Metastatic immune infiltrates correlate with those of the primary tumour in canine osteosarcoma
Funding information: American Kennel Club Canine Health Foundation, Grant/Award Number: Clinician-Scientist Fellowship Program; Center for Companion Animal Health, School of Veterinary Medicine, University of California, Davis. ; National Cancer Institute, Grant/Award Numbers: K12CA138464, P30CA093373, U01CA224166-01
Abstract
Our lack of understanding of the immune microenvironment in canine osteosarcoma (cOSA) has limited the identification of potential immunotherapeutic targets. In particular, our ability to utilize readily available tissue from a dog's primary tumour to predict the type and extent of immune response in their pulmonary metastatic lesions is unknown. We, therefore, collected 21 matched pairs of primary tumours and pulmonary metastatic lesions from dogs with OSA and performed immunohistochemistry to quantify T-lymphocyte (CD3), FOXP3+ cell, B-lymphocyte (Pax-5), and CD204+ macrophage infiltration. We found that T-lymphocytes and FOXP3+ infiltrates in primary tumours positively correlated with that of metastatic lesions (ρ = 0.512, P = 0.038 and ρ = 0.698, P = 0.007, respectively), while a strong trend existed for CD204+ infiltrates (ρ = 0.404, P = 0.087). We also observed T- and B-lymphocytes, and CD204+ macrophages to be significantly higher in a dog's pulmonary metastasis compared to their primary tumour (P = 0.018, P = 0.018, P = 0.016, respectively), while FOXP3+ cells were only significantly higher in metastases when all primary tumour and metastasis lesions were compared without pairing (P = 0.036). Together, these findings suggest that the metastatic immune microenvironment may be influenced by that of the primary cOSA, and that primary tumour immune biomarkers could potentially be applied to predict immunotherapeutic responses in gross metastatic disease. We, therefore, provide a rationale for the treatment of cOSA pulmonary metastases with immunotherapeutics that enhance the anti-tumour activity of these immune cells, particularly in dogs with moderate to high immune cell infiltration in their primary tumours.
1 INTRODUCTION
Pulmonary metastasis is the most common cause of death in dogs with appendicular canine osteosarcoma (OSA; cOSA) treated with amputation of the effected limb and adjuvant cytotoxic chemotherapy.1, 2 Despite aggressive adjuvant cytotoxic chemotherapy regimens, little advancement in the systemic treatment of OSA has led to stagnant survival rates in humans and dogs, of 70% at 5 years and 15% at 2 years, respectively, in cases that present without gross metastasis.3 Furthermore, the prognosis is particularly dire for humans and dogs with gross metastatic disease.4-8 Therefore, novel therapeutics designed to target a variety of host immune cells and enhance the anti-tumour immune response are being investigated in both species for their activity in OSA.9-12
The immune microenvironment in which tumour cells reside is a critical determinant of immunotherapeutic response.13-15 Therefore, the implementation of immunotherapeutic strategies in cOSA would be aided by a thorough understanding of the immune microenvironment both in primary tumours and metastases. Our group and others have evaluated the prognostic significance of T-lymphocytes (T-cell) and macrophages in the primary tumour.16, 17 For example, Biller et al identified a high CD8+/T-regulatory cell (Treg) ratio through flow cytometry in the blood as a positive prognostic factor for survival although this did not reach significance within the tumour.16 In addition, Modiano et al. reported that elevated T-cell infiltrates in cOSA after treatment with Fas-ligand gene therapy was associated with improved survival, while we identified elevated CD204+ macrophage tumour infiltration as being associated with a prolonged disease-free interval (DFI).17, 18 To the authors' knowledge, the degree of leukocyte infiltration into pulmonary metastatic cOSA is currently unknown however.
Studies evaluating infiltrating immune cells in primary and metastatic human OSA (hOSA) primarily focus on lymphocytic infiltrates.19-21 For example, Sundara et al described the presence of CD3+CD8−, CD3+CD8+, and CD3+FOXP3+ T-cells in primary tumours, local relapses, and metastases from 25 people with hOSA, with both primary tumours and metastases available from 14 patients.19 CD3+ cells were significantly higher within all metastases evaluated compared to all primary tumours, while the proportions of T-cell subsets remained remarkably stable between disease stages.19 These studies typically include multiple sites of metastasis, and it is unclear if differences were evaluated between sites of disease within individuals.19-21 Furthermore, metastatic hOSA tissue is likely to be obtained from heavily chemotherapy-treated individuals, which has the potential to significantly alter the types and amounts of infiltrating immune cells present. Alternatively, many dogs are not treated aggressively once gross pulmonary metastasis is evident. Therefore, the evaluation of immune microenvironment characteristics in metastatic cOSA may be a prime example of how the naturally-occurring canine disease can add to our understanding of this cancer in both species.
