Transplantation of donor lungs with pulmonary embolism – a retrospective study
Summary
Lung transplantation from donors with fulminant pulmonary arterial embolism as a cause of death remains controversial. An analysis was performed comparing preoperative characteristics and outcomes of 25 donors with a primary diagnosis of pulmonary arterial embolism to 1085 recipients of donor lungs without pulmonary arterial embolism. No early functional impairment of donor lungs with pulmonary embolism was detectable as depicted by the incidence of primary graft dysfunction immediately after surgery (P = 0.66), 24 (P = 0.79), 48 (P = 0.99) and 72 h (P = 0.99) after transplantation. Pulmonary function testing at 1 year (P = 0.003) and at last outpatient control (P < 0.05) showed superior results in the cohort receiving lungs from donors with pulmonary embolism. Incidence of chronic lung allograft dysfunction (CLAD) showed no difference within the first year after lung transplantation, however, 5 year-CLAD free survival was superior in recipients (70.4% vs. 55.1%, P = 0.006) of donor lungs with pulmonary embolism. Overall survival was similar in both groups. Lungs from donors with fulminant pulmonary embolism prior to brain death can safely be used for lung transplantation without impairing postoperative outcomes. Lung function testing shows favorable midterm results in recipients of donor lungs with pulmonary embolism.
Introduction
Lung transplantation remains the only curative treatment option for patients suffering from end-stage-lung disease. However, donor organ shortage remains an unresolved problem. European countries with active lung transplantation programs repeatedly report increasing numbers of patients on waiting lists in contrast to decreasing or stable numbers of potential donors 1, 2. Thus, enlarging the pool of donors is necessary to reduce waiting list mortality.
Extended criteria donor organs have been successfully used for lung transplantation with similar short- and long-term outcome as compared to standard donor organs 3-7. A majority of organs are classified as extended criteria donor lungs due to older age, impaired oxygenation, trauma, aspiration or drowning 8-12. These organs for selected recipients are now routinely accepted in high-volume centers. However, the use of donor organs with a structural defect as in donors with pulmonary thromboembolism as the leading cause of death remains debatable. We have previously published an initial experience with three donors with pulmonary arterial embolism (PAE) showing the feasibility of utilizing these organs for clinical routine 13. Since then, other case reports on utilization of these organs for lung transplantation have shown similar successful outcomes 14, 15.
Since the introduction of ex vivo lung perfusion (EVLP) in the field of lung transplantation, utilization of extended criteria donor lungs and their recruitment with EVLP has become the focus of the transplant community 16. Several case reports have reported successful utilization of EVLP for donor organs with pulmonary thromboembolism, suggesting the addition of lysing reagents to dissolve the remaining thrombi during ex vivo perfusion prior to transplantation 17-19.
In this study, we report the overall acceptance rate of lungs from donors with the initial diagnosis of pulmonary thromboembolism in Germany and our center experience on routine use of these organs for lung transplantation.
Patients and methods
An analysis was performed to assess the impact of donor pulmonary arterial embolism on the transplantability of the lungs as well as on recipient outcomes.
All donors reported by the German Organ Procurement Organization (DSO) as multi-organ donors between January 2006 and December 2015 with the primary diagnosis of acute pulmonary arterial embolism prior to brain death were recorded (Fig. 1). The diagnosis of PAE was confirmed by echocardiography or CT scan in all cases. Treatment of PAE varied between the different donor hospitals, and detailed treatment information was not available for all donors. Donor demographics and the cause of nonutilization were recorded. In addition, the decision path that led to the rejection of PAE lungs for transplantation was recorded, particularly if the decision was made at the donor side or within the allocation process by a lung transplantation center. Donors with prediagnosed or macroscopic signs of chronic thromboembolic pulmonary disease were not included in the study.
