Tranexamic acid is associated with post-injury mortality in a resource-limited trauma system: Findings from the epidemiology and outcomes of prolonged trauma care cohort study
The findings, views, and conclusions presented are those of the authors and do not represent the official policy or position of the US Department of Defense or the Western Cape Government.
Abstract
Background
Injury-related deaths claim millions of lives annually, with severe hemorrhage a leading cause. This study assesses tranexamic acid (TXA) administered within 3 h post-injury on mortality in trauma patients.
Study Design And Methods
We conducted secondary database analysis of EpiC, a multicenter, prospective cohort of trauma patients in South Africa. We compared mortality between severely injured patients at risk for traumatic hemorrhage receiving TXA within 3 h post-injury versus untreated patients. Inverse probability of treatment weighting adjusted for confounders, and multivariate logistic regression assessed 24-h mortality, with extended secondary outcome analyses.
Results
Of 3607 analyzed patients, 502 received TXA within 3 h. TXA reduced 24-h mortality by 38% (marginal odds ratio [mOR], 0.62; 95% confidence interval [CI], 0.49–0.78) versus untreated patients. Similar reductions were observed for longer-term mortality. Subgroup analyses revealed reduced mortality when TXA was given within 2 h post-injury (mOR, 0.57; 95% CI, 0.45–0.73), doses of 1 g of TXA within 3 h (mOR, 0.73; 95% CI, 0.56–0.94), and those with the highest risk of hemorrhage (mOR, 0.40; 95% CI, 0.30–0.53). The 24-h mortality reduction was significant for patients with penetrating injury (mOR, 0.58; 95% CI, 0.43–0.78) but not for blunt injury patients. Sensitivity analyses confirmed the robustness of these findings, with TXA consistently reducing mortality odds by 28%–39% across subgroups.
Discussion
Early TXA administration significantly reduced mortality in trauma patients, especially with penetrating injuries and those with the highest risk of hemorrhage. One-gram dosing was as effective as higher doses, and mortality reduction was notable when TXA was given within 2 h post-injury. These findings support TXA use in resource-limited trauma protocols.
1 INTRODUCTION
Trauma-related deaths claim 4.4 million lives each year worldwide with unintentional injuries causing 3.2 million deaths and violence-related injuries responsible for another 1.3 million deaths.1, 2 Uncontrolled bleeding is the leading preventable cause of death among trauma victims.3, 4 Among those with major bleeding, the risk of mortality rises due to the “lethal triad of trauma,” which includes coagulopathy, hypothermia, and acidosis. Treating trauma-induced coagulopathy is particularly challenging and continues to be an area of intense investigation by the global trauma research community.5, 6 The lethal triad, along with a host of other pathologic hyperimmune responses during trauma, collectively termed trauma-induced coagulopathy, result in physiologic perturbations that increase post-injury morbidity and mortality.7
Hyperfibrinolysis, the pathologic breakdown of the fibrin mesh that stabilizes blood clots, is a major physiologic driver of trauma-induced coagulopathy and death.5, 8, 9 Tranexamic acid (TXA), a synthetic derivative of the amino acid lysine, competitively inhibits the enzymatic breakdown of fibrin blood clots and preserves hemostatic clots.10-12 TXA has been useful in controlling excessive bleeding during various medical conditions and hemorrhage-prone surgical procedures. Additionally, TXA's ability to decrease fibrinolysis helps blunt the inflammatory response associated with hemorrhagic shock, potentially reducing the risk of multiple organ dysfunction.13, 14 TXA is also commonly used for minimizing and managing active hemorrhage during operative procedures with anticipated heavy blood loss.10, 14, 15
The existing literature on the impact of TXA for post-injury mortality generally demonstrates that TXA improves survival, but several reports demonstrate mixed results.16-19 A recent systematic review and bias-adjusted meta-analysis of randomized controlled trials suggests that TXA use for trauma leads to a reduction in 24-h and 30-day mortality.20 A few other studies exist that either did not find or were uncertain about an association between TXA treatment and mortality.21-26 Some studies reported that, among civilian adults hospitalized with traumatic hemorrhagic shock, those treated with TXA had a significant mortality reduction at 28 days, but not at 24 or 48 h, while others conversely reported an early survival advantage that is not maintained over the ensuing 6 months.27, 28 These varied findings may be partially attributable to study design and administration of TXA in patient populations with varying risk for fibrinolysis.29
In line with efficacy studies, several observational studies have investigated the impact of timing and dosing of TXA. While some studies demonstrate that TXA reduces bleeding and mortality from hemorrhage, its effectiveness is closely tied to the timing of treatment.30-32 Administering a “full” dose of TXA (i.e., completing a 1-g loading bolus and initiating a 1-g infusion) within 3 h of injury significantly lowers the risk of death from bleeding, whereas starting treatment after 3 h may be ineffective or even harmful. Several pharmacologic and clinical investigations suggest that 1 g of TXA may be as effective as a full conventional 2-g TXA dose.33, 34 Further evidence regarding the effectiveness of TXA is needed, including studies from diverse patient populations and regions of the world experiencing the highest trauma mortality with limited access to surgical care, such as low- and middle-income countries (LMICs).