We therefore aimed to determine if immune infiltrates in a dog's primary tumour could be used to predict the immune environment in pulmonary metastatic lesions by comparing immune infiltrates within paired primary and metastatic lesions. Given the previous report by Sundara et al we hypothesized that immune cell infiltration would be greater in cOSA metastatic lesions compared to primary tumours.19 In addition to evaluating primary and pulmonary metastatic cOSA samples for the presence of T-cells, FOXP3+ cells, and CD204+ macrophages, we also describe the distribution of B-cells within and peripheral to cOSA lesions. Finally, we explore for an association between infiltrating immune cells and recent chemotherapy exposure.
2 METHODS
2.1 Tissue collection and processing
Electronic medical records at the University of California, Davis William R. Pritchard Veterinary Medical Teaching Hospital (VMTH) were searched for dogs receiving a pathological diagnosis of both primary and pulmonary metastatic cOSA. Dogs presenting with disseminated disease, as well as those undergoing an amputation followed by a necropsy were included. Primary tumour samples were collected at the time of amputation or during necropsy after humane euthanasia. Tissue from lung metastases was either obtained through metastasectomy or necropsy. Formalin-fixed paraffin-embedded (FFPE) blocks of tissue from each dog's primary and pulmonary metastatic lesions were retrieved from the archives and cut into 5 μm sections. At least 1 slide from each tumour was stained with haematoxylin and eosin (H&E) and the remaining sections were adhered to charged slides in preparation for immunohistochemistry (IHC).
2.2 Clinical information collected
The medical records of dogs with available primary and pulmonary metastatic cOSA tissue were searched. Information collected included signalment, weight, primary tumour location, stage of disease at presentation, histological subtype, method of lung metastasis collection, treatment and the duration between chemotherapy treatment and collection of metastatic lesions. Dogs receiving inhaled interleukin-2 treatment were excluded. No other exclusions were made based on the treatments received. Survival times were evaluated in dogs receiving amputation and adjuvant injectable chemotherapy and calculated as the time between amputation and euthanasia.
2.3 IHC processing
Immunohistochemical staining of FFPE sections was performed as previously described by our group.18 Briefly, slides first underwent deparaffinization, rehydration and hydrogen peroxide quenching. Antigen retrieval was performed in pre-heated citrate buffer (H-3300, Vector; Burlingame, California) in a water bath at >95°C for 25 minutes. Samples were blocked with 2.5% normal goat serum (Vector; Burlingame, California) for 20 minutes, followed by 5% non-fat milk (Lab Scientific; Highlands, New Jersy) for 30 minutes at room temperature. Primary antibodies were diluted in Dako Antibody Diluent (Dako; Carpinteria, California). T-cells, FOXP3+ cells, B-cells and macrophages, were detected using anti-CD3 (1:50; rat anti-human, CD3-12; Moore Lab; Davis, California), anti-FOXP3 (1:25; rat anti-mouse/rat, FJK-16S; Thermo Fisher Scientific; San Diego, California), anti-Pax-5 (1:50; 24/Pax-5; BD Biosciences; San Jose, California), and anti-CD204 (1:400; mouse anti-human, SRA-E5; Cosmo Bio; Carlsbad, California), respectively. The anti-CD3 and -FOXP3 clones are stated as canine cross-reactive by their manufacturers, while the anti-Pax-5 and -CD204 have shown canine cross-reactivity in several previous studies.22-24 The ImmPRESS horseradish peroxidase (HRP) polymer detection kit was used for primary antibody detection (anti-mouse IgG, MP-7452; anti-rat IgG, MP-7444; Vector; Burlingame, CA), and Vector NovaRed HRP substrate kit (Vector; Burlingame, California) was used for chromogen development. Sections of canine lymph node were used as positive and negative controls for CD3, FOXP3, Pax-5 and CD204 staining by evaluating the distribution of their staining within the lymph node.
2.4 IHC quantification
Similar to a previous study by our group, we selected 3 cellular 100× fields on hematoxylin and eosin stained slides from each tumour to mimic the construction of tissue microarrays used in other studies to evaluate immune infiltrates in hOSA.15, 18, 25-27 Selected areas were marked on each IHC stained slide, enabling evaluation of each marker in similar areas on subsequent slides. Slides were excluded from analysis if the sample was too necrotic, if the tissue consistently fell off the slide, if staining was too weak to definitively identify positive cells, or if the marker was found to cross-react with tumour cells.
