Apheresis for chimeric antigen receptor T-cell production in adult lymphoma patients
Funding information: Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), Grant/Award Number: 324392634–TRR221; Else-Kröner Fresenius Foundation
Abstract
Background
To date, in-depth analysis of leukapheresis products as starting material for CAR T-cell manufacturing, specifically Tisagenlecleucel production, are scarce. In this study, we report on lymphapheresis data for production of Tisagenlecleucel for elderly and pretreated lymphoma patients.
Study Design and Methods
Spectra Optia from Terumo BCT, Lakewood, CO, was employed for apheresis using the cMNC program. Apheresis success was defined as meeting a target total nucleated cell (TNC) count of ≥2 × 109, a CD3-positive lymphocyte count of ≥1 × 109 and an overall viability of ≥70% in the lymphapheresis product.
Results
Twenty-three patients (age 37–77 years) and 24 apheresis runs were evaluated. The median CD3-positive lymphocyte count in peripheral blood at the beginning of apheresis was 565 cells/μl (range: 70–1345 cells/μl). Circulating lymphoma cells were detected in one patient prior to apheresis. Target criteria were met in 21 of 23 patients. The median TNC count in the apheresate was 11.2 × 109 (range: 2.9 × 109–47.4 × 109). The median CD3-positive lymphocyte count in the apheresate was 2.55 × 109 (range: 0.370 × 109–6.915 × 109), which resulted in a median collection efficiency for CD3-positive lymphocytes of 63.7% (range: 9.56%–93.6%). No adverse events associated with the apheresis process were observed.
Conclusions
Lymphapheresis with the Spectra Optia cMNC program provided a sufficient quantity of CD3-positive lymphocytes for CAR T-cell manufacturing for the majority of patients despite their heavy pretreatment and advanced age. Moreover, we are the first to advocate early pre-emptive lymphocyte collection in DLBCL-NOS patients intended to undergo treatment with Tisagenlecleucel.
1 INTRODUCTION
Driven by encouraging clinical results, adoptive T-cell therapy is gaining increasing significance in anti-cancer therapy.1 T cells genetically modified to express a synthetic chimeric antigen-receptor (CAR) are capable of detecting and destroying malignant cells.1 The CAR is a hybrid molecule consisting of an antibody-derived single chain fragment variable (scFv) fused to intracellular signaling domains, such as CD3ζ, CD28, and 4-1BB.2, 3 Antigen-specific binding to surface molecules on tumor cells is mediated by the extracellular scFv resulting in subsequent T-cell activation triggered by the intracellular signaling moieties.2, 3
In patients suffering from advanced hematological malignancies, CAR-T-cell therapy showed stunning anti-tumor efficacy.4 Long-lasting complete remissions were observed after a single infusion of autologous CD19-targeting CAR T cells in patients with relapsed or refractory diffuse large B cell lymphoma (DLBCL) and acute lymphoblastic leukemia (ALL).5-8 So far, FDA-approval has been issued for five CAR T-cell products transforming CAR T-cell therapy from an experimental procedure to an established oncologic therapy.9 In addition, a multitude of clinical trials is currently ongoing to evaluate the use of CAR T-cell therapy against a wide variety of tumor entities.4 Thus, the demand for CAR T-cell products is steadily rising.