The purpose of this study is to assess the association between TXA administration within 3 h of injury and subsequent 24-h mortality among severely injured patients with extra-cranial injuries with, or at risk for, major hemorrhage. This study uses secondary data from a prospective, multicenter cohort study in an LMIC population. Specifically, the study targets individuals with hemorrhage or those with a heightened risk of hemorrhagic shock to determine whether TXA treatment administered within 3 h from the time of injury has a significant effect on mortality.
2 METHODS
2.1 Study design and setting
This study is a secondary analysis of data collected in the “The Epidemiology and Outcomes of Combat-Relevant Prolonged Trauma Care (EpiC)” study. EpiC is a prospective, multicenter prehospital observational study in the Western Cape of South Africa aimed at advancing the understanding of epidemiology and outcomes of major trauma patients, with a heavy focus on hemorrhage. The EpiC study evaluates how the delivery and timing of life-saving interventions (LSIs) affect mortality and morbidity outcomes. The Western Cape emergency care system experiences among the world's highest burden of trauma with consequences which include ambulance delays to facility arrival, limited access to subspecialist and surgical care, and delayed access to radiology.35 The resource-limited context of the EpiC study has been separately reported.35-37 Ethics approval was received with a waiver of informed consent from the Health Research Ethics Committee at Stellenbosch University, the primary IRB (Project ID 14866; Ref. No. N20/03/036). The University of Colorado ceded oversight to Stellenbosch (Protocol 20-2176). The Defense Health Agency Office of Human Research Oversight provided second-level review and concurrence (OHRO Log Number E01863.1×).
2.2 Patient selection
EpiC includes adult trauma patients (≥18 years of age) who sustained injuries within 24 h prior to their first contact with the healthcare system and who were alive (i.e., had signs of life or attempted resuscitation) upon ambulance or hospital contact. Exclusions include prisoners, pregnant women, or individuals with trauma secondary to burns, hangings, or strangulation, drowning, envenomation, electrocution, and bites/stings. Those with substantial missing data or lost records were withdrawn from the study.
The target sub-cohort for this secondary analysis was severely injured patients from the EpiC database who had blunt or penetrating trauma with active hemorrhage or at heightened risk of hemorrhage, defined as systolic blood pressure (SBP) ≤ 100 mmHg or shock index ≥1.0 (either during primary emergency medical services (EMS) response or upon hospital arrival) or met one of the following criteria within 24 h of hospital arrival: initial lactate ≥4.0, initial base deficit ≥6, initial pH ≤ 7.2, or Sequential Organ Failure Assessment (SOFA) cardiovascular score ≥3. Patients were excluded if they had a head injury with an abbreviated injury scale (AIS) severity of 3 or more (i.e., severe traumatic brain injury).
2.3 Intervention/exposure and outcomes
The primary exposure group consisted of patients who received any intravenous dose (1 or 2 g) of TXA within 3 h of injury. The comparison group comprised patients who did not receive TXA at any point following the time of injury through their hospital stay. The study period was from August 2021 to July 2024. The primary outcome was all-cause in-hospital mortality within 24 h of injury. The secondary outcomes were 48-h, 7-day, and 30-day all-cause in-hospital mortality.