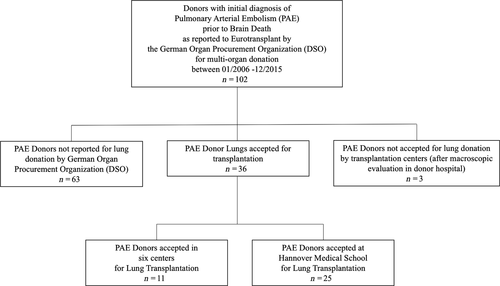
Then, donor data of all lung transplantations performed at our institution between January 2006 and December 2015 were analyzed for the primary donor cause of death. Outcomes of recipients of PAE-donors (PAE Group) were compared to the outcomes of recipients of lungs without pulmonary arterial embolism (NonPAE Group). The pre-, peri- and post-operative characteristics were retrospectively recorded.
Primary Graft Dysfunction was graded following ISHLT classification of 2005. Patients requiring extracorporeal support during surgery underwent implantation of veno-arterial extracorporeal membrane oxygenation (ECMO) via femoral vessels. ECMO was explanted at the end of surgery or left in place in case of per-protocol postoperative ECMO treatment in patients with pulmonary arterial hypertension, allowing left ventricular remodeling in the early postoperative period 20 . Postoperative care did not differ between recipients in the PAE- and nonPAE-group.
Lung transplantations with incidental findings of thrombi in the pulmonary artery during explantation or implantation were not included in the PAE Group.
All patients provided informed consent prior to lung transplantation, using a standardized consent form approved by the local ethics committee that approved the use of their data for scientific purposes.
Surgical protocol for PAE lung procurement
In donors with known PAE, standard procedure at the donor hospital included bronchoscopy and macroscopic assessment of the lung parenchyma as well as invasive measurement of the pulmonary artery pressure 21. Cold lung flush was performed using 4 l of Celsior Preservation Solution (Celsior®, WMS, LLC, Rochester, NY, USA) for antegrade flush via the pulmonary artery and 1 l of Celsior solution for retrograde flushing. Five lungs in the PAE cohort were preserved using LPD solution (Perfadex®; XVIVO Perfusion AB, Göteborg, Sweden). Retrograde flushing was performed after excision of the heart by directing the cannula directly into each pulmonary vein. Thereafter, a systematic inspection of the right and left pulmonary artery was performed with long forceps in order to remove residual thrombi. Upon arrival in the recipient OR, a second thorough inspection of the pulmonary artery was performed and heparinized saline was injected into the arteries to dissolve small distal thrombi before implantation. No additional anticoagulation was given to the recipients if not required otherwise (e.g. need for ECMO) and no specific postoperative imaging was performed in the recipients in the absence of elevated pulmonary artery pressure.
Statistics
Retrospective analysis of all parameters was performed using graphpadprism, Version 7.0 (San Diego, CA, USA). Variables were summarized as percentages, mean ± standard deviation (SD) or median + Interquartile Range (IQR), respectively. Mann-Whitney-U-Test was performed to test differences between continuous variables. Survival was calculated using the Kaplan-Meier method and compared using log-rank test. A donor age matched case control analysis was performed using spss 25.0 (IBM, New York, NY, USA). P values <0.05 were considered statistically significant.
Results
Between January 2006 and December 2015, a total of 102 donors with primary diagnosis of PAE were reported to Eurotransplant by the DSO. In 63 donors, the lungs were not reported for allocation by the DSO transplant coordinator in charge. In three donors, the lungs were allocated but not accepted by a transplantation center after macroscopic evaluation in the donor hospital (Fig. 1).
Donors who were not allocated for transplantation were significantly older than donors who were allocated for lung transplantation [37 (23.5–47) vs. 58 (44–67.3) years, P < 0.0001], showed significantly impaired oxygenation capacity (PaO2/FiO2 402.8 ± 120.4 vs. 303.8 ± 113.4 mmHg, P = 0.005) and were less often suitable for heart donation (50.0% vs. 16.7%, P = 0.0006; Table 1).