The process of CAR T-cell manufacturing, which is governed by strict laws and regulations, starts with obtaining sufficient T-cell numbers from patients via apheresis.10 Subsequently, the leukapheresis product is transferred to a GMP-facility, where CAR T cells are generated by viral or non-viral transduction of the CAR encoding vector.10 Finally, cytokine-driven expansion of engineered cells is executed before infusing CAR T cells back into the patient.10 While a growing number of clinical trials is currently analyzing the use of allogeneic T cells manufactured from healthy donors, autologous lymphapheresis still constitutes the standard of care underlying all of the FDA-approved CAR T-cell products and the majority of clinical trials.4
Over the last decade, leukapheresis has transformed into a standard procedure in clinical hematology providing both autologous and allogeneic stem cells for hematopoietic reconstitution after myeloablative chemotherapy. Lymphapheresis, which is a special subtype of leukapheresis, aims at maximizing the collection of CD3-positive T cells. With the advent of donor lymphocyte infusions (DLI) as therapeutic option after relapse post allogeneic stem cell transplantation, broad experience has been gathered about the large-scale collection of CD3-positive lymphocytes from previous stem cell donors.11 After the success of CAR T-cell therapy and the growing demand for CAR T-cell products, a lymphapheresis process for CAR T-cell patients was developed akin to DLI collection.12 Nevertheless, CAR T-cell patients frequently develop severe leukopenia and lymphopenia originating either from intensive chemotherapy pretreatment or from advanced hematological malignancies with a concurrent depression of healthy hematopoiesis. Contrary with DLI apheresis from healthy individuals, lymphapheresis for CAR T-cell patients poses the challenge of collecting sufficient CD3-positive cells from severely ill and pretreated patients with low lymphocyte counts.
So far, thorough analyses of the lymphapheresis process in CAR T-cell patients have been largely focused on pediatric patients.13-17 In elderly adult patients diagnosed with lymphoma, only few detailed reports on T-cell collection for CAR T-cell therapy were published.18, 19 In this study, we provide comprehensive lymphapheresis results from elderly and pretreated lymphoma patients intended to undergo treatment with Tisagenlecleucel.
2 MATERIALS AND METHODS
2.1 Patients
In this retrospective study, 23 patients suffering from relapsed/refractory Non-Hodgkin's lymphoma treated at the University Hospital Regensburg were included. All patients underwent autologous lymphapheresis as preparation for subsequent treatment with CD19-specific CAR T cells. We retrieved epidemiologic, demographic, clinical, laboratory, management, and outcome data from patients' medical records. Aphereses were conducted between April 2019 and December 2021. This retrospective study was approved by the Ethics Committee of the University Regensburg (ethics statement number: 21-2716-104), and written informed consent was obtained from all patients prior to apheresis.
2.2 Apheresis
This calculations will be done on the day of the apheresis itself, and these preliminary values can then be used to more easily adjust the blood volume to be processed based on the latest lymphocyte counts. Parameters defining a successful apheresis run comprised a TNC value of at least 2 × 109 (viability ≥70%) with a minimum number of 1 × 109 CD3-positive lymphocytes. After apheresis, TNC and CD3-positive lymphocytes were determined using a multi-parameter automated hematology analyzer (XN-550, Sysmex, Kobe, Japan) and flow cytometry (Navios, EX, Beckman Coulter, Indianapolis, IN, USA) respectively.
2.3 Laboratory analysis prior to lymphapheresis
Prior to apheresis, a multi-parameter automated hematology analyzer was utilized to generate complete blood counts with automated differentials. Lymphocyte subpopulations were distinguished via flow cytometry (Navios EX, Beckman Coulter) using the following markers: CD3 (T cells), CD19 (B cells), and CD16/56 (NK cells).
2.4 Statistical analysis
The collection efficiency for CD3-positive lymphocytes was calculated according to the following formula: CD3-positive lymphocytes collected (×106)/(Pre-apheresis CD3-positive lymphocytes (×106) × liters (L) processed) × 100. As indicated, variables were documented as mean, median, and range (minimum/maximum) values. Significant differences between study variables were determined using two-tailed Student's t-test. A p-value less than .05 was deemed statistically significant.
3 RESULTS
3.1 Patient characteristics
In aggregate, 23 adult patients (12 females and 11 males) with histologically confirmed Non-Hodgkin's lymphoma treated at our medical center underwent lymphapheresis as preparation for CAR T-cell therapy with Tisagenlecleucel. All patient characteristics are depicted in Table 1. The majority of patients suffered from diffuse large B cell lymphoma not otherwise specified (DLBCL-NOS). Moreover, three patients with transformed follicular lymphoma, one patient with transformed chronic lymphocytic leukemia (CLL) and one patient with follicular lymphoma were included. Median age of patients was 72 years (range 37–77 years, only five patients were younger than 60 years) at the time of apheresis. The median weight was 78 kg (range 54–110 kg). All patients completed at least one line of rituximab-based standard immunochemotherapy. Furthermore, 12 patients presented with relapsing or refractory disease after two lines of standard chemotherapy, and two patients underwent apheresis after pretreatment with more than two different standard chemotherapy regimens. Overall physical condition at the time of apheresis according to ECOG performance status indicated a slightly reduced performance (ECOG 1) in 18 patients, full function (ECOG 0) in two patients, and moderately reduced performance (ECOG 2) in three patients. Initial lymphapheresis was successful in 22 of 23 patients. One patient had to undergo a second apheresis owing to an insufficient lymphocyte count after the first run. Hence, 24 apheresis runs were available for analysis.