2.4 Statistical analysis
In the selected cohort, patient demographics, injury profiles, and clinical characteristics were descriptively analyzed by treatment group (TXA-treated vs. untreated). Continuous variables were summarized as means and standard deviations (SD), while categorical variables were reported as frequencies and percentages. To reduce confounding, common in observational studies, we applied inverse probability of treatment weighting (IPTW) based on propensity scores (PS). The PS represents the conditional probability of receiving TXA within 3 h of injury, given covariates. To estimate the average treatment effect (ATE), patients were weighted by the inverse of their PS estimates, mitigating confounding.38, 39 The covariates in the PS model included sex, age, injury force type (blunt vs. penetrating), mechanism of injury, shock index, New Injury Severity Score (NISS), initial Glasgow Coma Scale (GCS), and blood product volumes during the first 24 h (whole blood, packed red blood cells or platelets, plasma, freeze-dried plasma, or cryoprecipitate).40, 41 Key assumptions for PS modeling included the Stable Unit Treatment Value Assumption (SUTVA), no unmeasured confounders, and positivity, that is, all patients had a nonzero probability of receiving TXA or remaining untreated.42 To enhance accuracy, we optimized PSweights by trimming extreme PS values, following Crump et al. (2009), and reestimated PS for the remaining cohort to avoid model misspecification.43-46 Covariate balance between TXA-treated and untreated groups was assessed using weighted standardized mean differences (SMDs), considering balance achieved when SMD < 10%.
A multivariable logistic regression was fitted to the weighted cohort to assess differences in 24-h mortality between patients treated with TXA within 3 h of injury and untreated patients. A double adjustment approach, using all covariates from the PS model, enhanced precision and ensured robustness as doubly robust estimators while alleviating residual confounding from potential PS model misspecification.47, 48 Results are presented as marginal odds ratios (mOR) with 95% confidence intervals [CIs], using g-methods.49, 50 Secondary outcomes for 48-h, 7-day, and 30-day mortality followed the same approach.
2.5 Subgroup analyses
The effects of TXA on outcomes in specific sub-cohorts were examined. Outcomes for patients treated within 2 and 1 h of injury were compared to those of untreated patients. To account for dosage, a total of 1 g of TXA given within 3 h of injury was additionally compared to untreated patients. Patients with the highest risk for hemorrhage (SBP ≤ 90 mmHg or shock index ≥1.2) were analyzed, with those treated with 3-h TXA compared to untreated patients. A sub-cohort of patients without any blood product transfusion was also analyzed, comparing those who received TXA within 3 h of injury to untreated patients. Further, the 3-h TXA treatment effect by injury force type was also assessed. Finally, in the TXA-treated cohort, we examined the impact of treatment dosage by comparing the administration of 1 versus 2 g within 3 h of injury. Each sub-cohort was re-weighted by the inverse of its reestimated propensity scores.
2.6 Sensitivity analyses
Three sensitivity analyses were conducted to assess the robustness of the primary statistical method. First, we analyzed the full cohort before PS modeling. Second, we repeated PS modeling and IPTW without trimming. Third, as an alternative to the IPTW method, we applied propensity score matching (PSM) using an optimal full matching method. Primary and secondary outcomes were evaluated in all sensitivity analyses.51, 52
All analyses were performed using R (version 4.3.3)53 with the PSweight,54 MatchIt,55 and marginal effects56 packages for weighting, matching, covariate balance, and marginal effect estimation. Tests were conducted at a 5% significance level.
3 RESULTS
A total of 4007 eligible patients met the cohort inclusion criteria, but 12 patients (0.3%) had missing values for one or more key covariates (missing at random) and were subsequently excluded from the analysis (Figure 1). The remaining 3995 patients, including 502 (13%) who received TXA within 3 h of injury and 3493 (87%) who did not receive any TXA, were included in the PS model and IPTW computation (see Tables S1 and S2). Following the IPTW trimming approach, 388 untreated patients were excluded.