A Leica DM2000 was used to image each 100× field. Images were digitalized using the attached Jenoptik ProgRes C5 camera. The scale was set using an image of a haemocytometer and Image J 1.51 seconds (National Institutes of Health, USA), and determined to be 2041.8 pixels/mm at 100× magnification. The area of each 100× magnification image equalled to 1.2 mm2.
First, the cellular area was measured and selected as the region of interest (ROI) using ImageJ. CD3, FOXP3 and Pax-5 were then counted in each of the three cellular fields from each primary and metastatic lesion. The number of cells/cellular area in pixels was then converted to the number of cells/mm2 by dividing by 4 169 024 pixels2/mm2 (2041.8 × 2041.8 pixels/mm2). Macrophages were abundant and therefore measured as the percent area of CD204+ staining within each cellular (%CD204+) area using Image J, similar to that previously described by our group and others.18, 28 The average density of CD3, FOXP3 and Pax-5 infiltration (cells/mm2), and %CD204+ (%) was then determined across the cellular fields evaluated for each sample. The intensity of IHC staining was not evaluated.
2.5 Statistical analysis
Correlation was determined between primary tumour and metastasis infiltrates using Spearman's correlation coefficient. Immune infiltrate measurements were then compared between an individual dog's primary and metastatic lesions in a paired analysis using a Wilcoxon matched-pairs signed rank test. In addition, we compared all primary tumour immune infiltrates to that of all metastatic lesions in an unpaired analysis using a Mann-Whitney test. The magnitude of immune cell infiltration was also evaluated for an association with recent chemotherapy exposure (administration within 3 months vs not) and primary tumour location (humerus vs other) with a Mann-Whitney test. Statistical analyses were performed using commercial software (GraphPad Prism version 7.0a) and 2-sided P-values <0.05 were considered significant.
3 RESULTS
3.1 Clinical characteristics
Tissue blocks retrieved from the VMTH archives were collected between 2006 and 2017 and included paired primary and metastatic cOSA lesions from 21 dogs. The mean age at amputation was 7.4 years (median = 7 years; range = 2-15 years). Patients included 11 spayed females, 8 castrated males, and 2 intact males. Breeds included 7 mixed breeds, 2 each of Rottweilers, Great Danes and Labrador retrievers, and one each of 8 other breeds. Histological subtype was described in 12 dogs, and consisted of 7 osteoblastic, 2 telangiectatic, 2 mixed and 1 chondroblastic. Four dogs had primary tumours arising from the proximal humerus, 16 were appendicular from other locations, and 1 arose from the chest wall. In 1 dog, pulmonary metastatic tissue was obtained through metastatectomy, while the remaining metastatic samples were collected at necropsy. Six dogs presented with clinically-evident disseminated disease and were euthanized prior to any therapeutic intervention. Of the remaining 15 dogs presenting with localized disease, 11 were treated with amputation and adjuvant injectable chemotherapy, 1 was treated with amputation and adjuvant toceranib and 3 were treated with amputation alone. Injectable chemotherapies administered in the adjuvant setting included carboplatin alone in 6 dogs, alternating carboplatin and doxorubicin in 2 dogs, and 1 dog each received carboplatin followed by metronomic cyclophosphamide, combined carboplatin and inhaled gemcitabine, and combined doxorubicin and thiomolybdate. All dogs died of cOSA related causes, and the median survival time of the 11 dogs treated with amputation and injectable chemotherapy was 197 days (range = 60-754 days).