| Donor with PAE (n = 25; 01/2006–12/2015) | Not-allocated donors with PAE (n = 66) | P value | |
|---|---|---|---|
| Donor age [median (IQR) years] | 37 (23.5–47) | 58 (44–67.3) | <0.0001 |
| Female (%) | 76.0 | 100.0 | 0.0003 |
| Mechanical ventilation (days; mean ± SD) | 8.8 ± 12.6 | 4.9 ± 3.7 | 0.26 |
| BMI (mean ± SD) | 27.7 ± 6.5 | 30.01 ± 7.3 | 0.10 |
| PaO2/FiO2 (mean ± SD) | 402.8 ± 120.4 | 303.8 ± 113.4a | 0.005 |
| History of smoking (%) | 28.0 | 28.8 | >0.99 |
| Heart used for transplantation (%) | 50.0 | 16.7 | 0.0006 |
- a Available only in 22/66 donors.
Thirty-six lungs were reported and accepted for lung transplantation. The remaining lungs were successfully transplanted in six different transplant centers across Eurotransplant countries (Fig. 1). The recipients of these centers were excluded for a more homogenous data analysis.
This analysis compared all lung transplantations performed at our institution using lungs from PAE-donors (PAE group, n = 25) with lung transplantations using donors without the diagnosis of PAE prior to donation (NonPAE group, n = 1085) that were performed in the same time period in our center.
Donor characteristics
Pulmonary arterial embolism-donors were significantly younger than nonPAE-donors [37 (23.5–47) vs. 46 (33.8–54) years, P = 0.03] and more often females (76.0% vs. 48.7%, P = 0.008). BMI (P = 0.09) as well as history of smoking (P = 0.16) were similar in both groups. Also, the length of mechanical ventilation (P = 0.30), incidence of aspiration (P = 0.64) as well as PaO2/FiO2 ratio (P = 0.70) prior to organ donation showed no significant differences between both groups. The diagnosis of PAE was made through Computer tomography in 15 (60%) donors, pulmonary angiography was documented in three (12%) donors as the used methodology. The remaining donors were diagnosed with PAE using echocardiography (n = 7, 28%) showing severe right heart distension in combination with a known history of thromboembolism. One patient showed blood clots in the right atrium and another patient had a blood clot in the inferior vena cava. In two patients, large clots were detected using echocardiography in the main pulmonary artery. In PAE-donors, an initial lysis therapy using alteplase before or immediately after admission to the hospital was documented in 52% of the donors. One donor underwent mechanical embolectomy. All donors were additionally anticoagulated using heparine (Table 2).
| Donor with PAE (n = 25; 01/2006–12/2015) | NonPAE-donor (n = 1085; 01/2006–12/2015) | P value | |
|---|---|---|---|
| Donor age [median (IQR) years] | 37 (23.5–47) | 46 (33.8–54) | 0.03 |
| Female (%) | 76.0 | 48.7 | 0.008 |
| Mechanical ventilation (days; mean ± SD) | 8.8 ± 12.6 | 5.5 ± 4.7 | 0.30 |
| BMI (mean ± SD) | 27.7 | 25.3 | 0.09 |
| PaO2/FiO2 (mean ± SD) | 402.8 ± 120.4 | 396.5 ± 110.4 | 0.70 |
| History of smoking (%) | 28.0 | 42.3 | 0.16 |
| Lung contusion (%) | 4.0 | 9.9 | 0.50 |
| Aspiration (%) | 8.0 | 5.3 | 0.64 |
Recipient characteristics
Recipients receiving PAE-donor lungs showed no differences in age [52 (39.5–58.5) vs. 50 (35–57.5) years, P = 0.60] and distribution of underlying diseases, but were more often female (72.1% vs. 46.4%, P = 0.01) than recipients of nonPAE-donor organs.
