Anti-tumour effect of lapatinib in canine transitional cell carcinoma cell lines
Abstract
Transitional cell carcinoma (TCC) accounts for >90% of canine malignant tumours occurring in urinary bladder, and the prognosis is poor. Our previous study, using RNA sequencing, showed that human epidermal growth factor 2 (HER2) was the most activated upstream regulator related to carcinogenesis in canine TCC. The aim of this study was to examine the anti-tumour effect of lapatinib, a tyrosine kinase inhibitor of HER2, on canine TCC cell lines in vitro and in vivo. Five canine TCC cell lines (TCCUB, Love, Sora, LCTCC, and MCTCC) were used. Western blotting showed that HER2 protein expression was observed in all of the canine TCC cell lines. Lapatinib inhibited phosphorylation of HER2 and cell growth in a dose-dependent manner. Cell cycle analyses using flow cytometry showed that lapatinib significantly increased the sub-G1 and G0/G1 phase fractions and significantly decreased the S and G2/M phase fractions in the cell lines (Sora and TCCUB). For the in vivo experiments, the canine TCC cells (Sora) were subcutaneously injected into nude mice. Six days after inoculation, lapatinib (100 mg/kg) or vehicle was administered daily via intraperitoneal administration for 14 days. Tumour volume was significantly smaller in the lapatinib group compared with the vehicle control group. Histologically, lapatinib significantly increased necrotic areas in the tumour tissues. These findings suggest that lapatinib exerts anti-tumour effects on canine TCC cells by inhibiting HER2 signalling and inducing cell cycle arrest.
1 INTRODUCTION
Transitional cell carcinoma (TCC), also known as urothelial carcinoma, accounts for approximately 1% to 2% of all canine malignant tumours and is the most common tumour affecting the urinary tract in dogs.1, 2 Because of nonspecific clinical signs such as hematuria, pollakiuria, and stranguria, early detection of canine TCC is rare. By the time of diagnosis, in most cases of canine TCC, the cancer has invaded the bladder wall, with 20% of cases displaying invasion into neighbouring organs as well as distant metastasis.1 Curative-intent surgery has demonstrated limited benefit in canine TCC. Regimens using chemotherapy, cyclooxygenase inhibitors, and a combination of these compounds are considered the mainstay treatment for canine TCC. Although there are several reports on the available pharmacotherapy for canine TCC, the median survival time associated with these therapies is less than 1 year.3 Thus, a novel therapeutic option with increased survival time is currently needed for treatment of canine TCC.
Our previous study, using RNA sequencing for urinary bladder (UB) tissues in dogs with TCC and healthy dogs, showed that human epidermal growth factor 2 (HER2) was the most activated upstream regulator related with carcinogenesis in canine TCC.4 The HER2 protein is a 185-kD transmembrane glycoprotein part of the HER family of tyrosine kinase receptors, which include HER1 (also known as epidermal growth factor receptor [EGFR]), HER2, HER3, and HER4. In normal circumstances, HER2 is involved in proliferation, differentiation, angiogenesis, and migration through two principal signalling pathways: the mitogen-activated protein kinase/extracellular-regulated kinase (MAPK/Erk) and the phosphatidylinositol 3-kinase (PI3K)/Akt.5, 6 HER2 protein overexpression is observed in a variety of human malignancies such as breast, bladder, gastric, ovarian, lung, and colon cancers,7 and is associated with poor prognosis.8-10
HER2 protein overexpression is a potential therapeutic target in human breast cancers. The representative HER2-targeted therapies are trastuzumab and lapatinib. Trastuzumab is an anti-HER2 humanized monoclonal antibody. Treatment with trastuzumab significantly improves 33% to 52% of disease-free survival and 34% to 41% of overall survival in early human breast cancer patients.11, 12 Lapatinib is a dual small-molecule tyrosine kinase inhibitor of HER2 and EGFR.13, 14 Treatment with lapatinib has been shown to prolong progression-free survival in human breast cancer patients who had progressed on trastuzumab monotherapy.15, 16
As in humans, HER2 protein overexpression is observed in 56% of canine TCC.17 However, it remains unknown whether HER2-targeted therapy can exert anti-tumour effects on canine TCC. In general, it is difficult to use humanized antibody drugs in dogs because of failure of cross-reactivity.18 Alternatively, small-molecule drugs often cross-react with various species.19 As EGFR protein overexpression is also observed in 72% of canine TCC,20 the HER2 and EGFR tyrosine kinase inhibitor lapatinib may be effective in canine TCC.