| Parameter | Patients (N = 23) |
|---|---|
| Age (years) | |
| Mean | 68 |
| Median | 72 |
| Range | [37; 77] |
| ≥60 | 18 |
| <60 | 5 |
| Female:male (patients) | 12:11 |
| Weight (kg) | |
| Mean | 80 |
| Median | 78 |
| Range | [54; 110] |
| ECOGa (patients) | |
| 0 | 2 |
| 1 | 18 |
| 2 | 3 |
| Lymphoma entity | |
| Diffuse-large B cell lymphoma, NOSb | 18 |
| Transformed Follicular Lymphoma | 3 |
| Transformed Chronic lymphocytic leukemia | 1 |
| Follicular lymphoma | 1 |
| Disease stagea (patients) | |
| I | 1 |
| II | 4 |
| III | 5 |
| IV | 12 |
| CR | 1 |
| Number of previous lines of therapy (patients) | |
| 1 | 9 |
| 2 | 12 |
| ≥3 | 2 |
| Patients undergoing T-cell Apheresis | |
| Once | 22 |
| Twice | 1 |
| Patients receiving CAR T cellsc | 13 |
- a At the time of apheresis.
- b Not otherwise specified.
- c Tisagenlecleucel.
3.2 Apheresis process
Upon informed consent, the venous status of apheresis patients was assessed to determine which venous access was appropriate for lymphocyte collection. A hemodialysis catheter was required for half of the apheresis runs, while the other half was conducted with peripheral venous access (Table 2). Neither central venous access nor peripheral venous access was associated with any significant complications, such as vasovagal reactions. The processed blood volume varied from 5.0 to 13.9 L with a median of 9.9 L (Table 2). The median total blood volume (TBV) processed amounted to 1.9 TBV (range 1.3–3.0 TBV) (Table 2), taking into account the German regulations (“Richtlinie zur Gewinnung von Blut und Blutbestandteilen und zur Anwendung von Blutprodukten”) in their most recent version, which generally limits leukapheresis to 3 TBV. Anticoagulation was performed with ACD-A using a median volume of 1012 ml per apheresis (range 518–1538 ml) (Table 2). The evaluation of the cell separators' internal time-tracking protocols yielded a median collection time of 167 min (range 103–259 min). Possible serious side-effects reported in the context of lymphapheresis including paresthesia, nausea, headache, pain, hypotension, or cardiovascular events were not observed in any of the evaluated patients.
| Parameter | Apheresis runs (N = 24) |
|---|---|
| Venous access (patients) | |
| Peripheral venous catheter | 12 |
| Hemodialysis catheter | 12 |
| Liters processed | |
| Mean | 9.683 |
| Median | 9.931 |
| Range | [5.000; 13.981] |
| Total blood volumes processed | |
| Mean | 1.97 |
| Median | 1.90 |
| Range | [1.30; 3.00] |
| Anticoagulationa consumption (ml) | |
| Mean | 987 |
| Median | 1012 |
| Range | [518; 1538] |
| Timeb (min) | |
| Mean | 171 |
| Median | 167 |
| Range | [103; 259] |
- a Acic Citrate Dextrose Solution.
- b Collection time on apheresis machine.
