancer cells with no influence on viability of normal cells, we further focused on its mechanism of cancer cell inhibition through apoptosis evaluation. After treatment with 100 ng/mL izTRAIL for 36 hours, JuB4, Re12 and Ud6 cells showed clear detachment from the dishes and the number of cells had decreased (Figure 4A). Flow cytometric analysis further showed an increase in the cell number in the grey area, representing Annexin V+/PI− apoptotic cells, 36 hours after izTRAIL treatment in JuB4, Re12 and Ud6 (Figure 4B), which was statistically significant for JuB4, Re12 and Ud6 cells (Figure 4C). In contrast, MDCK cells remained tightly attached as a monolayer to the dish, with no morphological change after izTRAIL treatment (Figure 4A).
DAPI staining further revealed nuclear fragmentation in JuB4, Re12 and Ud6 cells 36 hours after treatment with 100 ng/mL izTRAIL (Figure 5A), whereas MDCK cells displayed no nuclear fragmentation 36 hours after treatment with 100 ng/mL izTRAIL. Cell cycle analysis revealed a marked increase in cells in the sub-G1 phase and a corresponding decrease in G0/G1-phase cells in the JuB4, Re12 and Ud6 cell lines 48 hours after treatment with 100 ng/mL izTRAIL (Figure 5B,C).
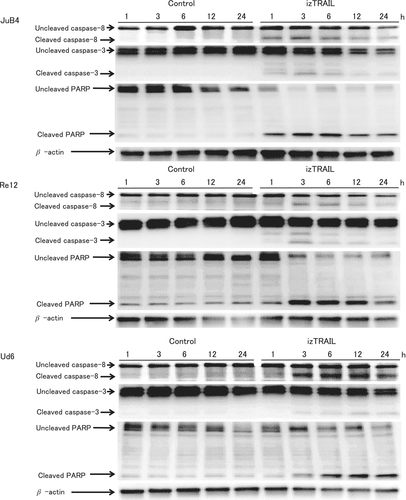
3.3 Western blotting for caspase-8, cleaved-caspase-3, and PARP
Caspase-8, caspase-3, and PARP were detected by western blotting in JuB4, Re12 and Ud6 cells after treatment with izTRAIL, but the times at which these proteins were detected differed among the cell lines (Figure 6). In JuB4 cells, cleaved caspase-8 expression increased at 1 to 24 hours after treatment, cleaved caspase-3 increased from 1 hour after treatment, and cleaved PARP increased at 1 to 24 hours after treatment. In Re12 cells, cleaved caspase-8 increased at 3 to 24 hours after treatment, cleaved caspase-3 increased from 1 hour after treatment. Cleaved PARP was increased slightly at 3 to 24 hours after treatment, and uncleaved PARP decreased from 3 hours after treatment. In Ud6 cells, cleaved caspase-8 increased at 3 to 24 hours after treatment, and cleaved caspase-3 and cleaved PARP increased at 3 to 24 hours after treatment.
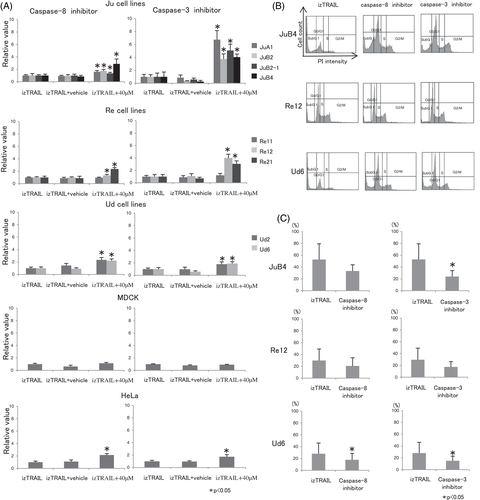
3.4 Caspase-8 and caspase-3 inhibitors prevented TRAIL-induced apoptosis in canine HSA cells
The WST-1 assays showed that both caspase inhibitors recovered the TRAIL-induced loss of cell viability at 40 μM in all HSA cell lines and in HeLa cells (Figure 7A). The caspase-3 inhibitor was more effective in preserving cell viability under izTRAIL treatment than the caspase-8 inhibitor in JuA1, JuB2, JuB2-1 and JuB4 cells. The flow cytometry results further showed that caspase-8 and caspase-3 inhibitors decreased the numbers of cells in the sub-G1 phase and increased the numbers of cells in the G0/G1 phase (Figure 7B). The number of cells in the sub-G1 phase significantly decreased in JuB4 and Ud6 cells after addition of the caspase-3 inhibitor. Consistently, although not statistically significant, the decrease in the number of sub-G1 phase cells was also observed in Re12 cells (Figure 7C).