In this study, we investigated the anti-tumour effect of lapatinib on canine TCC cell lines in vitro and in vivo. To understand the mechanisms associated with the anti-tumour effects of lapatinib, we also examined phosphorylation of Erk and Akt as downstream molecules in the HER2 and EGFR signalling pathways.
2 MATERIALS AND METHODS
2.1 Reagents and antibodies
Lapatinib used in the in vitro experiments and in vivo animal experiments were obtained from Selleck Chemicals (Houston, Texas) and LC Laboratories (Woburn, Massachusetts), respectively. Lapatinib was dissolved in dimethyl sulfoxide (DMSO). Recombinant human epidermal growth factor (EGF) was purchased from R&D Systems (Minneapolis, Minnesota). EGF was dissolved in phosphate buffered saline.
For western blotting, mouse primary antibodies against HER2 and Tyr 1112-phosphorylated HER2 were obtained from nanoTools (Teningen, Germany). Other mouse primary antibodies against Tyr 1068-phosphorylated EGFR and rabbit primary antibodies against EGFR, Erk 1/2, Tyr 202/Tyr 204-phosphorylated Erk 1/2, Akt, Ser 473-phosphorylated Akt, and β-Actin were obtained from Cell Signaling (Danvers, Massachusetts). IRDye 680RD donkey anti-mouse and IRDye 800CW donkey anti-rabbit secondary antibodies were purchased from M&S TechnoSystems Inc. (Osaka, Japan). All antibodies used for western blotting were diluted according to the manufacturer's recommendations. For immunohistochemistry (IHC), a rabbit primary antibody against HER2 and EnVision+System-HRP Labelled Polymer Anti-Rabbit were obtained from Dako (Glostrup, Denmark).
2.2 Cell culture
Five canine TCC cell lines (TCCUB, Love, Sora, LCTCC, and MCTCC) were used. TCCUB, Love, and Sora were developed in our previous study,21 whereas LCTCC and MCTCC were provided by Dr. Y. Hoshino, Hokkaido University.22 All cell lines were determined to be free of Mycoplasma using EZ-PCR Mycoplasma Test Kit (Biological Industries, Connecticut). Cells were maintained in RPMI 1640 medium (Sigma-Aldrich, St. Louis, Missouri) supplemented with 10% fetal bovine serum (Gibco, Carlsbad, California) and 1% penicillin—streptomycin (Gibco) at 37°C with 5% CO2.
2.3 Cell proliferation assay
Cell proliferation was determined by the methylthiazolyl tetrazolium (MTT) assay using Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan), according to the manufacturer's instructions. Briefly, canine TCC cells (TCCUB, Love, Sora, LCTCC, and MCTCC) were seeded in 96-well plates with 5 × 103 cells per well. After 1 day, the cells were treated with various concentrations of lapatinib (0.001-10 μM) for 24, 48, or 72 hours. MTT solution was then added to each well. The survival fraction was determined based on the absorbance detected at 450 nm using the iMark Microplate Reader (Bio-Rad, Hercules, California). All the samples were examined in quadruplicate, and three independent experiments were performed.
