Preoperative transfusion versus no transfusion policy in sickle cell disease patients: a randomized trial
ABSTRACT
BACKGROUND
Many children with sickle cell disease (SCD) indicated for adenotonsillectomy receive pre-operative transfusion therapy, either simple or exchange transfusion, in order to reduce surgical and sickle cell disease-related complications.
SUBJECTS AND METHODS
This is a prospective randomized controlled clinical trial aiming to compare between preoperative simple transfusion and no transfusion in pediatric patients with sickle SCD admitted in Sultan Qaboos University Hospital, Muscat, Oman for adenotonsillectomy during the period from January 2014 through June 2018. They were randomly assigned into two arms (simple transfusion and no transfusion).
RESULTS
Postoperative SCD-related complications have been encountered in 6 out of 138 patients (4.3%). There was no statistically significant difference between the two studied groups as regards the development of surgical or SCD-related complications (p = 0.6 and 0.8 respectively). The length of postoperative hospital stay was comparable in the two groups. (p = 0.607). SCD-related complications occurred exclusively in cases with homozygous sickle anemia (4 out of 81 = 4.9%).
CONCLUSION
Sickle cell disease patients with a hemoglobin level above 7.5 g/dL do not need PRBCs transfusion prior to adenotonsillectomy. This approach did not increase the risk of postoperative surgical or SCD-related complications.
Sickle Cell Disease (SCD) is a chronic hematological disorder characterized by chronic anemia and recurrent painful episodes.1 Patients with SCD commonly have adenotonsillar hypertrophy and chronic tonsillitis, with a prevalence ranging from 25 to 55%.2, 3 Though the causes of adenotonsillar hypertrophy are still unclear, numerous mechanisms are proposed such as compensatory hypertrophy of the lymphoid tissue for functional asplenia, reactive enlargement due to repeated infections and increased hematopoietic needs because of chronic hemolytic anemia.4, 5 Adenotonsillar hypertrophy can increase the risk of obstructive sleep apnea, hypoxemia, dehydration, and predisposes to several complications in patients with SCD including vaso-occlusive crisis (VOC), pulmonary hypertension, acute chest syndrome (ACS), priapism or even stroke.3, 6-8 There are many preoperative concerns in patients with SCD; the most debatable one is the need for transfusion. A number of reports have demonstrated that simple transfusion is as effective as exchange transfusion in reducing postoperative complications in patients with SCD and it requires much less blood volume.2, 7, 9, 10
The aim of the current work is to compare between simple packed red cell (PRBCs) transfusion and non-transfusion policy as a preoperative management strategy in stable pediatric patients with SCD undergoing adenotonsillectomy and to study the risks and benefits of both approaches.
Hypothesis
We hypothesize that the proportion of patients with post-operative complications is higher in the non-transfused group.
METHODS
Trial design
This is a prospective randomized controlled study including all pediatric patients with SCD admitted to Sultan Qaboos University Hospital (SQUH) for tonsillectomy, adenoidectomy, or both procedures during the period from January 2014 through June 2018. Sultan Qaboos University Hospital is the largest tertiary referral center for pediatric hematology and ENT surgery in Oman.
Participants and randomization
Patients were randomly assigned using block randomization with variable block sizes. The study was un-blinded. No stratification was done. Clinicians chose which patients were randomly assigned after assessment of their eligibility criteria. Inclusion criteria were age ranging from 2 to 13 years and hemoglobin level ranging from 7.5 to 8.9 g/dL. Excluded patients with Hb level less than 7.5 were transfused and those with Hb ≥ 9 g/dL were not transfused preoperatively. Other exclusion criteria included patients who are already on long term exchange transfusion for other indications (e.g., history of cerebrovascular accidents), history of acute chest syndrome within the previous 1 year, and patients who received blood transfusion within the previous 6 months.