Median time on waiting list prior to lung transplantation as well as incidence of preoperative admission to ICU, preoperative mechanical ventilation or ECMO/ECLA treatment showed no significant differences between groups (Table 3).
| Donor with PAE (n = 25; 01/2006–12/2015) | NonPAE-donor (n = 1085; 01/2006–12/2015) | ρ value | |
|---|---|---|---|
| Age [years; median (IQR) years] | 52 (39.5–58.5) | 50 (35–57.5) | 0.60 |
| Female (%) | 72.0 | 46.4 | 0.01 |
| BMI (mean ± SD) | 21.8 | 21.8 | 0.96 |
| Lung allocation scorea (mean ± SD) | 46.6 ± 17.1 | 43.2 ± 16.4 | 0.23 |
| Previous chest surgery (%) | 16.0 | 17.1 | 0.99 |
| Chronic bacterial colonization (%) | 32.0 | 30.9 | 0.99 |
| Waiting list (days; median; IQR) | 243; 82.5–517.5 | 124; 31.5–392 | 0.19 |
| Underlying disease | |||
| Emphysema (%) | 16 | 31.8 | 0.13 |
| IPF (%) | 32 | 28.9 | 0.82 |
| Cystic fibrosis (%) | 36 | 22.0 | 0.14 |
| Primary pulmonary hypertension (%) | 8.0 | 4.8 | 0.62 |
| CLAD (%) | 4.0 | 7.4 | 0.72 |
| Other (%) | 4.0 | 5.1 | 0.99 |
| Sek. PHT preoperatively (PAPmean > 25 mmHg; %) | 56.0 | 41.5 | 0.16 |
| Preoperative admission to ICU (%) | 12.0 | 13.6 | 0.99 |
| Preoperative mechanical ventilation (%) | 4.0 | 6.4 | 0.72 |
| Preoperative ECMO/ECLA (%) | 8.0 | 8.1 | 0.99 |
- a LAS allocation since November 2011, LAS therefore only available in 11/25 patients in PAE cohort and 489/1085 patients in nonPAE cohort.
Perioperative characteristics
All PAE-donor lungs were used for bilateral procedures, whereas nonPAE-donors were utilized in 95.3% of the cases for bilateral transplantations. The need for intraoperative use of cardiopulmonary bypass (PAE group: 8.0% vs. NonPAE group: 13.2%, P = 0.56) or ECMO (PAE group: 28.0% vs. NonPAE group: 19.5%, P = 0.31) as a result of hemodynamic or respiratory impairment showed no significant differences between both groups. Similarly, cold ischemic time of the first (P = 0.44) and second lungs (P = 0.96) were not different (Table 4).
| Donor with PAE (n = 25; 01/2006–12/2015) | NonPAE-donor (n = 1085; 01/2006–12/2015) | ρ value | |
|---|---|---|---|
| Bilateral TX (%) | 100 | 95.3 | 0.41 |
| Interaoperative use of CPB (%) | 8.0 | 13.2 | 0.56 |
| Intraoperative use of ECMO (%) | 28.0 | 19.5 | 0.31 |
| Cold ischemic time 1st side (min; mean ± SD) | 411.3 ± 119.7 | 427.3 ± 123.9 | 0.44 |
| Cold ischemic time 2nd side (min; mean ± SD) | 536.8 ± 120 | 531.3 ± 133.8 | 0.96 |
| ECMO intra- and post-operative (%) | 8.0 | 6.5 | 0.99 |
| Primary graft dysfunction | |||
| PGD grade 2/3 @T0 (%) | 28.0 | 34.0 | 0.66 |
| PGD grade 2/3 @T24 (%) | 20.0 | 17.8 | 0.79 |
| PGD grade 2/3 @T48 (%) | 16.0 | 17.9 | 0.99 |
| PGD grade 2/3 @T72 (%) | 12.0 | 14.8 | 0.79 |
| Rethoracotomy (%) | 12.0 | 14.1 | 0.99 |
| Postoperative dialysis (%) | 20.0 | 11.2 | 0.19 |
| Reperfusion edema (%) | 12.0 | 11.5 | 0.99 |
| De-novo ECMO postoperative (%) | 12.0 | 3.6 | 0.06 |
| Mechanical ventilation postoperative (days; median; IQR) | 1; 1–2.5 | 1; 1–2 | 0.71 |
| ICU stay postoperative (days; median; IQR) | 2; 1–7.5 | 3; 2–7.75 | 0.44 |
| Total hospital stay postoperative (days; median; IQR) | 23; 21–33 | 23; 21–35 | 0.73 |
Postoperative characteristics
Median postoperative length of mechanical ventilation [PAE group: 1 (1–2.5) vs. NonPAE group: 1 (1–2) days, P = 0.71] as well as ICU [PAE group: NonPAE group: 3 (2–7.75) days, P = 0.44) and total hospital stay [PAE group: 23 (21–33) vs. NonPAE group: 23 (21–35) days, P = 0.73] was similar in both groups. Incidence of primary graft dysfunction grade 2 or 3 (ISHLT classification) on arrival at ICU (T0: 28.0% vs. 34.0%, P = 0.66), at 24 h (T24: 20.0% vs. 17.8%, P = 0.79), 48 h (T48: 16.0% vs. 17.9%, P = 0.99) and 72 h (T72: 12.0% vs. 14.8%, P = 0.79) after surgery was similar in both groups.