3.2 Quantification of infiltrating immune cells in primary and metastatic cOSA
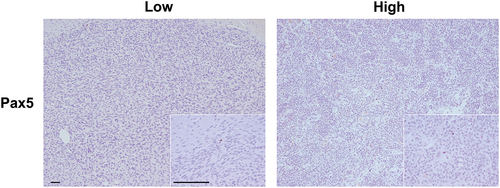
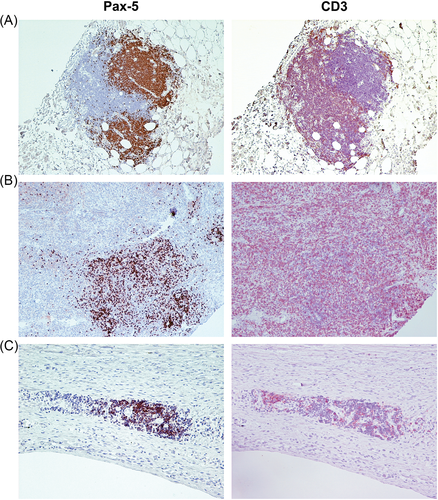
Of the 21 paired samples included, interpretable slides were available for evaluation of CD3, FOXP3, Pax-5 and CD204 expressing cells from 19, 19, 19 and 20 primaries, and from 20, 15, 17, and 19 metastases, respectively. The overall median densities of CD3+, FOXP3+, Pax-5+, and CD204+ infiltrates in primary and metastatic lesions are listed in Table 1. Representative images of low and high Pax-5+ infiltration are shown in Figure 1, while representative images of CD3+, FOXP3+ and CD204+ infiltrates in cOSA are presented in a previous manuscript by our group.18
| Median CD3+ (range; cell/mm2) | P-value | Median FOXP3+ (range; cell/mm2) | P-value | Median Pax5 (range; cell/mm2) | P-value | Median CD204 (range; %) | P-value | |
|---|---|---|---|---|---|---|---|---|
| Primary tumours | 20.1 (0-836.5) |
0.041 | 3.8 (0-85.9) |
0.036 | 0.8 (0-22.2) |
0.188 | 2.0 (0.2-12.7) |
0.021 |
| Metastases | 64.6 (8.0-1880.0) |
27.8 (0-148.6) |
1.7 (0-11.4) |
4.2 (0.7-18.2) |
- P values are italicized, p values indicating statistical significance are bolded.
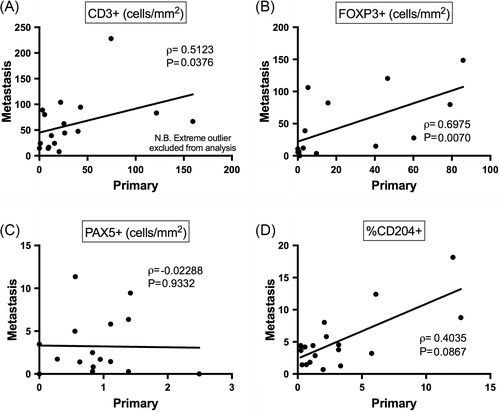
Firstly, we evaluated whether immune infiltrate levels in the primary tumour might predict levels in metastatic lesions. We therefore plotted infiltrate levels within each dog's primary tumour against that of their metastatic lesions. CD3+ infiltrate levels from one dog was excluded from analysis of the correlation between primary tumours and metastases since it was an extreme outlier with a value of 836 cells/mm2 within the primary tumour, and 1034 cells/mm2 in the metastatic lesion. Significant positive correlations were observed between primary tumours and metastases for both CD3+ and FOXP3+ infiltrates (P = 0.038 and P = 0.007, respectively; Figure 2 A-B). A clear trend towards a positive correlation was also observed for CD204+ infiltrates (P = 0.087; Figure 2 D), while no correlation was evident for Pax-5+ infiltrates (P = 0.933; Figure 2 C).
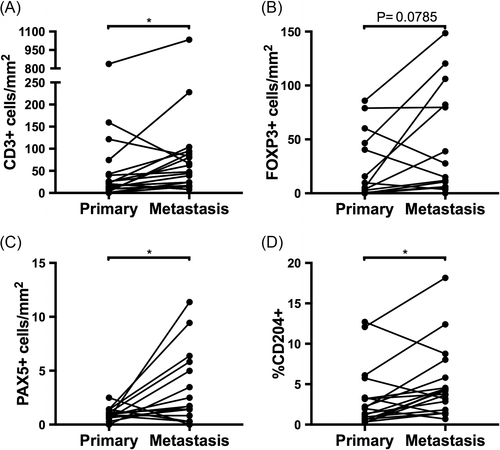
We then examined whether infiltrating leukocytes within metastatic lesions tended to be consistently higher or lower compared to paired primary tumours. Dogs displayed significantly greater CD3+, Pax-5+, and CD204+ infiltrates in metastases compared to paired primary tumours (P = 0.018, P = 0.018, and P = 0.016, respectively; Figure 3 A, C, D). While FOXP3+ infiltrates in metastases were not significantly higher than in paired primary tumours (P = 0.079; Figure 3 B), evaluation of all available data points in an unpaired analysis revealed significantly higher FOXP3+ infiltration in metastases compared to primary tumours (P = 0.036; Suppl. 1 B; Table 1). All parameters except for Pax-5+ cell counts (Suppl. 1 C) were significantly higher in metastases compared to primary cOSA when all data points were evaluated (Suppl. Figure 1; Table 1).