3.3 Preapheresis laboratory values
Automated differential blood counts as well as flow-cytometry based analyses of lymphocyte subpopulations and circulating malignant cells were obtained from all patients within 2 weeks prior to apheresis (Table 3). Flow-cytometric analysis of CD3-positive lymphocytes, NK cells, and B cells are available from 22 of 24 runs. Before apheresis, the patients showed a median WBC of 4610 cells/μL (range: 1670–19,200 cells/μl) with a median neutrophil count of 2800 cells/μl (range: 950–15,400 cells/μl) and a median monocyte count of 520 cells/μl (range: 250–2740 cells/μl) (Table 3). Moreover, the median lymphocyte count of 740 cells/μl (range: 120–1490 cells/μl) at the time of apheresis was found below the reference range, which was largely attributed to B-cell aplasia (median B-cell count 0 cells/μl) following CD20-directed chemoimmunotherapy (Table 3). Median CD3-positive lymphocyte counts and NK cell counts were within reference range at 565 cells/μl (range: 70–1345 cells/μl) and 140 cells/μl (range: 31–504 cells/μl) respectively (Table 3). Circulating lymphoma cells as assessed by flow cytometry were only detected in one patient with DLBCL. Preapheresis laboratory requirements were set by institutional guidelines encompassing a measurable CD3-positive cell count in the peripheral blood, and immediately prior to apheresis hemoglobin levels greater than 8 g/dL as well as a platelet count of greater than 30 G/L. Apheresis collection yields.
| Parameter | Apheresis runs (N = 24)a | Reference range |
|---|---|---|
| WBCb (×103/μl) | 4.0–10 | |
| Mean | 5.85 | |
| Median | 4.61 | |
| Range | [1.67; 19.2] | |
| Neutrophils (×103/μl) | 1.56–6.13 | |
| Mean | 3.96 | |
| Median | 2.80 | |
| Range | [0.95; 15.4] | |
| Neutrophils (%) | 34–71 | |
| Mean | 66.2 | |
| Median | 68.8 | |
| Range | [39.0; 81.0] | |
| Monocytes (×103/μl) | 0.24–0.36 | |
| Mean | 0.70 | |
| Median | 0.52 | |
| Range | [0.25; 2.74] | |
| Monocytes (%) | 4.7–12.5 | |
| Mean | 13.0 | |
| Median | 13.1 | |
| Range | [4.0; 22.8] | |
| Lymphocytes (×103/μl) | 1.18–3.74 | |
| Mean | 0.74 | |
| Median | 0.80 | |
| Range | [0.12; 1.49] | |
| Lymphocytes (%) | 19–52 | |
| Mean | 14.5 | |
| Median | 16.1 | |
| Range | [4.1; 34.3] | |
| CD3-positive Lymphocytes (/μl) | 500–2300 | |
| Mean | 564 | |
| Median | 565 | |
| Range | [70; 1345] | |
| CD3-positive Lymphocytes (%) | 55–83 | |
| Mean | 73.0 | |
| Median | 78.5 | |
| Range | [30; 91] | |
| NK-cells (/μl) | 90–600 | |
| Mean | 145 | |
| Median | 140 | |
| Range | [31; 504] | |
| NK-cells (%) | 7–31 | |
| Mean | 21.4 | |
| Median | 17.0 | |
| Range | [5.0; 69] | |
| B cells (/μl) | 100–500 | |
| Mean | 27 | |
| Median | 0 | |
| Range | [0; 202] | |
| B cells (%) | 6–9 | |
| Mean | 3.3 | |
| Median | 0 | |
| Range | [0; 31] | |
| Circulating lymphoma cells (patients) | 1 | 0 |
- a Results for CD3-positive Lymphocytes, NK-cells, and B cells are only available from 22 of 24 runs.
- b White blood cell count.