4 DISCUSSION
Apoptosis is induced by irradiation, certain infectious agents,and various chemical compounds. There are two main apoptosis signalling pathways: the intrinsic apoptotic pathway, which is the p53-dependent mitochondrial pathway, and the extrinsic apoptotic pathway, which is p53-independent and occurs through death receptors.33 The p53 protein regulates the repair of cellular DNA and induces apoptosis when the DNA damage is too serious to repair.34 Thus, p53 has an important role in the apoptosis-inducing pathway, and is the most commonly silenced or mutated gene in most cancers, with 50% to 55% of all human cancers characterized by loss of wild-type p53 activity.35 Thus, it is difficult to induce apoptosis in cancer cells through activation of p53. TRAIL and its receptor belong to the p53-independent extrinsic pathway of apoptosis induction, and TRAIL can causes apoptosis dependently and/or independently of p53. Therefore, these proteins have come into the spotlight for the development of novel therapeutic molecules for the treatment of human cancers, including mechanisms involving inactivation of p53.33, 36, 37 Compared with human cancers, there have been few reports of canine cancers with p53 inactivation. In the Re12 and Ud6 canine HSA cell lines used in this study, p53 activity was reported to be decreased by downregulation of miR-214 and COP1 E3 ubiquitin protein ligase-overexpression.30 Therefore, the demonstration that TRAIL can induce the apoptosis of Re12 and Ud6 cells indicates its apoptosis-inducing effect in neoplastic cells of canine cancers in which p53 is inactivated.
We evaluated the cytotoxic effects of three different types of rhTRAILs. The human and dog TRAIL sequence identity is 81.3%, and the amino acid sequences of the trimeric or receptor-binding domains of TRAIL are highly conserve.25, 38 A previous study showed that rhTRAIL, the same as TRAIL-TEC used in this study, induced apoptosis in a canine mastocytoma cell line.24 Of the three rhTRAIL proteins examined, izTRAIL showed the strongest ability to induce apoptosis in canine HSA cells. Based on a previous report, it is likely that TRAIL trimer formed through the zipper motifs as in izTRAIL would be more stable than the monomer form, and thus more effective against neoplastic cells.20 TRAIL-TL has a His-Tag at the N-terminal region, which is able to bind to the zinc ion necessary for TRAIL trimerization; thus, the formation of a TRAIL-TL trimer is more probable than the formation of a TRAIL-TEC trimer, which has no His-tags.39-41 Therefore, TRAIL-TEC may also affect HSA cell lines but at higher concentrations than tested herein.