2.4 Western blotting
Canine TCC cells (TCCUB, Love, Sora, LCTCC, and MCTCC) were cultured in petri dishes. When the cells reached 70% to 80% confluence, the cells were washed with Hanks’ Balanced Salt Solution (HBSS; Sigma-Aldrich) and were incubated in serum free medium with each concentration of lapatinib (0.1, 1, and 10 μM) for 1 hour. The cells were then treated with 10 ng/mL EGF for 30 minutes according to manufacturer's datasheet. After washing with HBSS, the cells were incubated with RIPA buffer (Cell Signaling) containing protease and phosphatase inhibitor cocktail tablets (Roche Diagnostics, Mannheim, Germany). The cell lysates were centrifuged at 14 000 rpm at 4°C for 10 minutes. Then, total protein concentrations were measured using a BCA Protein Assay kit (Thermo Scientific, Waltham, Massachusetts).
Equal sample concentrations (12 μg) were loaded in 7.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels for electrophoresis. After separation, proteins were transferred to nitrocellulose membranes and were blocked in Tris-buffered saline (TBS) containing 5% skim milk at room temperature for 1 hour. Primary antibodies were incubated at 4°C overnight. Then, the membranes were washed using TBS with 0.2% Tween 20 (Sigma-Aldrich) and were incubated with the appropriate secondary antibodies at room temperature for 1 hour. Proteins were detected using the ODYSSEY CLx (M&S TechnoSystems Inc.). Expression of β-Actin was used as a protein loading control. The cell lysate of a human ovarian cancer cell line (SKOV3; nanoTools) was used as a positive control according to the manufacture's protocol.
2.5 Cell cycle analyses
Canine TCC cells (Sora and TCCUB) were seeded in 6-well plates with 2 × 105 cells per well. After 1 day, the cells were treated with 10 μM lapatinib or vehicle for 24 hours. The cells were washed with HBSS, centrifuged at 3000 rpm for 3 minutes after trypsinization, fixed in 75% alcohol and kept at −20°C until analyses. The samples were then washed with HBSS and incubated with a staining solution containing 50 μg/mL propidium iodide (Sigma-Aldrich), 0.1 mg/mL RNase A (QIAGEN, Hilden, Germany), and 0.05% Triton X-100 (Sigma-Aldrich) at 37°C for 40 minutes. The DNA contents of the cells were evaluated using a FACS flow cytometer (BD Biosciences, Tokyo, Japan). All the samples were examined in triplicate, and three independent experiments were performed.
2.6 Terminal deoxynucleotidyl transferase deoxyuridine Triphosphate (dUTP) nick end labeling staining
Canine TCC cells (Sora and TCCUB) were seeded on cover glasses with 2 × 105 cells per cover glass. After 1 day, the cells were treated with 10 μM lapatinib or vehicle for 24, 48, or 72 hours. The cells were washed with HBSS and fixed in 4% paraformaldehyde at 4°C for 25 minutes. Transferase dUTP nick end labeling (TUNEL) staining was then performed using the DeadEnd Fluorometric TUNEL System (Promega, Madison, Wisconsin) according to the manufacturer's protocol. Cells treated with 1 U DNase I (Promega) were used as positive controls.
2.7 Xenograft mouse model
Female BALB/cSlc nude mice (4 weeks old) were purchased from Japan SLC, Inc. (Shizuoka, Japan). The mice were acclimated to our facilities for 2 weeks prior to the experiment. To develop tumour-engrafted mice, canine TCC cells (Sora) were subcutaneously injected with 2 × 106 cells into the left side flank of each mouse. The greatest longitudinal and transverse diameters of tumours were measured using a vernier calliper every 2 days, and tumour volume was calculated based on the following formula: tumour volume = (long diameter) × (short diameter)2 × 0.52. Six days after tumour cell inoculation, the mice were treated with lapatinib (100 mg/kg; n = 10) or vehicle control (saline containing 10% DMSO and 10% Tween 20; n = 9) daily via intraperitoneal administration for 14 days.23 Clinical signs such as decreased activity, anorexia, vomiting, diarrhoea, or weight loss were daily evaluated to assess toxicity of lapatinib. At the endpoint, the mice were euthanized, and the tumours were collected. After measurement of tumour weight, the tumours were fixed in 10% neutral buffered formalin and embedded in paraffin. All of the experimental procedures using mice were approved by the Animal Care Committee of the Graduate School of Agricultural and Life Sciences at the University of Tokyo (authorization number: P17-127).