3.3 Pax-5 staining in cOSA
While the infiltrating Pax-5+ cells were relatively uncommon in the cellular areas that were selected for quantification, clusters of Pax-5+ cells were present at the perimeter of the tumour or within connective tissue adjacent to the tumour in 10 primary tumours and 7 metastases. In three cases, adjacent sections stained for CD3 revealed an encircling population of T cells around these B cell clusters, suggesting a lymphoid follicle-like organization. Examples of several of these clusters are shown in Figure 4. Of note, one dog with a chondroblastic cOSA displayed a considerable amount of strong nuclear staining for Pax-5 in the tumour cells of both their primary and metastatic lesions (Suppl. 2). This dog was excluded from analysis of Pax-5 infiltrates.
3.4 Association of immune cell infiltrates with clinical characteristics
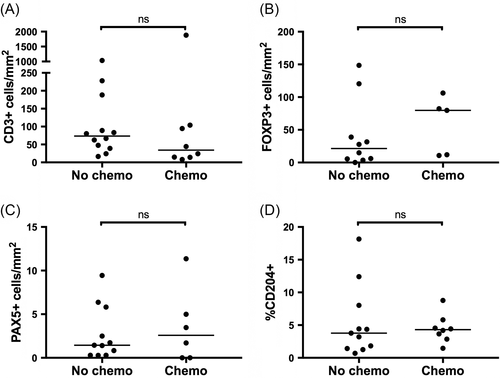
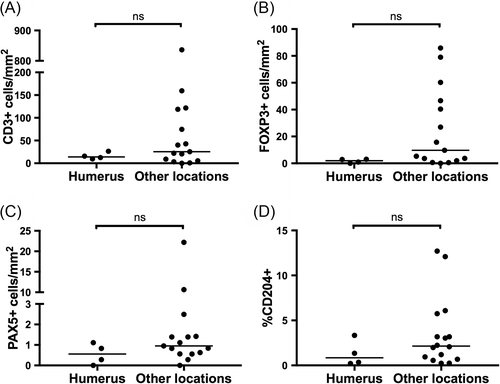
Finally, we evaluated potential associations between prior treatment with chemotherapeutics and immune infiltrates within metastatic lesions. Nine dogs were treated with chemotherapy within 3 months of the collection of metastatic tissue, including 4 treated with carboplatin and 1 each treated with doxorubicin, ifosfamide, toceranib, rapamycin, and inhaled gemcitabine. None of the remaining 12 dogs were exposed to chemotherapy within 10 months of sample collection. No significant differences in CD3+, FOXP3+, Pax-5+, or CD204+ infiltration were detected within metastatic lesions from dogs with recent exposure to chemotherapy (Figure 5). In this cohort, the difference in immune cell infiltrates between tumours arising from the humerus compared to other locations did not reach significance, although values were numerically lower in humeral lesions for each cell type evaluated (Figure 6).
4 DISCUSSION
A thorough understanding of the immune microenvironment in pulmonary metastatic cOSA is necessary to develop immunotherapeutic strategies targeting this site of disease, since the majority of dogs succumb to pulmonary metastases even after amputation and adjuvant cytotoxic chemotherapy.1, 2 While direct sampling of metastatic lesions would ideally be evaluated to provide the most representative impression of the metastatic immune microenvironment, the invasiveness of collecting cOSA pulmonary metastases from dogs in a clinical setting often precludes this. Therefore, we sought to determine if a dog's primary cOSA could provide insight into the immune infiltrates present within subsequent and concurrent metastatic disease. To do so, we quantified the infiltration of T cells, FOXP3+ cells, B cells, and CD204+ macrophages in 21 matched pairs of primary cOSA and pulmonary metastases. We observed correlations between primary tumours and metastases were positive and significant for T cells and FOXP3+ cells, while this trend did not quite reach significance for CD204+ cells (P = 0.087). In addition, all 4 cell types evaluated were higher in metastases compared to primary tumours, thus supporting our original hypothesis. B cells were the least prevalent immune cell type, particularly in primary tumours, although almost half of all lesions demonstrated clusters of B cells at the periphery of the tumour or within adjacent connective tissue. These lymphoid follicle-like structures were reminiscent of tertiary lymphoid structures (TLS) and were somewhat of a surprise to see in our cOSA samples, given the rarity of reports describing them in any human sarcomas. Together these data reveal the presence of targetable immune cell types in cOSA metastases and suggest the ability to predict the degree of their infiltration by evaluation of paired primary tumours.