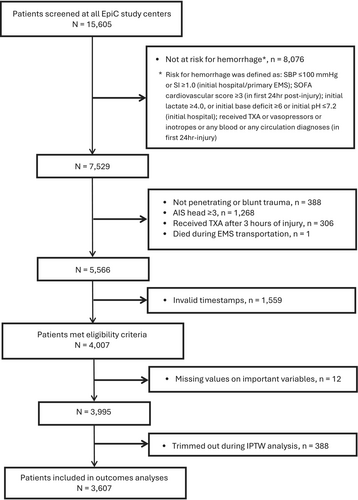
Table 1 provides the demographic, injury, and clinical characteristics of the weighted cohort (N = 3607) by treatment group. IPTW effectively balanced the distribution of key covariates between the treated and untreated groups (SMDs < 10% for all covariates). Overall, the average age of patients was 32.9 years (SD, 10.3 years), with 83.9% being male. Penetrating injuries comprised 72.3% of the cohort; 15.9% of the cohort had wounds. About 62% of patients had an NISS of <9 (mild), while 12.9% and 25.1% had an NISS of 9–15 (moderate) and >15 (severe), respectively. In terms of blood product transfusions, 3.1% of patients received whole blood, 11% received packed red blood cells or platelets, and 15.2% received plasma, freeze-dried plasma, or cryoprecipitate. Patients in the TXA group received significantly more blood products than individuals in the non-TXA groups. Among patients who received TXA within 3 h (n = 502), the average time from injury to initial TXA administration was 1.64 h (SD: 0.76 h). Regarding TXA dosage, 76.1% received 1 g of TXA, and 23.9% received 2 g (i.e., 1-g initial bolus followed by initiation of a 1-g infusion).
| Characteristics | No TXA | TXA (3 h) | SMD |
|---|---|---|---|
| N = 3105 | N = 502 | ||
| Sex, male, n (%) | 2564 (82.6) | 462 (92.0) | 0.01 |
| Age (in years), mean (SD) | 33.0 (10.4) | 31.9 (9.1) | 0.05 |
| Injury force type, n (%) | |||
| Penetrating | 2170 (69.9) | 437 (87.1) | 0.01 |
| Blunt | 726 (23.4) | 43 (8.6) | 0.01 |
| Blunt + Penetrating | 209 (6.7) | 22 (4.4) | <0.01 |
| Injury mechanism, n (%) | |||
| Stabbing/cut | 1835 (59.1) | 263 (52.4) | 0.04 |
| Firearm/gunshot | 394 (12.7) | 180 (35.9) | 0.03 |
| Vehicular | 368 (11.9) | 35 (7.0) | 0.02 |
| Struck/other | 508 (16.4) | 24 (4.8) | 0.01 |
| Shock index, mean (SD) | 0.89 (0.29) | 0.89 (0.36) | 0.09 |
| NISS, categorized, n (%) | |||
| Minor, (<9) | 2028 (65.3) | 210 (41.8) | 0.06 |
| Moderate, (9–15) | 399 (12.9) | 66 (13.1) | <0.01 |
| Severe, (>15) | 678 (21.8) | 226 (45.0) | 0.07 |
| Initial hospital GCS, categorized, n (%) | |||
| Severe (<8) | 83 (2.7) | 25 (5.0) | 0.01 |
| Moderate (9–12) | 79 (2.5) | 34 (6.8) | <0.01 |
| Mild (13–15) | 2897 (93.3) | 430 (85.7) | <0.01 |
| Missing | 46 (1.5) | 13 (2.6) | <0.01 |
| Initial lactate, mean (SD) | 4.42 (3.49) | 5.66 (4.32) | 0.05 |
| Initial pH, mean (SD) | 7.31 (0.14) | 7.28 (0.14) | 0.04 |
| Initial hosp. heart rate, mean (SD) | 93.38 (22.19) | 91.92 (22.35) | <0.01 |
| Any circulation LSI, n (%) | 598 (19.3) | 71 (14.1) | 0.01 |
| Whole blood (mL) | |||
| None | 3028 (97.5) | 467 (93.0) | 0.01 |
| (0, 500) | 26 (0.8) | 12 (2.4) | 0.01 |
| (500, 1000) | 32 (1.0) | 15 (3.0) | <0.01 |
| >1000 | 19 (0.6) | 8 (1.6) | <0.01 |
| PRBC and/or platelets (mL) | |||
| None | 2878 (92.7) | 332 (66.1) | 0.02 |
| (0, 500) | 92 (3.0) | 65 (12.9) | 0.01 |
| (500, 1500) | 113 (3.6) | 79 (15.7) | 0.01 |
| >1500 | 22 (0.7) | 26 (5.2) | <0.01 |
| FDP, or plasma, or cryoprecipitate (mL) | |||
| None | 2783 (89.6) | 274 (54.6) | 0.02 |
| (0, 300) | 113 (3.6) | 80 (15.9) | 0.01 |
| (300, 600) | 141 (4.5) | 94 (18.7) | 0.01 |
| >600 | 68 (2.2) | 54 (10.8) | <0.01 |
| Time to TXA receival (h), mean (SD) | 1.64 (0.76) | ||
| TXA dose, for the treated group | |||
| 1 g, n (%) | 382 (76.1) | ||
| 2 g, n (%) | 120 (23.9) | ||
- Abbreviations: FDP, freeze-dried plasma; GCS, glasgow coma scale; LSI, life-saving intervention; NISS, new injury severity score; pH, potential of hydrogen; PRBC, packed red blood cells; SMD, standardized mean difference; TXA, tranexamic acid.