2.8 Histopathology
Sections of 4 μm were cut from the formalin-fixed paraffin-embedded tumour tissues from the lapatinib and vehicle control groups, and haematoxylin and eosin staining was performed. The tumour tissues were photographed with a 2× objective and a 10× eyepiece to capture the tumours in whole. Using image analysis software (ImageJ, U.S. National Institutes of Health, Bethesda, MD),24 percentage of necrosis in each tumour tissue was calculated by a single evaluator (K.S).
2.9 IHC for HER2
The 4-μm sections were deparaffinized in xylene and hydrated in graded ethanol. Antigen retrieval was performed at 121°C for 10 minutes using 10 mM citrate buffer (pH 6.0). After cooling for an hour, the slides were treated with peroxidase-blocking solution (Dako) at room temperature for 10 minutes, followed by three washes in TBS with 0.1% Tween 20 (TBS-T). After blocking with TBS-T containing 5% skim milk (Wako, Osaka, Japan) for 1 hour, the sections were incubated with the anti-HER2 rabbit polyclonal antibody (diluted to 1:100 in TBS) at 37°C for 40 minutes. The sections were sequentially incubated with the secondary antibody at 37°C for 40 minutes. The peroxidase reaction was conducted with 3,3′-diaminobenzidine tetra-hydrochloride (Dako) for 10 minutes. The reaction was stopped by two washes in distilled water, and counterstaining using Mayer's haematoxylin was then performed. Incubation of sections with the antibody diluents served as negative controls. The IHC protocol used for canine TCC tissues was described in a previous study with a slight modification.17
2.10 Statistical analyses
Student's t tests were used for comparisons between two groups. Prism software version 5.0.1 (Graph Pad Software, San Diego, California) was used for the statistical analysis. Statistical significance was defined as P < 0.05.
3 RESULTS
3.1 Basal protein expressions of HER2, EGFR, and their phosphorylated forms
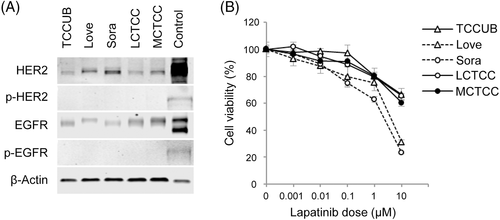
HER2 and EGFR protein expressions were observed in all canine TCC cell lines (Figure 1A). The HER2 protein level was high in Love and Sora, whereas that of EGFR was high in TCCUB and MCTCC (Table 1). Autophosphorylation of HER2 and EGFR was not observed in any of the canine TCC cell lines (Figure 1A).
| TCCUB | Love | Sora | LCTCC | MCTCC | |
|---|---|---|---|---|---|
| HER2 | 1.00 | 1.91 | 2.53 | 0.62 | 1.08 |
| EGFR | 1.00 | 0.41 | 0.40 | 0.60 | 1.07 |
| IC50 (μM) | >10 | 3.73 | 2.10 | >10 | >10 |
- Abbreviations: EGFR, epidermal growth factor receptor; HER2, human epidermal growth factor receptor 2; IC50, 50% inhibitory concentrations; TCC, transitional cell carcinoma.
- The protein levels of HER2 and EGFR are normalized to β-actin in each canine TCC cell line. Data are presented as relative value when the protein levels of HER2 and EGFR in TCCUB are set at 1.00.