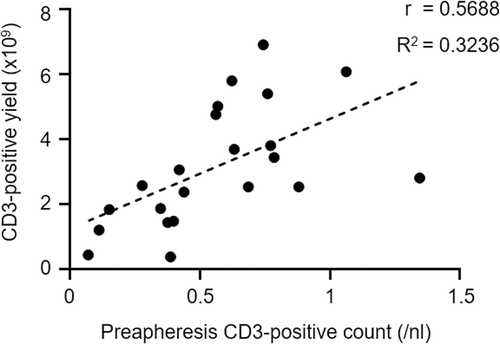
Apheresis success, defined as meeting the requirements of the manufacturer (Novartis) comprising a target TNC of ≥2 × 109, a CD3-positive lymphocyte count of ≥1 × 109 with an overall viability of ≥70%, was achieved in 21 of 23 patients (Table 4). Apheresis collection yields are summarized in Table 4. The median TNC count in the apheresate was 9.2 × 109 (range: 2.9 × 109–47.4 × 109). The median CD3-positive lymphocyte count in the apheresate amounted to 2.55 × 109 (range: 0.370 × 109–6.915 × 109) with an overall viability of 94% (range: 82.7%–97.2%). In general, the peripheral blood CD3-positive cell count correlated with the product CD3-positive cell yield (Figure 1). The median collection efficiency for CD3-positive lymphocytes was 63.7% (range: 9.56–93.6%). In total, 17 apheresis products obtained from 16 patients (including the two patients formally not achieving apheresis success) were dispatched to Novartis for Tisagenlecleucel manufacturing. Unfortunately, three patients died from rapidly progressing disease during CAR T-cell production. Due to death, CAR T-cell manufacturing was prematurely aborted in all three patients, and no CAR T-cell products were generated. Consequently, CAR T-cell products were successfully administered to a total number of 13 patients, while seven patients continued with standard chemotherapy treatment. Their remaining apheresis products were stored below −140°C in our transfusion medicine facility for CAR T-cell production in case of relapse or chemo-refractory disease. At the time of this writing, those seven patients do not require CAR T-cell therapy due to ongoing responses, thus their apheresis products are still frozen. An early lymphapheresis with subsequent on-site storage has become standard of care at our medical center, as this strategy permits CAR T-cell production even for patients that become lymphopenic upon sequential immunochemotherapies. Post-apheresis analysis of the apheresis products' composition revealed a median lymphocyte percentage of 38.3% (range: 8.4%–60.3%), a median CD3-positive cell percentage of 35.9 (range: 4.4%–55.6%), a median granulocyte percentage of 7% (range: 1.7%–62.5%), and a median monocyte percentage of 50.1% (range: 11.0%–62.4%) (Table 4). Hemoglobin levels well below 1 g/dl in the apheresate demonstrated the successful targeting of the WBC layer during the apheresis process (Table 4). In aggregate, T-cell apheresis and subsequent CAR T-cell production were feasible in the majority of patients included in this study.
| Parameter | Apheresis runs (N = 24) | Target |
|---|---|---|
| Hemoglobin (g/dl) | ||
| Mean | 0.45 | |
| Median | 0.40 | |
| Range | [0.3; 0.9] | |
| TNC (×109) | ≥2 | |
| Mean | 11.2 | |
| Median | 9.2 | |
| Range | [2.9; 47.4] | |
| Viability (%) | ≥70 | |
| Mean | 92.5 | |
| Median | 94.0 | |
| Range | [82.7; 97.2] | |
| Granulocytes (%) | ||
| Mean | 13.4 | |
| Median | 7.0 | |
| Range | [1.7; 62.5] | |
| Monocytes (%) | ||
| Mean | 47.7 | |
| Median | 50.1 | |
| Range | [11.0; 62.4] | |
| Lymphocytes (%) | ||
| Mean | 38.9 | |
| Median | 38.3 | |
| Range | [8.4; 60.3] | |
| CD3-positive Lymphocytes (%) | ||
| Mean | 32.1 | |
| Median | 35.9 | |
| Range | [4.4; 55.6] | |
| CD3-positive Lymphocytes (×109) | ≥1 | |
| Mean | 3.00 | |
| Median | 2.55 | |
| Range | [0.370; 6.915] | |
| CD3-positive Lymphocytes (×106/kg) | ||
| Mean | 38.40 | |
| Median | 33.15 | |
| Range | [4.11; 109.8] | |
| Collection efficiency (%)a | ||
| Mean | 63.8 | |
| Median | 63.7 | |
| Range | [9.56; 93.6] | |
| Successfulb Apheresis (patients) | 21 | |
| Aphereses submitted for CAR T cell production | 17 | |
| Successful CAR T-cell production (patients) | 13 | |
- a CD3-positive Lymphocytes collected (x106)/(Pre-apheresis CD3-positive Lymphocytes (x106) × L processed) x 100.