Recipients of PAE-donor lungs required more often intra- and post-operative ECMO support (12.0% vs. 3.6%, P = 0.06). However, 8% of these recipients received a per-protocol ECMO for postoperative LV remodeling since Pulmonary Arterial Hypertension was identified as the underlying disease 20. Also, more patients required de-novo ECMO therapy postoperative in the absence of intraoperative extracorporeal support as a result of primary graft dysfunction (n = 2) or hemorrhagic shock (n = 1; Table 4). Right ventricular dysfunction or elevated pulmonary arterial pressure was not detected in the recipient cohort.
Postoperative lung function results
FEV1 (% predicted) values at hospital discharge (69.9 ± 20.9% vs. 67.7 ± 21.1%, P = 0.46), at 1 year after transplantation (100.6 ± 21.2% vs. 83.9 ± 25.8%, P = 0.003) as well as at last outpatient control (78.1 ± 34.0% vs. 69.2 ± 32.8%, P = 0.05) were better in the PAE than the nonPAE cohort. Last FEV1% predicted values were assessed after a median time after transplantation of 33.6 and 35.3 months, respectively (Fig. 2a).
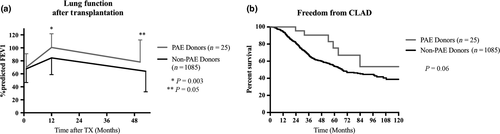
Recipients of PAE-donor lungs show a CLAD-free survival of 70.4% at 5 years after transplantation, whereas recipients of nonPAE-donor lungs have a CLAD-free survival of 55.1% at 5 years (Fig. 2b).
Donor age case matched analysis revealed no difference in postoperative spirometry results or CLAD-free survival between the two groups.
Postoperative survival
In-hospital mortality was higher in recipients from PAE-donors (12.0% vs. 7.6%, P = 0.43), although not statistically significant. The first patient, a 37 year old cystic fibrosis patient required mechanical ventilation and ECMO support prior to transplantation, was transplanted on CPB requiring massive blood transfusion and subsequently three re-thoracotomies as well as postoperative dialysis because of kidney failure. The initial primary graft dysfunction may be related to the preoperative state of the recipient and possible in parts to the donor organ, which was from a 37 years old female donor, who was on mechanical ventilation for 7 days following CPR for massive PAE. The recipient died on POD 26 as a result of sepsis and multi-organ failure. The second patient, a 45 year old patient with Sarcoidosis, underwent lung transplantation uneventfully but developed severe graft failure only hours after surgery requiring urgent veno-venous ECMO therapy. T- and B-Cell crossmatches were positive and the patient died on POD 1 because of severe rejection despite all initiated treatment efforts (e.g. Plasma exchange). The donor was a 36 year old female with a positive history of smoking, who was resuscitated for PAE 3 days prior to the organ procurement showing an impaired oxygenation index (PaO2: 273 mmHg; FiO2 1.0, Peep 5 mmHg) at procurement. The third patient, a 54-year-old IPF patient underwent lung transplantation and was weaned off mechanical ventilation. The patient then developed Status Epilepticus and died on POD 22 as a result of cerebral edema. The donor was a 41 year old female patient with good oxygenation (PaO2: 324 mmHg; FiO2 1.0, Peep 5 mmHg), who was on mechanical ventilation for 4 days, negative smoking history and central PAE at the time of hospital admission prior to lysing therapy. This organ was preserved using the ex vivo lung perfusion and showed no functional deficits during this period, leading to the decision to use the organ for transplantation.