3.2 Effects of lapatinib on cell proliferation
Lapatinib showed dose-dependent growth inhibitory activity in all of the canine TCC cell lines at 24 hours (Figure 1B). Love and Sora were more sensitive to lapatinib than TCCUB, LCTCC, and MCTCC. In particular, in Sora, cell viability decreased to 23.1% of the control after treatment with 10 μM lapatinib. The 50% inhibitory concentrations (IC50) of lapatinib correlated with the protein levels of HER2 in the canine TCC cell lines, whereas the protein level of EGFR was not associated with the sensitivity to lapatinib (Table 1). Similar results were obtained at 48 or 72 hours (data not shown).
3.3 Effects of lapatinib on signalling pathways of HER2 and EGFR
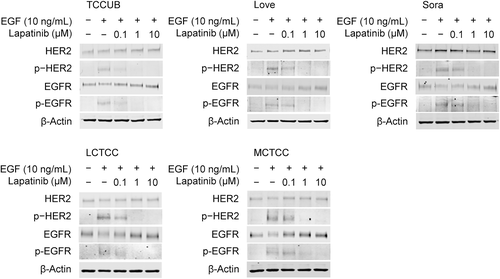
HER2 and EGFR were phosphorylated in all of the canine TCC cell lines through treatment with EGF (Figure 2). Lapatinib inhibited the EGF-induced phosphorylation of HER2 and EGFR in a dose-dependent manner (Figure 2). The total protein expression of HER2 and EGFR did not alter with lapatinib treatment.
As shown in Figure 3A, phosphorylation of Erk 1/2 and Akt was observed in unstimulated Sora cells. EGF treatment mildly enhanced the phosphorylation of Erk 1/2 and Akt. Lapatinib inhibited the phosphorylation of Erk 1/2 and Akt in a dose-dependent manner. In particular, phosphorylation of Erk 1/2 was more strongly suppressed than that of Akt. The total protein expression of Erk 1/2 and Akt did not alter with lapatinib treatment. Cell cycle analyses using flow cytometry showed that the sub-G1 and G0/G1 phase fractions were significantly increased, whereas the S and G2/M phase fractions were significantly decreased in Sora cells after treatment with lapatinib compared with the vehicle control (Figure 3B and C). After the treatment with lapatinib for 24 hours, there were a few TUNEL-positive cells in Sora (Figure 3D). Similar results were obtained at 48 or 72 hours (data not shown). Results of western blotting, cell cycle analysis, and TUNEL staining in TCCUB were similar to those in Sora (Figure S1).

3.4 Effects of lapatinib on tumour growth in canine TCC-engrafted mice
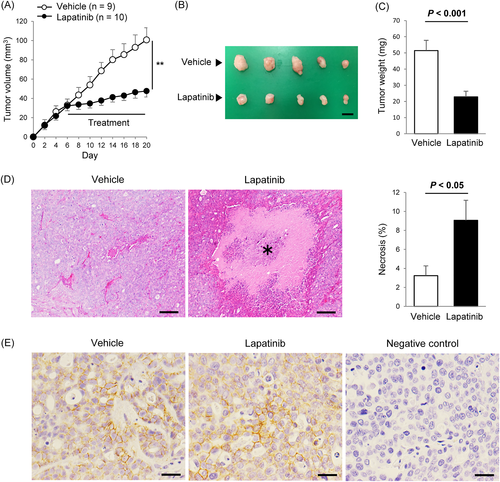
Six to fourteen days after treatment, the tumour volume in the lapatinib group was significantly smaller than those in the vehicle control group (P < 0.01, Figure 4A). The tumour weight collected from the mice was significantly reduced in the lapatinib group compared with the vehicle control group (P < 0.001, Figure 4B and C). During the treatment, mild soft stools and weight loss (<5%) were observed in the lapatinib group. The mean ± SD of the body weight at the end of the study was 25.1 ± 1.1 g and 23.9 ± 0.8 g in the control and lapatinib groups, respectively. There was no significant difference in the body weight of the mice between the two groups. Histological analyses showed that lapatinib significantly increased the necrotic area in the tumour tissues (P < 0.05, Figure 4D). HER2 protein overexpression was observed on the membrane of tumour cells in both of the lapatinib treatment and vehicle control groups (Figure 4E).