The results from the covariate-adjusted weighted logistic regression models for both primary and secondary outcomes, expressed as marginal effects, are summarized in Table 2. The group treated with TXA had a 38% lower odds of 24-h all-cause mortality compared to the untreated group (mOR, 0.62; 95% CI, 0.49–0.78). This significant reduction in all-cause mortality was similarly observed for secondary outcomes, including 48-h mortality (mOR, 0.57; 95% CI, 0.42–0.77), 7-day mortality (mOR, 0.61; 95% CI, 0.45–0.83), and 30-day mortality (mOR, 0.63; 95% CI, 0.48–0.82). Detailed results from the adjusted logistic regression models for these outcomes are provided in Table S3. Further, recognizing the importance of early time points in hemorrhage control, we analyzed TXA's impact at 6- and 12-h post-injury. While the 6-h reduction in mortality odds (mOR, 0.73; 95% CI, 0.49–1.09) was not significant, the 12-h reduction by 35% (mOR, 0.65; 95% CI, 0.49–0.86) was significant, as presented in Table S4.
| Death/N (%)a | mORb (95% CI) | p-value | |
|---|---|---|---|
| Primary outcome: 24 h mortality | |||
| Untreated | 69/3105 (2.2) | 0.62 (0.49, 0.78) | <.001 |
| Treated | 27/502 (5.4) | ||
| Secondary outcomes | |||
| 48-h mortality | |||
| Untreated | 78/3105 (2.5) | 0.57 (0.42, 0.77) | <.001 |
| Treated | 30/502 (6.0) | ||
| 7-day mortality | |||
| Untreated | 95/3105 (3.1) | 0.61 (0.45, 0.83) | .002 |
| Treated | 35/502 (7.0) | ||
| 30-day mortality | |||
| Untreated | 108/3105 (3.5) | 0.63 (0.48, 0.82) | <.001 |
| Treated | 39/502 (7.8) | ||
- Abbreviation: CI, confidence interval.
- a Ratio from the raw dataset (without weighting).
- b mOR: marginal odds ratio from IPTW weighted, covariate-adjusted models.
The subgroup analyses largely aligned with the primary analysis, showing considerably lower all-cause mortality odds among patients who received TXA, except in two cohorts: those treated within 1 h of injury and those with blunt trauma. As per Table 3A, patients receiving TXA within 2 h of injury showed 43% lower odds of 24-h mortality compared to untreated patients (mOR, 0.57; 95% CI, 0.45–0.73), while those treated within 1 h showed no significant difference (mOR, 0.85; 95% CI, 0.68–1.07), likely due to the small size of the treated group, with less than 5% receiving TXA within this time frame in the weighted cohort. Patients receiving 1 g of TXA within 3 h were associated with lower 24-h mortality odds compared to untreated patients (mOR, 0.73; 95% CI, 0.56–0.94). Among patients with the highest risk of hemorrhage, TXA within 3 h was associated with a 60% lower 24-h mortality (mOR, 0.40; 95% CI, 0.30–0.50). In the no-blood-products subgroup, mortality was rare among TXA-treated patients, with no deaths at 24 h, 48 h, or 7 days, and one death at 30 days, preventing statistical modeling due to complete separation and insufficient events (Table S5). Regarding injury type, patients with penetrating injuries receiving TXA within 3 h showed lower 24-h mortality odds (mOR, 0.58; 95% CI, 0.43–0.78), while those with blunt injuries showed no significant difference (mOR, 0.83; 95% CI, 0.50–1.38), noting that patients with both injury types were excluded due to small sample size (Table 3B). These mortality reduction trends persisted across 48-h, 7-day, and 30-day intervals. Among TXA-treated patients, no statistically significant difference in mortality odds was observed between those receiving 1 and 2 g of TXA, as shown in Table 3B.