Five TRAIL-receptor variants have been identified in humans.42-48 Of these receptors, only death receptor (DR) 4 and DR5 have the death domains necessary for the induction of apoptosis, while the other receptors such as decoy receptor (DcR)1, DcR2 and osteoprotegerin (OPG) have no region that transmits a signal into the cell.42-46, 48 Previous studies have revealed that when TRAIL binds to DR4 or DR5, it activates the Fas-associated death domain protein and induces apoptosis through activation of caspase-8/10 and caspase-3.42, 49, 50 Decoy receptors are also expressed in normal cells, but the balance of expression of each receptor differs from that in neoplastic cells.51 Therefore, many normal cells are resistant to apoptosis induced by TRAIL. In veterinary medicine, a study on the effect of TRAIL on canine mast cell tumour cell lines revealed a role of the activation of caspase-3.24 In addition, caspase-8 was shown to be activated by flavopiridol (FVP) in lymphoma cell lines exhibiting TRAIL resistance, but it is not clear whether TRAIL activated caspase-8 as well.26 At present, the only TRAIL receptor identified in dogs is OPG, and its transfer signals have only recently been predicted based on gene sequence analysis.24, 52 Therefore, how TRAIL receptors act in canine tumour cell lines and the mechanism of action of TRAIL remain unclear. In this study, all cell lines used for western blotting analysis were found to express cleaved PARP with activation of caspase-8 and caspase-3 under TRAIL treatment. In addition, the clear inhibition of apoptosis, but not fully inhibition, following treatment with caspase-8 and caspase-3 inhibitors in all cell lines suggests that caspase-8 and caspase-3 are at least partially involved in TRAIL-induced apoptosis. One possible reason for the greater effectiveness of the caspase-3 inhibitor in the Ju cell lineage could be through compensatory activation of caspase-10, which performs the same role as caspase-8, or that a small amount of cleaved caspase-8 is able to continuously activate caspase-3, which has been demonstrated to occur in various cascade reactions.53, 54 Alternatively, in the Re and Ud lineages, caspase-8 may be prevented by various factors before activating caspase-3. In addition to directly activating caspase-3, caspase-8 can activate caspase-3 through caspase-9.55 Moreover, Bcl-2 has been reported to be overexpressed in canine HSA.56 When Bcl-2 is expressed, activation of caspase-3 through caspase-9 is suppressed, and caspase-8 remains the only direct activator of caspase-3.28 This prevention of caspase-8 is consistent with the fact that Ju cells showed lower IC50 values than the others; however, further study on the expression levels of Bcl-2 among the HSA cells lines is needed to verify this hypothesis.
Many reports have noted that neoplastic cells expressing decoy receptors show resistance to TRAIL.28, 57, 58 However, other reports suggest that other factors also contribute to TRAIL resistance, including anti-apoptotic factors such as Bcl-2 and cFLIP.28, 59, 60 Moreover, survivin expression has also been confirmed in canine HSA, which belongs to the same IAP family as XIAP that has been shown to be related to TRAIL resistance in canine mammary cell tumours and osteosarcoma.27, 56 Thus, the difference in expression of anti-apoptotic molecules in canine HSA is considered to contribute to the difference in TRAIL sensitivity among lineages observed in this study. TRAIL-sensitivity may increase by inhibition of some anti-apoptotic factors such as Bcl-2, survivn or XIAP which related to the apoptosis pathway through caspase-9.27, 56 Inhibition of cFLIP, which allows for the activation of caspase-8, has also been reported in canine lymphoma cells; however, since we detected activation of caspase-8 with TRAIL treatment, it is considered that there is no influence of cFLIP on the apoptosis mechanism at this time.26, 28 For exploration of the application potential of TRAIL to a wider range of canine tumours, it is necessary to study the factors related to the acquisition of TRAIL resistance, including decoy receptors and anti-apoptotic factors. It is expected that TRAIL may be widely applied to TRAIL-resistant canine tumours in combination with FVP inhibiting cFLIP or TIC10 which also increases TRAIL death receptor expression.23, 26
Studies with experimental animals have shown tumour regression following intraperitoneal or intravenous TRAIL administration; however, the half-life of TRAIL in the blood is extremely short.20, 22 Therefore, development of low-molecular-weight molecules that show TRAIL receptor specificity, and the administration of TIC10 to induce TRAIL production in tumour cells have been studied in the human medicine field.23, 61-65 Thus, further research into these small-molecule drugs is necessary for the clinical application of TRAIL in dogs.
In conclusion, we demonstrated a clear cytotoxic effect of TRAIL to canine HSA cell lines through apoptosis induction, whereas no cytotoxicity was detected against canine normal endothelial cells. Our results further suggest that this apoptosis induction at least partially involves caspase-8. Further studies of the anti-apoptotic factors that contribute to differences in TRAIL susceptibility between cell lines are necessary to further understand the mechanism and explore the clinical and therapeutic potential of TRAIL.
ACKNOWLEDGEMENTS
This study was supported by the Grants-in-Aid for Scientific Research (C) Grant No.17 K08101 from the Japan Society for the Promotion of Science.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.