4 DISCUSSION
Lapatinib is a tyrosine kinase inhibitor belonging to the 4-anilinoquinazoline class that targets the kinase domain of both HER2 and EGFR.13, 14 Because HER2 and EGFR protein overexpression was observed in more than half of dogs with TCC,17, 20 we aimed to evaluate the potential anti-tumour effects of lapatinib on canine TCC cell lines in this study. Our results showed that lapatinib inhibited growth activity in these cells. The growth inhibitory activity of lapatinib was dependent on the protein level of HER2 but not on that of EGFR, which is in concordance with the observed properties of human cancer cell lines such as breast and gastric.25, 26 Among the HER family, HER2 has the most robust catalytic kinase activity and functions as the most active signalling complex.27, 28 The HER2 protein level may be an important predictor of sensitivity to lapatinib in canine TCC. Further study will be necessary to confirm the association between HER2 expression and efficacy of lapatinib in clinical cases.
The anti-tumour effect of lapatinib is also observed in human bladder cancer cell lines.29, 30 Using IHC, HER2 protein overexpression has been reported in 8% to 81% of human bladder cancer.31-40 However, a phase III randomized trial failed to demonstrate clinical benefits of lapatinib in human bladder cancer patients.41 In this study, lapatinib exerted anti-tumour effects on canine TCC not only in vitro but also in vivo. A previous study using IHC showed that HER2 protein overexpression was observed in 56% of canine TCC.17 Although the clinical trial of lapatinib failed in human bladder cancer, these findings provide a rationale for clinical trials of lapatinib monotherapy or in combination with other anti-tumour drugs in dogs with TCC.
Lapatinib inhibited the phosphorylation of HER2, EGFR, and downstream molecules (Erk 1/2 and Akt) in the canine TCC cell lines. The MAPK/Erk pathway is involved in cell proliferation,42 whereas the PI3K/Akt pathway is involved in cell survival.43 The present study demonstrated that lapatinib induced cell cycle arrest, whereas induction of apoptosis was weak in the canine TCC cells, suggesting that the anti-tumour effect of lapatinib is caused mainly by inhibition of the MAPK/Erk pathway. Similar analysis in vivo was not performed but might have strengthened our conclusion. Further studies are needed to examine the inhibitory effect of lapatinib on the phosphorylation of HER2/EGFR signalling in vivo.
There was a limitation to this study. We used the ectopic mouse model because it does not require surgery to engraft tumour cells, is easy to monitor tumour growth, and is less costly. In ectopic models, tumour microenvironments including surrounding blood vessels, immune cells, fibroblasts, and extracellular matrix are different from the originals. Recently, tumour microenvironments have been considered as an important factor for tumour growth, progression, and drug response.44-48 Thus, the different microenvironment from the UB might influence on the tumour development.
In conclusion, we demonstrated the growth inhibitory activity of lapatinib in canine TCC cell lines, which is mainly mediated through inhibition of HER2-MAPK/Erk pathway. In addition, we showed that lapatinib exerted anti-tumour effect in the canine TCC-engrafted mouse model without severe side effects. These results suggest that lapatinib has therapeutic potential for dogs with TCC, particularly in HER2-overexpressing cases.
ACKNOWLEDGEMENTS
We thank Dr. Y. Hoshino (Hokkaido University) for providing canine TCC cell lines (LCTCC and MCTCC). This study was supported by Japan Society for the Promotion of Science (JSPS) Fellows of JSPS, a Grant-in-Aid for Science Research (KAKENHI Grant Number 16H06208), and Anicom Capital Research Grant (EVOLVE).
Conflict of interest
None of the authors have any financial or personal relationships that would inappropriately influence or bias the content of the paper.