The immunological explanation for our finding of increased leukocyte infiltrates in progressive metastatic lesions is likely multifactorial. Broadly, host-tumour interactions can vary from tumour-promoting inflammation to effective anti-tumour immunity.29 This may begin to explain the somewhat paradoxical increase in CD204+ macrophage infiltration in pulmonary metastases in this study given our previous findings identifying CD204+ macrophages in primary tumours as a positive prognostic factor in cOSA.18 This shift towards tumour-promoting inflammation can involve alterations to the functionality of these macrophages between sites of disease.30, 31 In addition, the increase in T cells in metastatic lesions may reflect the recruitment of tumour-permissive T cell subsets such as T helper 2 (Th2) cells32 and T regulatory cells,33 and/or a strong cytotoxic T cell response that has been inhibited by the expression of inhibitory factors such as programmed death ligand-1 (PDL-1),34 indoleamine dioxygenase (IDO),35 and transforming growth factor β (TGFβ).36 Indeed, several studies have shown the therapeutic inhibition of programmed death-1 (PD-1/PDL-1) interactions to be particularly effective in lymphocyte-infiltrated tumours, suggesting a functional endogenous T cell response underlies these inhibitory signals.37 Interestingly, Remark et al. reported differing proportions of pro- and anti-tumour genes expressed by tumour infiltrating lymphocytes (TILs) in renal cell carcinoma (RCC) compared to colorectal carcinoma (CRC), which may account for reports that generally associate TILs with a favourable prognosis in CRC and a poor prognosis in RCC.13 This study highlighted how the heterogeneity of tumour infiltrating CD3+ cells results in the variable clinical significance of their presence with a tumour. Taken together, our findings may suggest potentially effective immunotherapeutics in the treatment of cOSA gross pulmonary metastases could include macrophage-activating agents, inhibitors of T cell suppressive factors, or vaccines promoting an effective cytotoxic immune response. Further investigation utilizing molecular techniques will likely aid in the identification of precise deficits in the host's immune response however.
Our findings are in line with most human studies evaluating lymphocyte infiltration in primary and metastatic OSA.19, 20 Specifically, Lussier et al indirectly suggested an increase in T cell infiltrates in 16 matched pairs of hOSA metastases compared to primary tumours.20 They showed a strong correlation between infiltrating CD8+ cells and tumour PDL-1 expression and that PDL-1 was present in the majority of metastases but absent in the primary tumours.20 Increased lymphocytic infiltrates in hOSA metastases was later confirmed directly by Sundara et al as previously described.19 Alternatively, Koirala et al. reported an association between PDL-1 expression and the presence of CD3+ cell infiltrates, while also finding no difference in PDL-1 expression between primary tumours and metastases, which indirectly suggested no difference in CD3+ infiltration between primary tumours and metastases.21 However, in this study metastases were only included in the tissue microarray and not the whole slide evaluation, which was subsequently found to better represent the tumour microenvironment.21 While studies of other human tumour types generally report a positive correlation in lymphocytic infiltrates between primary and metastatic lesions, the trend towards greater infiltration in metastases is clearly not consistent across leukocyte subsets and tumour origins.38-40
To the authors' knowledge macrophages have not been evaluated in metastatic OSA in either dogs or humans. Reports in both species, however, tend to support the notion that macrophages within the primary tumour have an anti-metastatic role in OSA.15, 18, 27, 41 Furthermore, macrophages are thought to be a key mediator of the efficacy of liposomal muramyl tripeptidyl phosphatidylethanolamine (L-MTP-PE), a treatment that has prolonged survival in cOSA and hOSA clinical trials.42-45 In addition, macrophages have recently been found to play a role in the regression of metastases in a mouse model of OSA treated with PD-1/PDL-1 inhibition.46 Herein we have shown a modest correlation between primary cOSA tumour macrophages and those within pulmonary metastatic cOSA. Our findings could therefore potentially be used to evaluate primary tumour macrophages as a possible predictive biomarker of response to macrophage-activating therapies in the setting of pulmonary metastatic cOSA. Interestingly, we observed a significantly increased amount of infiltrating macrophages within metastatic cOSA compared to paired primary lesions. One potential explanation for why macrophages could both be anti-metastatic within the primary tumour, in addition to being preferentially recruited into metastatic lesions would be that differences in macrophage function exist between the two microenvironments. In this study we utilized CD204 as a marker for macrophages due to the antibody's well-documented canine cross-reactivity. While CD204 is typically associated with an M2 macrophage phenotype, classically considered permissive of tumour progression, this functional correlate has never been evaluated in canine macrophages. Therefore, future studies evaluating canine macrophage functional subsets, in addition to macrophage function within primary and metastatic cOSA lesions would likely be enlightening.