Intervention
Patients were randomly assigned into one of two arms; Arm 1 patients received simple top-up PRBC transfusion before surgery with a target hemoglobin of 10 g/dL, and Arm 2 patients did not receive any preoperative transfusion. To calculate the transfusion requirements, we used the following formula: weight (kg) × desired increment in Hb (g/dL) × 3/(hematocrit [Hct] level of transfused RBCs).11
Outcome
The primary outcome measure was the proportion of patients with postoperative complications. The primary hypothesis was tested using proportions and a 95% CI. We completed a power calculation comparing patients with preoperative simple transfusion to patients without preoperative blood transfusion in terms of the proportion of patients who have adverse events (AEs) after 37 days of follow-up.
- Primary Outcome: Presence/Absence of AEs (SCD-related complications).
- Predictor: Presence/Absence of transfusion.
- Secondary outcome measures: surgical complications and blood requirement difference in both groups pre- and postoperatively.
The study was conducted in accordance with the declaration of Helsinki and the International Conference on Harmonization, and approved by the Ethics Committee, College of Medicine and Health Sciences, Sultan Qaboos University (MREC acceptance number # 89/2013). Informed consents were obtained from the legal guardians of all enrolled patients.
Sample size
As far as sample size calculation is concerned and based on preliminary data,12, 13 we believe that 15% of preoperative simple transfusion patients and 40% of “no transfusion” patients will have an adverse event (AE) respectively. With a sample of 43 subjects per group, we have 80% power to detect a difference of 0.25 between the null hypothesis that the proportion with a relapse in each group is 0.15 and the alternative hypothesis that the proportion of patients with AE in the non-transfusion group is 0.4 with a significance level of 0.05 using a two-sided two-sample test of proportions.
Indications for surgery were recurrent tonsillitis, adenoid hypertrophy with upper airway obstruction, or sleep apnea (characterized by sleep pause, snoring, and restlessness in sleep). All the patients with recurrent tonsillitis had had at least four episodes per year. The medications which were allowed during the study were hydroxyurea (HU), folic acid, antibiotics, and analgesics as required. Five Patients in Group 1 and six patients in Group 2 were on HU. All patients were on folic acid prophylaxis. Preoperative laboratory studies included a hemogram, prothrombin time, activated partial thromboplastin time, and automated multiple analyses (liver function tests, urea, and electrolytes). Lateral neck soft tissue X-ray was done for the patients to document the adenoid enlargement. Extended red cell phenotyping and cross matching were performed and included alloantibody screening, Hb F level and Hb S level collected before simple or exchange transfusion. Apart from PRBC transfusion, a standardized preoperative care and anesthesia protocol were applied to the studied groups.7, 14, 15 On admission, all patients were hydrated with pediatric saline, administered at a rate of 1.5-2 times maintenance. The patients were reviewed by the anesthesiologist. The intraoperative care of the patient with SCD included the use of warming blankets and the operating room ambient temperature was increased to 26-27°C. Axillary temperature was continuously monitored. Pediatric saline infusion with the same rate continued during surgery. Oxygen saturation of the arterial blood and end-tidal carbon dioxide levels with pulse oximetry were monitored and standard anesthetic agents were used. Tonsillectomy has been done either by dissection–ligature technique or bipolar cautery.16 The adenoids were curetted. The children were extubated when fully awake and breathing normally. Postoperative management included continuous pulse oximetry with monitoring for apnea and bradycardia, supplemental oxygen, incentive spirometry, warming in blankets, hydration, and pain management in the form of paracetamol, or non-steroidal anti-inflammatory drugs (NSAIDs) if needed. Patients who developed VOCs received continuous opioid infusion. The patients were encouraged to follow a soft fluid diet and were discharged 2-3 days following surgery. All complications occurring from the time of surgery through a 30-day follow-up period were monitored and classified as surgical (primary hemorrhage; occurring within 24 hours of the operation or secondary hemorrhage; occurring within 1-14 days postoperatively)16, 17 and non-surgical (complications that required prolonged hospitalization or life threatening complications like VOC, ACS, CVA). Severe VOC is defined as excruciating pain of sudden onset4 in the extremities, chest, or back, not responding to paracetamol or NSAIDs, and requiring opioid infusion. Patients were assessed during follow-up clinic visits with history taking, physical examination, and complete blood counts.