- b Defined as meeting target values (right column).
3.4 Apheresis related changes in hematocrit, hemoglobin, and platelet count
Blood counts were closely monitored before and after apheresis. The median starting hematocrit prior to apheresis was 33.1% (range: 25.2%–42.2%) and the mean post-apheresis hematocrit amounted to 28.6% (range: 21.3%–38.6%) (Table 5). Moreover, a hemoglobin level of greater than 8 g/dl and a platelet count of greater than 30 G/L were prerequisites to begin apheresis, which were fulfilled in all patients without the need to administer blood products. The lowest hemoglobin level and the lowest platelet count at the start of apheresis were 8.4 g/dl and 63 G/L respectively (Table 5). The median hemoglobin level prior to apheresis was 11.3 g/dl (range: 8.4–14.4 g/dl), and the median pre-apheresis platelet count was 234 G/L (range: 63–318 G/L) (Table 5). Post-apheresis median hemoglobin levels and platelet counts amounted to 9.8 g/dl (range: 7.1–13.2 g/dl) and 150 G/L (range: 51–244 G/L) (Table 5). While no patient required thrombocyte transfusion after apheresis, four patients received RBCs post apheresis due to hemoglobin levels dropping below 8 g/dl. In all those four patients, pre-apheresis hemoglobin levels ranged between 8.4 and 8.7 g/dl. Among others, one reason for the drop in hemoglobin levels may rely on dilution effects owing to ACD-A addition during apheresis. Irrespective of symptoms of anemia, which were not present in any of those four patients, blood transfusions will be administered as soon as hemoglobin levels drop below 8 g/dl as stipulated by institutional guidelines. In sum, lymphapheresis expectedly caused drops in hematocrit, hemoglobin, and platelet counts, and close monitoring ensured appropriate RBC substitution before patients became symptomatic.
| Parameter | Pre-apheresis | Post-apheresis | Δa | p valueb |
|---|---|---|---|---|
| Hematocrit (%) | ||||
| Mean | 33.0 | 29.2 | −3.82 | |
| Median | 33.1 | 28.6 | −3.90 | .0111 |
| Range | [25.2; 42.2] | [21.3; 38.6] | [−6.30; −1.80] | |
| Hemoglobin (g/dl) | ||||
| Mean | 11.2 | 9.98 | −1.23 | |
| Median | 11.3 | 9.80 | −1.30 | .0274 |
| Range | [8.40; 14.4] | [7.10; 13.2] | [−2.10; −0,50] | |
| Platelet count (G/L) | ||||
| Mean | 203 | 147 | −57 | |
| Median | 234 | 150 | −64 | .0019 |
| Range | [63; 318] | [51; 244] | [−130; −2] | |
| Red cell transfusion required (patients) | 0 | 4 | n.a. | |
| Platelet transfusion required (patients) | 0 | 0 | n.a. | |
- a Difference post-apheresis-pre-apheresis.
- b p values were determined using two-tailed Student's t-test.
3.5 Apheresis related changes in selected electrolytes
Calcium and potassium levels were also regularly checked during the apheresis process to avoid hypocalcemia and hypokalemia, which may both result in potentially relevant arrhythmias or even cardiogenic shock. The median total calcium level measured before apheresis was 2.32 mM (range: 2.08–2.45 mM). All patients, including those with normal calcium levels, received prophylactic calcium substitution during apheresis by calcium gluconate infusions (usually 2–6 g calcium). Hence, total serum calcium levels post-apheresis were significantly higher than pre-apheresis with a median calcium level at the end of apheresis of 2.47 mM (range: 2.27–2.73 mM). Potassium was also prophylactically substituted during apheresis (usually 20–40 mM potassium chloride were administered) ensuring similar median potassium levels pre- (3.75 mM, range: 3.2–4.5 mM) and post-apheresis (3.56 mM, range: 3.0–4.56 mM). In sum, close monitoring and prophylactic substitution prevented severe electrolyte dysbalances during apheresis. Moreover, no side-effects associated with electrolyte dysbalances were observed.