However, the overall 30-day (88.0% vs. 96.2%) as well as 1- (88.0% vs. 87.3%) and 5-year (59.8% vs. 64.3%) survival was similar in both groups (P = 0.38; Fig. 3), this finding was confirmed in the donor age case matched analysis.

Discussion
Donor scarcity in western countries has been recognized in the past decades as a major burden in transplantation medicine, leading to a more extensive utilization of extended criteria donor organs. In lung transplantation, upon utilizing extended criteria lungs, careful donor and recipient selection has repeatedly shown to lead to similar outcomes as compared to utilizing standard donor lungs 3, 7, lowering the threshold for routine use of nonideal donors.
Structural defects such as fibrotic or bullous parenchymal changes in donor lungs remain unacceptable for lung donation. However, PAE represents a different pathogenicity to the organ. Thrombo-emboli in the pulmonary arteries cause an inflow obstruction to the lung leading to subsequent afterload increase of the right ventricle and right heart failure. Even large thrombi that cause total or subtotal occlusion of a lung do not lead to lung tissue alterations or ischemia, given a normal bronchial arterial blood supply is maintained. Therefore, presuming a complete removal of the thrombi is feasible, these organs may be considered to function as good as nonemboli organs 13.
However, efforts to achieve a complete removal of residual thrombemboli in these donors should include antegrade and retrograde flush of the donor lung, yet no recommendations with larger series on perfusion strategies in lung donors with known pulmonary arterial embolism are published to our knowledge. The Melbourne group reporting on the incidence of unexpected thrombemboli in lung donors used a retrograde flush with 50–70 ml saline in each lung vein 22. In donors without known PAE, retrograde flush of the lungs has no benefit as compared to standard antegrade flush 23. Similar findings were published by Ferraro et al. 24, finding no significant decrease in primary graft dysfunction when performing a retrograde flush immediately before implantation. Moreover, in our experience, the macroscopic inspection of the pulmonary arteries after separation of the right and left lung using long forceps is equally important, allowing the recovery of small emboli that are adhered to the endothelium of the pulmonary vasculature.
Given the comparable survival as well as superior spirometry outcome in our reported data, a persistent structural defect in the donor lungs that would impair the recipient's postoperative outcome is not present. Thus, these organs utilized for lung transplantation are in majority young donor organs with a lower incidence of smoking history as compared to the control cohort (Table 2). The donor age matched case control analysis supported this finding, showing similar spirometry as well as CLAD-free and overall survival in a sub-cohort of younger donors.