B cell responses appear to play an important role in the antitumor immune response in several human tumour types. Both infiltrating B cells and TLS within and around primary and metastatic tumours have been associated with an improved prognosis.47-50 Their evaluation in sarcomas, including OSA, however, has been limited. In this study, we identified significantly elevated B cell infiltrates in pulmonary metastatic lesions compared to primary cOSA, in addition to lymphoid follicle-like structures surrounding many of the primary and metastatic cOSA lesions evaluated. Morphologically, these follicle-like structures are reminiscent of TLS described in several human cancers. TLS are morphologically characterized by distinct B cell and T cell zones.51-53 B cell zones have a similar structure to that of a lymphoid follicle, consisting of predominantly naïve B cells surrounding a germinal center of rapidly proliferating B cells and interspersed follicular dendritic cells (FDCs). The T cell area surrounds the cluster of B cells and contains specialized blood vessels known as high-endothelial venules (HEVs) that facilitate the extravasation of circulating lymphoid cells.51-53 TLS have been suggested to potentiate a local immune response to the tumour. High CD8+ infiltrates in non-small cell lung cancer (NSCLC) with the concurrent presence of TLS are associated with an improved prognosis compared to high CD8+ infiltrates in the absence of TLS, suggesting TLS may mediate the positive effect of CD8+ cells on survival.54, 55 Furthermore, TLS appear to facilitate the oligoclonal expansion of B cells targeting tumour neoantigens.55-57 The in situ education of host immune cells is thought to result in the positive prognostic significance of TLS that has been described in several solid human cancers including lung, colorectal and breast carcinomas.53 However, some reports of their negative association with prognosis and their association with more advanced stages of disease exist for certain human tumour types, including liposarcoma.58-60 While we have shown distinct B and T cell compartments in these lymphoid structures surrounding cOSA, further studies are necessary to determine whether other characteristics of TLS are present such as FDCs, T follicular helper cells, HEVs, and chemokines including CCL19, CCL21, CXCL12 and CXCL13 that facilitate the homing of specific cell types to their various zones. Furthermore, the prognostic significance of these peritumoral lymphoid follicle-like structures described in this study need to be evaluated in a larger cohort with standardized therapy and follow up.
In this study, measures were taken to attempt to control several confounding factors in the immune microenvironment that contribute to the heterogeneity of intra-tumoral immune infiltrates. Firstly, we utilized whole slides of tissue sections created from the chemotherapy-naïve tumour after amputation, enabling evaluation of the largest possible area of tumour tissue. We then selected 3 cellular 100x magnification areas and eliminated any portions of necrosis from this field by creating an ROI of just the cellular area. Immune cells were then quantified per mm2 of cellular tissue, thus eliminating the area of cellular tumour tissue as a confounding variable from our analysis. Similar methods have been used in several studies quantifying immune infiltrates in hOSA, however frequently only small biopsy samples are available from chemotherapy-naïve humans tumours.15, 26, 27 This implies that studies of the immune microenvironment in cOSA could be better suited to account for tumour heterogeneity. However, variation in immune cell infiltrates within cellular areas is likely to occur throughout the tumour, and the use of tissue archives in this study eliminated our ability to correlate findings with the location of the sample within tumour (ie, center vs invasive margin). Future studies should consider the prospective collection of large portions of tumour from randomly selected sites in order to best represent the tumour as a whole.61
An important limitation of this study is the selection for cases undergoing a necropsy at the VMTH. This resulted in an over-representation of cOSA cases presenting with metastasis at diagnosis, in addition to those experiencing rapid cancer progression since they are more likely to still have a relationship with our hospital. This is evident in the relatively low median survival time of only 197 days in the 11 dogs treated with amputation and injectable chemotherapy. Therefore, the findings in this study are not necessarily applicable to less aggressive cOSA until further studies can confirm this. Future studies should address whether the time to metastasis effects the correlation of immune infiltrates between primary and metastatic lesions. Furthermore, the correlations observed in this study were only evaluated in the setting of gross metastasis, and therefore cannot be applied to microscopic disease without verification of a similar paradigm in that situation. In addition, we evaluated pulmonary metastases only. Given previous reports of a heterogeneous immune response between locations of metastasis, it is possible that bone and non-pulmonary soft tissue metastases may demonstrate an alternative pattern of immune infiltration.62 Finally, we evaluated a limited number of immune cell subsets in this study due to the availability of only FFPE tissue in these dogs. Further investigation utilizing IHC on frozen tissue specimens to evaluate CD4+ and CD8+ T cell subsets, as well as CD1a + dendritic cell subsets is warranted in future studies evaluating prospectively collected tissue samples. Additionally, given the heterogeneity of FOXP3+ cell function, the identification of Tregs is best performed by staining for additional markers simultaneously with FOXP3, such as CD4.63 Due to the current lack of reagents identifying phenotypic markers of canine macrophage subsets, the addition of molecular techniques to specify macrophage function (eg, iNOS, IL12, arginase, IL10) may also prove useful in subsequent studies. Finally, the differential expression of transcripts encoding immunomodulatory proteins such as PD-1, PD-L1 and IDO, is warranted until canine-specific reagents become available. Furthermore, future studies that include IHC assessment in combination with flow cytometric analysis of OSA lysates and peripheral blood mononuclear cells will allow a more robust and complete assessment of the immune landscape in cOSA.