| Subgroup analysis 1a | Subgroup analysis 2b | Subgroup analysis 3c | Subgroup analysis 4d | |||||
|---|---|---|---|---|---|---|---|---|
| mOR (95% CI) | p-value | mOR (95% CI) | p-value | mOR (95% CI) | p-value | mOR (95% CI) | p-value | |
| Primary outcome: 24 h mortality | ||||||||
| Treated versus untreated | 0.57 (0.45, 0.73) | <.001 | 0.85 (0.68, 1.07) | .165 | 0.73 (0.56, 0.94) | .016 | 0.40 (0.30, 0.53) | <.001 |
| Secondary outcomes | ||||||||
| 48-h mortality | ||||||||
| Treated versus untreated | 0.56 (0.42, 0.75) | <.001 | 0.80 (0.60, 1.06) | .124 | 0.66 (0.48, 0.89) | .006 | 0.42 (0.26, 0.67) | <.001 |
| 7-day mortality | ||||||||
| Treated versus untreated | 0.76 (0.55, 1.06) | .101 | 0.81 (0.60, 1.09) | .169 | 0.73 (0.55, 0.98) | .034 | 0.38 (0.21, 0.67) | <.001 |
| 30-day mortality | ||||||||
| Treated versus untreated | 0.77 (0.59, 1.00) | .053 | 0.71 (0.54, 0.93) | .015 | 0.72 (0.56, 0.94) | .016 | 0.38 (0.20, 0.70) | .002 |
- Abbreviations: CI: confidence intervals; mOR: marginal odds ratio.
- a Patients treated with TXA within 2 h of injury versus no TXA treatment.
- b Patients treated with TXA within 1 hour of injury versus no TXA treatment.
- c Patients who received 1 g of TXA within 3 hours of injury versus no TXA treatment.
- d Patients with the highest risk of hemorrhage (defined as systolic blood pressure /SBP/≤90 mm Hg or shock index /SI/≥1.2).
| Subgroup analysis 6a | Subgroup analysis 7b | ||||||
|---|---|---|---|---|---|---|---|
| Penetrating injury | Blunt injury | ||||||
| mOR (95% CI) | p-value | mOR (95% CI) | p-value | mOR (95% CI) | p-value | ||
| Primary outcome: 24-h mortality | |||||||
| Treated versus untreated | 0.58 (0.43, 0.78) | <.001 | 0.83 (0.50, 1.38) | .473 | 2 g versus 1 g of TXA | 1.18 (0.65, 2.14) | .593 |
| Secondary outcomes | |||||||
| 48-h mortality | |||||||
| Treated versus untreated | 0.58 (0.40, 0.84) | .004 | 0.59 (0.32, 1.10) | .099 | Treated versus untreated | 1.14 (0.84, 1.56) | .400 |
| 7-day mortality | |||||||
| Treated versus untreated | 0.57 (0.37, 0.86) | .007 | 0.86 (0.47, 1.58) | .629 | Treated versus untreated | 0.99 (0.69, 1.42) | .946 |
| 30-day mortality | |||||||
| Treated versus untreated | 0.62 (0.44, 0.87) | .006 | 0.78 (0.45, 1.32) | .352 | Treated versus untreated | 1.01 (0.70, 1.46) | .962 |
- Abbreviations: CI, confidence intervals; mOR, marginal odds ratio.
- a Injury type (penetrating versus blunt) by TXA treatment interaction.
- b 2 g of TXA versus 1 g of TXA within 3 h of injury.
Regarding the sensitivity analyses, results were largely consistent with the primary analysis, supporting the robustness of the IPTW-weighted with trimming approach. Patients' baseline characteristics in each of the three sensitivity analyses are provided in Table S6. Table 4 summarizes the marginal treatment effects on primary and secondary outcomes for each of the three sensitivity analyses. In the first analysis, considering the full cohort without covariate balancing, patients treated with TXA within 3 h showed lower, though not statistically significant, 24-h mortality odds compared to untreated patients (mOR, 0.85; 95% CI, 0.64–1.13), likely due to covariate imbalance. The second analysis, using untrimmed IPTW, yielded results closely aligned with the primary analysis (mOR, 0.61; 95% CI, 0.48–0.77 for 24-h mortality), indicating that excluding extreme PS estimates did not impact the weighting process or the treatment effect estimation. Finally, the PSM with optimal full matching produced marginal odds ratio estimates for primary and secondary outcomes closely comparable to the primary analysis, with TXA administration within 3 h associated with 28% lower odds of 24-h mortality (mOR, 0.72; 95% CI, 0.56–0.91). Ratios of raw mortality counts by treatment group in each of these sensitivity analyses can be found in Table S7.