Over the past 5 years, the acceptance rate of lungs that were reported to Eurotransplant has been stable between 53% and 57% 1. However, the acceptance rate of lungs from donors with known pulmonary embolism has been low in the past with only approx. 35% of the lungs found suitable for transplantation in our analysis. A vast majority of lungs were not even reported to Eurotransplant for potential donation by the transplant coordinator. Only three lungs of the reported donor cohort were rejected by transplant surgeons upon macroscopic evaluation. Cautiousness in selecting donor lungs from this sub-cohort of nonideal donors remains important. Oto et al. 22 reported on the high incidence (38%) of unexpected thromboembolism in donor lungs and the linked postoperative complications. Unexpected donor thrombemboli (clot and fat-emboli) caused significant oxygenation impairment, elevated pulmonary vascular resistance as well as longer postoperative mechanical ventilation and intensive care stay in the recipients as compared to a control cohort. Interestingly, although the group performed a retrograde flush of these lungs for the detection of emboli, the overall outcome was impaired. Of note, the highest risk for undiagnosed pulmonary embolism prior to organ retrieval was found in donors after trauma, suggesting other confounding causes for potential graft impairment, such as lung contusion. In a second analysis of unexpected donor emboli, the risk of developing primary graft dysfunction was significantly higher in transplant recipients receiving lungs from donors with fat-emboli, suggesting a more deleterious effect of fat-emboli, which are commonly seen in traumatized donors 25. These findings are in line with multiple case reports on fat embolism in donor lungs leading to severe graft dysfunction with often fatal outcomes 26-28. The major difference of the herein described donor cohort as compared to the cohort of Oto et al. is the initial diagnosis of pulmonary arterial embolism upon admission to the hospital. All donors, that have been diagnosed with pulmonary embolism also received specific treatment upon admission to hospital (e.g. thrombolytic therapy) prior to fatal diagnosis of brain death. These therapeutic steps have not completely dissolved all emboli, since some were still detectable during lung retrieval. However, the diagnosis of emboli most likely led to a therapy focused on pulmonary embolism (e.g. anticoagulation) and therefore minimized the lung volume with compromised perfusion.
Results of our analysis indicate that utilization of lungs from donors with known pulmonary arterial embolism does not lead to a higher incidence of primary graft failure, prolonged mechanical ventilation or intensive care stay when efforts are made perioperatively to completely remove any residual thrombemboli from the lung vasculature. In contrast, recipients of PAE-donor lungs show a significant better lung function as compared to recipients of nonPAE-donor lungs. Moreover, CLAD-free survival up to 5 years after transplantation was lower in lungs from PAE-donors. An explanation for these findings might be found in the donor demographics. Donor organs from donors with PAE that were accepted for lung transplantation were significantly younger and showed a better oxygenation capacity than PAE-donors that were either not reported for lung donation or rejected by transplant surgeons 29. Younger PAE-donors most likely had few other major risk factor besides pulmonary embolism, as suggested by the lower percentage of smoking history, incidence of contusion and the excellent oxygenation capacity. This is even more important, since lung allocation was not directed towards low-risk recipients. The PAE cohort showed a similar distribution of underlying diseases, incidence of preoperative ICU admission, extracorporeal therapy and mechanical ventilation as the nonPAE cohort.
The overall question that remains is which PAE lung is acceptable for transplantation and what characteristics suggest to reject the donor lung. The PAE-donors, that were either not reported or rejected for lung transplantation, were significantly older and showed a lower oxygenation index than utilized PAE-donor lungs. The possible outcome of these older organs after transplantation remains speculative. However, mean FiO2/PaO2 was >300, duration of mechanical ventilation was lower and incidence of smoking history similar to the group that donated lungs, suggesting that some of these organs may have been of acceptable quality for transplantation. The decision of which organ is suitable for transplantation and which organ should be rejected remains up to the procuring surgeon, however, we recommend to evaluate the number of risk factors associated with each donor. We consider advanced age (>65 years), impaired oxygenation capacity after recruitment in the OR and macroscopic evaluation of the organ during procurement, history of smoking and structural defects (contusion, inflammatory processes) as relevant risk factors and would be cautious if two or more additional risk factors are present.
Study limitations
The data displays the usual limitations of a retrospective, single-center analysis. The cohort size of recipients of PAE-donor lungs is small, rendering statistical analysis of long-term outcome data difficult. Also, detailed donor data was not accessible in all cases.
Conclusion
In conclusion, lung transplantation from donors with the initial diagnosis of pulmonary arterial embolism can be performed without compromising outcomes of recipients. Donors with the diagnosis of PAE should be reported for organ allocation as potential lung donors, since the final decision of transplantability can only be made during final macroscopic assessment by the procuring surgeon.
Authorship
WS, HK, FI, AH, IT and GW: designed the study, performed the research, analyzed data and wrote the paper. JS, TS, DB, MA, CK MG, JG, AR and TW: collected data and critically revised the manuscript.
Funding
The authors have declared no funding.
Conflicts of interest
The authors have declared no conflicts of interest.