Statistical analysis
Quantitative variables were expressed by mean (±SD). They were compared by X 2 test or ANOVA whenever appropriate. A p value of ≤0.05 was considered significant. Statistical analyses were performed using Statistical Package for the Social Science (SPSS) software version-15 for Microsoft Windows and GraphPad Prism-5.0 (GraphPad Software). We used a Bonferroni correction to reduce the chances of obtaining false-positive results.18
RESULTS
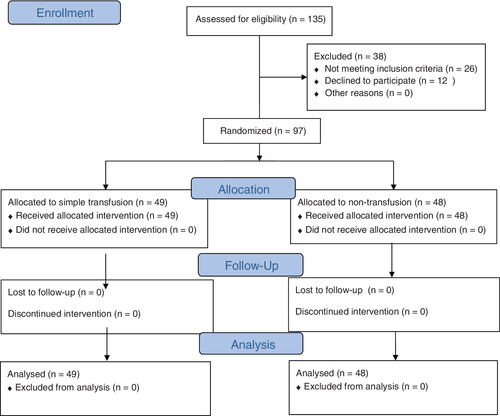
Overall, 135 patients with SCD have been admitted to Sultan Qaboos University Hospital for adenoidectomy, tonsillectomy, or adenotonsillectomy throughout the period of study. Twenty-two patients were excluded for having an Hb level less than 7.5 or more than 9 g/dL and four patients were excluded, being on regular exchange transfusion for other indications. Of the eligible 109 patients, 12 parents refused or declined consent. A total of 97 patients have been enrolled in the study, and randomly assigned into 2 arms (Fig. 1). Table 1 demonstrates the baseline demographic and clinical characteristics of the two studied groups. The pre-transfusion average hemoglobin value and Hb S level were comparable in the two studied groups. Patients on simple transfusion required a mean of 7.14 mL/kg PRBC transfusion (Table 2). No significant difference was found among the two studied arms as regards SCD-related postoperative complications, namely VOC and acute chest syndrome (Table 3). Surgical complications were more relatively encountered in the simple transfusion group, though the difference was not significant.
| Simple transfusion (Group 1)n = 49 | Non-transfusion (Group 2) n = 48 | |
|---|---|---|
| Sex | ||
| Male: n (%) | 28 (57.1) | 26 (54.2) |
| Female: n (%) | 21 (42.9) | 22 (45.8) |
| Age, in years at the time of surgery (mean ± SD) | 6.6 ± 2.35 | 5.9 ± 3.31 |
| Hematological diagnosis | ||
| Hb SS/HbS-β thal | 42/7 | 39/9 |
| Surgical procedure (n) | ||
| Tonsillectomy | 6 | 5 |
| Adenoidectomy | 4 | 5 |
| Adenotonsillectomy | 39 | 38 |
- Values are expressed in number (%) or mean ± standard deviation.
| Simple transfusion (group 1) n = 49 | Non-transfusion (group 2) n = 48 | p value | |
|---|---|---|---|
| Hb (g/dL) Mean ± SD | 8.11 ± 0.387 | 8.09 ± 0.365 | 0.794 |
| Hemoglobin S (%) (Pre-transfusion) | 76.3 ± 16.3 | 75.6 ± 15.5 | 0.829 |
| Preoperative transfused PRBCs | |||
| Total (ml) | 6685 | ||
| (Mean ± SD) | (141.3 ± 115.7) | ||
| ml/kg (Mean ± SD) | 7.14 ± 0.3 | ||
- PRBCs = packed red blood cells.
- Values are expressed in number (%) or mean ± standard deviation.
- *The result is highly significant at p ≤ 0.5.
| Complication | Simple transfusion group (n = 49) | Non-transfusion group (n = 48) | p value |
|---|---|---|---|
| Surgical | |||
| No complications | 45 | 46 | 0.606 |
| Primary hemorrhage | 3 | 1 | |
| Secondary hemorrhage | 1 | 1 | |
| Non-surgical | |||
| No complications | 46 | 47 | 0.85 |
| VOC | 2 | 1 | |
| ACS | 1 | 0 | |
| Average postoperative hospital stay: (days) | |||
| Mean ± SD | 2.26 ± 0.784 | 2.17 ± 0.519 | 0.607 |
- Values are expressed in number (%) or mean ± standard deviation.