| Sensitivity analysis 1a | Sensitivity analysis 2b | Sensitivity analysis 3c | ||||
|---|---|---|---|---|---|---|
| mOR (95% CI) | p-value | mOR (95% CI) | p-value | mOR (95% CI) | p-value | |
| Primary outcome: 24-h mortality | ||||||
| Treated versus untreated | 0.85 (0.64, 1.13) | .269 | 0.61 (0.48, 0.77) | <.001 | 0.72 (0.56, 0.91) | .007 |
| Secondary outcomes | ||||||
| 48-h mortality | ||||||
| Treated versus untreated | 0.78 (0.56, 1.08) | .139 | 0.56 (0.41, 0.76) | <.001 | 0.67 (0.50, 0.90) | .008 |
| 7-day mortality | ||||||
| Treated versus untreated | 0.74 (0.53, 1.02) | .066 | 0.64 (0.47, 0.88) | .006 | 0.64 (0.47, 0.85) | .002 |
| 30-day mortality | ||||||
| Treated versus untreated | 0.74 (0.54, 1.00) | .047 | 0.66 (0.50, 0.86) | .002 | 0.62 (0.48, 0.81) | <.001 |
- Abbreviations: CI, confidence interval; mOR, marginal odds ratio.
- a Full cohort, without any weighting.
- b IPTW weighted cohort, but with no trimming.
- c Propensity score optimal full matched cohort.
4 DISCUSSION
This multicenter observational study contributes additional evidence in support of TXA, as advocated for by the US National Trauma Research Action Plan and trauma experts worldwide.57 We observed an association of a 38% reduction in odds of 24-h all-cause in-hospital mortality among severely injured South African patients with, or at heightened risk for, extra-cranial hemorrhage who received TXA within 3 h of injury versus those who did not. Consistent mortality benefits were also observed across secondary endpoints, subgroup analyses, and sensitivity analyses, underscoring a robust association between TXA administration within 3 h and lower mortality. Notably, while the mortality benefit remained significant for patients with penetrating injuries, it did not reach significance among those with blunt trauma. This study uniquely extends the evidence for TXA to trauma populations experiencing a high proportion of penetrating trauma (72% in our cohort) and within resource-limited settings where access to blood products and timely surgical care are limited. Importantly, the mortality benefit from TXA was independent of blood products or other life-saving interventions. Notably, the median time from injury to TXA administration was 1.6 h, and a 1-g dose was given in the majority (76%) of cases.
We acknowledge that the association with mortality reduction in our study is higher than that reported in other observational and interventional studies. A recent meta-analysis of seven randomized controlled trials found that TXA reduced death risk by 24% at 24 h and 11% at 1 month.20 In the older CRASH-2 randomized placebo-controlled trial, TXA significantly reduced mortality—TXA given within 1 h of injury decreased bleeding death risk by 32%, and by 21% if administered between 1 and 3 h.58 Further, the MATTERs Study, a retrospective analysis of wartime injuries, similarly showed lower in-hospital mortality of TXA versus no TXA patients (17.4% vs. 23.9%, respectively), a 27% decrease in all-cause mortality.59 These and other findings indicate that TXA significantly decreases mortality in trauma patients, particularly when administered within 3 h of injury. The large mortality reduction in our study likely reflects the high proportion of penetrating injuries (72%), with over one-half of all patients experiencing stab wounds predominantly to the torso. This injury profile increases the likelihood of noncompressible bleeding, and/or an inflammatory response from visceral injury, which may make the population more responsive to TXA therapy. However, this mechanism of injury differs from the predominant firearm and collision mechanisms seen in other studies, which may further contribute to the larger difference seen in our study. Additionally, access to facilities is delayed, and access to surgical care and blood products is limited in this population.36, 60 The cumulative result may be a selection bias in which patients with major noncompressible hemorrhage die before reaching the facilities, selecting for patients' whose hemorrhage is more amenable to nonsurgical therapies, such as TXA. Those reasons may help explain why the benefit of TXA is more magnified in our study population compared to studies conducted in high-income settings that are not as resource-constrained. Hence, our study has implications for similar resource-constrained health systems, for example, low-income nations, austere and remote settings, and combat casualty care.