4 DISCUSSION
Successful lymphapheresis is the first step in CAR T-cell manufacturing and in-depth analysis of clinical scale lymphocyte collection therefore crucial to further refine the generation of CAR T-cell products. With detailed reports on T-cell apheresis for CAR T-cell production in pediatric patients published in the past, this report is exclusively focused on adult patients. In this retrospective study, we analyzed the lymphapheresis process of 23 patients diagnosed with aggressive Non-Hodgkin's lymphoma progressing after first-line chemotherapy, who were considered for in-label CAR T-cell therapy with CD19-targeting CAR T cells (Tisagenlecleucel). Sufficient (≥1 × 109 CD3-positive lymphocytes) T-cell collection could be achieved in the vast majority of patients. Additionally, apheresis material from 16 patients was submitted for CAR T-cell manufacturing, and 13 patients were eventually treated with CAR T cells with three patients dropping out due to death from rapidly progressing disease resulting in premature abortion of CAR T-cell manufacturing. Finally, no serious side-effects associated with lymphapheresis were observed, which is in line with majority of previously published reports.20
Both patients failing successful T-cell collection presented with severe lymphopenia and depressed T-cell counts prior to lymphapheresis. The first patient was treated with five different lines of chemotherapy before undergoing apheresis. Refractory disease combined with heavy pretreatment are unfavorable preconditions for successful lymphocyte collection, as insufficient yields (<1 × 109 CD3-positive lymphocytes) were obtained despite two consecutive apheresis runs. Nevertheless, the material from the two consecutive apheresis runs was pooled, and, albeit not meeting specifications, was submitted for CAR T-cell manufacturing. Unfortunately, the patient soon passed away from rapidly progressing disease and thus CAR T-cell production was aborted. The second patient began lymphapheresis upon relapse after two lines of chemotherapy, including immune checkpoint blockade. This patient had circulating malignant cells in his blood. Leukemic DLBCL is often associated with a high tumor burden and bone marrow involvement, both favor lymphopenia. Similar to the first patient, CAR T-cell manufacturing was initiated after apheresis despite an insufficient yield (<1 × 109 CD3-positive lymphocytes). Unfortunately, this patient could not be treated with CAR T cells due to rapid deterioration of his physical condition prompting initiation of palliative care. Taken together, intensive pretreatment and extensive disease at the time of apheresis pose severe risk factors for failing lymphapheresis. Seeking to mitigate those risks, we try to pre-emptively collect T cells at early time points in lymphoma therapy (usually before the initiation of second line therapy), and freeze the apheresis material until CAR T-cell therapy is required. Given the absence of severe side-effects emanating from lymphapheresis, early T-cell collection has become standard of care for DLBCL patients at our institution.
To date, comprehensive reports on T-cell collection for CAR T-cell manufacturing were predominantly focused on pediatric cancer patients.13-17 Investigators from the National Institutes of Health (NIH) published apheresis data pooled from three CAR T-cell trials enrolling children diagnosed with B-cell leukemia, lymphoma, or neuroblastoma.13 Of 71 apheresis runs, 97% (69 patients) met the target of 2 × 109 CD3-positive lymphocytes, and 77% (55 patients) attained the minimum of 0.6 × 109 CD3-positive lymphocytes. Both patients failing the minimum CD3-positive lymphocyte yield showed a sizable portion of circulating blasts, which underscores the risk of low T-cell collection yields in the presence of circulating malignant cells as seen in one patient from our study.13 In a second study, Ceppi and colleagues presented apheresis results from two clinical trials evaluating CAR T-cell therapy for children and young adults suffering from leukemia or neuroblastoma.14 All 99 patients included in this study achieved the target of 1 × 109 total mononuclear cells per kilogram in the first apheresis run. Nevertheless, lymphapheresis had to be repeated in three patients, due to concerns about bacterial contamination in one patient, and insufficient T-cell expansion during CAR T-cell manufacturing in the other two patients. Complications associated with the apheresis process were rare in both studies. Overall, this previously published experience from pediatric patients underpins that lymphapheresis for CAR T-cell production is safe and successful in the majority of patients, which is in line with our results from elderly and pretreated patients.