With the number of dogs included in this study, we elected to evaluate a limited number of clinical associations with immune infiltrates. Given that an advantage of the canine model involves the less intensive treatment with chemotherapy, we explored for an association between chemotherapy exposure within 3 months and immune cell infiltration with metastatic lesions. We detected no difference in the degree of any immune cell infiltration in cOSA metastasis between dogs with recent chemotherapy exposure compared to those without recent chemotherapy exposure. While this could suggest that, chemotherapy schedules in dogs are not aggressive enough to cause any changes to the immune microenvironment, we did not evaluate a sufficient number of cases to power the observation of a difference between groups and no conclusions can truly be made in this regard. In addition, dogs were treated with a range of chemotherapy agents that may have led to heterogenous effects on the immune microenvironment. While the differences between immune infiltrates in tumours arising from the proximal humerus compared to other locations did not reach significance in this cohort, a clear trend towards decreased inflammation in the 4 cases of proximal humeral cOSA was detected. These results are in line with our previous report describing decreased CD3+, FOXP3+, and CD204+ cells in proximal humeral cOSA compared to other appendicular locations.18
Finally, the finding of one case in which the tumour cells in both the primary and metastatic cOSA lesions were positive for Pax-5 is interesting but of unknown significance. In humans, no Pax-5 expression was detected in 99 osteosarcoma, 6 undifferentiated sarcoma, and 9 embryonal rhabdomyosarcoma samples, whereas the majority of alveolar rhabdomyosarcomas and Wilms tumours stained positive, using the same clone as we have reported here.64 These authors subsequently confirmed the cross-reactivity of this antibody clone with Pax-2 indicating that positively labelled cells express either Pax-2 or Pax-5, or both. Whether this cross reactivity exists in canine tissue remains to be determined. Pax genes encode several transcription factors that regulate cell proliferation, self-renewal, and resistance to apoptosis.65 The dysregulation of Pax proteins have implied their involvement in the progression of several cancer types.65 Interestingly, Pax-3 was overexpressed in a mouse model of metastatic osteoblastic osteosarcoma.66 The further study of Pax proteins in cOSA will require the verification of the cross-reactivity clone 24 across Pax family transcription factors.
5 CONCLUSION
In summary, our data reveals that immune cells infiltrating metastatic cOSA can be predicted by evaluation of the immune cells infiltrating a dog's primary cOSA, suggesting the potential benefit of utilizing primary tumour immune biomarkers to predict immunotherapeutic response in metastases. Furthermore, metastatic lesions have consistently higher immune infiltrates than primary tumours, highlighting several potential immunotherapeutic targets for the treatment of metastatic cOSA.
ACKNOWLEDGEMENTS
The present study funded by the Center for Companion Animal Health, School of Veterinary Medicine, University of California, Davis. http://www.vetmed.ucdavis.edu/CCAH/, NIH-NCI P30CA093373 and NIH-NCIU01 CA224166-01. S.W. is partially supported by the American Kennel Club Canine Health Foundation Clinician-Scientist Fellowship Program. J.H.B. is supported by the National Cancer Institute of the National Institutes of Health under Award Number K12CA138464. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLICT OF INTEREST
The authors have no conflicts of interest in regards to the content of this manuscript.