Existing literature suggests that hemostatic resuscitation with blood components or whole blood confers a significant survival benefit in hemorrhage. In our study cohort, 19% of patients received blood components or whole blood, raising the question of whether the observed mortality benefit was attributable to blood transfusions rather than TXA. However, in our IPTW-weighted models, patients who received specific blood components or whole blood demonstrated higher odds of mortality compared to those who did not receive transfusions, a finding that contrasts with the observed mortality-reducing effect of TXA but should be interpreted cautiously due to the covariate imbalance of blood products (Table S3). Thus, we can reasonably conclude that the mortality benefit and the substantial effect sizes associated with TXA in our study were not influenced by the administration of blood products.
Among several subgroup analyses in our study, one key finding revealed that administration of 1 g of TXA demonstrated comparable effectiveness to 2 g doses in reducing mortality odds. These findings align with recent literature, including Gunn et al. (2024), who found no significant differences in 28-day mortality across three TXA dosing strategies (1-g bolus vs. 1-g bolus plus 1-g infusion vs. 2-g bolus).34 Similarly, El-Menyar et al.61 evaluated the safety and efficacy of a second in-hospital TXA dose versus placebo and reported that the additional dose provided no advantages over a single prehospital dose in terms of mortality rates, blood transfusion requirements, thromboembolic complications, organ failure, or hospital length of stay. Further validation in pharmacokinetic studies is needed. However, a 1 g TXA dose becoming the standard could yield pragmatic benefits for early prehospital administration (e.g., EMS transport times may only accommodate a 1-g loading dose) and increased feasibility of intramuscular TXA administration (current TXA concentrations permit a maximum of 1 g per intramuscular injection, not 2 g). A 1 g TXA dose has pragmatic implications.
4.1 Limitations
Our study had several limitations. First, although our multicenter study was of hospitals within the same government healthcare system, we did not account for heterogeneity among facilities. Additionally, the observational design limits our ability to establish causal relationships. Third, although the EpiC study is not heavily impacted by missing data, we used complete case analysis to handle missing values, which may introduce bias, particularly if the data are not missing completely at random. Another limitation is the potential presence of unobserved or unmeasured covariates, such as specific clinical characteristics of patients, which may be associated with both TXA treatment and mortality outcomes. This limitation challenges the assumption of propensity score estimation, which requires that the PS model includes all covariates related to both the treatment and the outcome. Nevertheless, we believe our comprehensive covariate analysis and sensitivity assessments mitigate this concern. Additionally, while we can identify hemorrhage-related deaths, the dataset has limited details on other specific causes of death, precluding a detailed analysis of causes of late mortality, such as sepsis or organ failure. Finally, several of our subgroup analyses were limited by small sample sizes and issues of complete separation and require validation in large cohort samples.
5 CONCLUSION
This study suggests that early administration of TXA in severely injured trauma patients is significantly associated with reduced mortality risk across multiple time points. The protective effect of TXA was particularly pronounced in patients with penetrating injuries and those at the highest risk of hemorrhage, when administered within 1 to 3 h post-injury. Notably, a 1-g dose was effective in reducing mortality. The mortality reduction benefits were consistent across different analytical approaches, strengthening our findings. While TXA showed remarkable effectiveness in most subgroups, its impact varied by injury type. These findings support incorporating TXA into early trauma care protocols in LMIC environments, though further research is needed to optimize its use.
ACKNOWLEDGMENTS
We are immensely grateful to the research team at the Collaborative for Emergency Care in Africa who diligently collected data for this study. We are also profusely thankful for the clinicians, staff, and administrators at the Western Cape Government Health and Wellness facilities and agencies whose support and collaboration enabled this work to occur.
FUNDING INFORMATION
This work was supported by United States Department of Defense award numbers/grant log numbers: W81XWH-20-2-0042/BA190049, W81XWH-22-1-0883/RC210170, HT9425-23-1-1025/PR220453.
CONFLICT OF INTEREST STATEMENT
The authors have disclosed no conflicts of interest.