The first detailed report on leukapheresis for CAR T-cell production in adult patients with B cell lymphoma was published by Korell et al. Of 41 patients, sufficient lymphocytes could be obtained from 37 patients in the first attempt, and four patients required a second collection session.18 The leukapheresis products were characterized by a median of 98 × 108 (9–341 × 108) total nucleated cells (TNC) with 38 × 108 (4–232 × 108) CD3+ T cells.18 Moreover, CAR T-cell products could be manufactured for all but one patient.18 In conclusion, these early results are further corroborated by the findings in this study that it is mostly feasible to collect sufficient lymphocytes for CAR T-cell production in adult lymphoma patients. While the median age in Korell et. al was 56 years, and only four patients were intended for Tisagenlecleucel treatment, we demonstrate feasibility of Tisagenlecleucel manufacturing in a larger and older (median age 72 years) cohort. Furthermore, Korell et al included a sizable portion of patients undergoing lymphapheresis within a clinical trial, which by the application of exclusion and inclusion criteria specifically selects a certain patient group. In our report, we just incorporated patients intended for treatment with the commercially available FDA-approved CAR T-cell product Tisagenlecleucel. Thus, the data presented here are all real-world data without specifically stated inclusion and exclusion criteria.
Interestingly, combining our experience with the data reported by Korell et al., all patients requiring a second apheresis due to low T-cell yield in the first run, and all patients failing the CAR T-cell manufacturing process owing to insufficient apheresis quality or premature death from rapidly progressing disease were patients diagnosed with DLBCL-NOS. Even though the overall risk for a DLBCL-NOS patient to fail CAR T-cell manufacturing is low, it seems to be somewhat higher than in ALL or other Non-Hodgkin lymphomas. In order to further mitigate this a priori low risk, pre-emptive lymphocyte collection at an early time point in lymphoma therapy as described in this publication could be beneficial.
In aggregate, lymphapheresis for CAR T-cell manufacturing did not cause any serious side effects in this study. Moreover, a sufficient quantity of CD3-positive lymphocytes for CAR T-cell production was collected in the majority of patients despite heavy pretreatment and advanced age and disease. Additionally, pre-emptive lymphocyte collection at an early time point in lymphoma therapy might improve the quantity and quality of collected T cells, especially in patients with DLBCL-NOS, to enhance the chances of successful Tisagenlecleucel manufacturing. However, for CAR T-cell products directly generated from fresh aphereses, this approach cannot be applied to.
List of Abbreviations
-
- ACD-A
-
- Acid Citrate Dextrose Solution A
-
- ALL
-
- Acute Lymphoblastic Leukemia
-
- CAR
-
- Chimeric Antigen Receptor
-
- CD
-
- Cluster of Differentiation
-
- CLL
-
- Chronic Lymphocytic Leukemia
-
- DLBCL
-
- Diffuse Large B Cell Lymphoma
-
- DLI
-
- Donor Lymphocyte Infusion
-
- ECOG
-
- Eastern Cooperative Oncology Group
-
- FDA
-
- Food and Drugs Administration
-
- GMP
-
- Good Manufacturing Practice
-
- Hb
-
- Hemoglobin
-
- NIH
-
- National Institutes of Health
-
- NOS
-
- Not otherwise specified
-
- PLT
-
- Platelets
-
- scFv
-
- single chain Fragment variable
-
- TBV
-
- Total Blood Volume
-
- TNC
-
- Total Nucleated Cells
ACKNOWLEDGMENTS
We thank the Else-Kröner Fresenius Foundation for financial support (Dennis Christoph Harrer). This study was also funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Projektnummer 324392634–TRR221 to Dennis Christoph Harrer. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
Simone Thomas declares Honoraria from Novartis, BMS, Gilead, Janssen, EUSA Pharma, and Abbvie, as well as research funding from Gilead and BMS. Daniel Wolff declares research support from Novartis (unrelated to CAR-treatment), and honoraria from Novartis, Gilead and BMS. The other authors declare that they have no conflict of interest.