One patient in the non-transfusion group, and three patients in the simple transfusion group suffered a primary hemorrhage due to mild tonsillar vein bleeding that required no blood transfusion. In addition, one patient in each group developed secondary hemorrhage due to local infection at the site of adenotonsillectomy within 5-7 days of surgery respectively. This hemorrhage was controlled effectively with IV amoxicillin/clavulinic acid without transfusion. Collectively, four patients developed postoperative SCD-related complications and all of them were Hb SS disease. VOCs occurred in a comparable number of patients in the two groups. These episodes responded to IV hydration and analgesia. Acute chest syndrome developed in one patient in the simple transfusion group. The patient in the simple transfusion group was an 8-year-old boy and his pre-operative Hb was 8.3 g/dL. He underwent partial exchange transfusion with 375 mL PRBCs during the attack. The child improved and was discharged on the fifth postoperative day with no further transfusions. Overall, the length of hospital stay did not vary significantly among the two studied arms.
As shown in Table 4, SCD-related complications occurred exclusively in cases with Hb SS disease. However, this result was not significant. On the other hand, surgical complications were found in 4 out of 81 cases (4.9%) with Hb SS, compared to 2 out of 16 cases (12.5%) with S/β thalassemia, and the difference also was not significant.
| Complications | Hb SS disease (81 cases) | S/β Thalassemia (16 cases) | p value |
|---|---|---|---|
| Surgical | |||
| Yes | 4 | 2 | 0.256 |
| No | 77 | 14 | |
| SCD-related | |||
| Yes | 4 | 0 | 1 |
| No | 77 | 16 |
DISCUSSION
In addressing the need for PRBC transfusion prior to surgery in stable patients with SCD undergoing adenotonsillectomy and documenting its risks and benefits, we have performed a prospective randomized controlled study including all pediatric patients with SCD admitted to Sultan Qaboos University Hospital (SQUH) for tonsillectomy, adenoidectomy, or both procedures. The main inclusion criterion was a preoperative Hb level ranging from 7.5 to 8.9 g/dL. Ninety-seven patients were eligible for the study and were randomly assigned into two arms; simple top-up PRBC transfusion to a target Hb of 10 g/dL and no transfusion.
In the current study, the incidence of postoperative surgical and SCD-related complications was comparable in the two studied arms. None of the patients in the non-transfusion arm developed acute chest syndrome, which is one of the most fearful postoperative complications of SCD. In addition, the surgical outcome of both study arms was almost similar. Moreover, the preoperative transfusion strategy did not affect the length of average postoperative hospital stay in the two studied groups.
Although the difference did not reach a significant level, it is noteworthy that all the four patients who developed SCD-related complications have been found to have homozygous sickle cell anemia (Hb SS disease), constituting 4.9% of this subgroup of patients.
Avoiding preoperative PRBC transfusion in SCD patients prevents all known hazards of blood products including circulatory overload, transmission of blood-borne infections, alloimmunization, hyperviscosity, iron overload, and transfusion reactions.19
There is no agreed upon consensus on optimum perioperative management of children with SCD. A wide variation is noted among clinicians in different centers. Decision is governed mainly by physician preference, arbitrary measures of disease severity and the type of surgical procedure. In a survey of North American Pediatric anesthetists, it has been found that 17 to 51% of the contributing clinicians would transfuse the patient to a hemoglobin concentration of 10 g/dL preoperatively. This wide range (17%-51%) depends on disease severity and the type of surgery.20 Moreover, the National Institute of Health (NIH) evidence-based guideline of sickle cell disease management recommends that expert opinion should be sought for appropriate preoperative transfusion decision in patients with Hb SS disease who have a hemoglobin level more than 8.5 g/dL, indicating that transfusion decision can be individualized based on various factors.8
A number of previous studies have elucidated the need for preoperative transfusion in SCD. In (TAPS) study, Howard et al12 have found that preoperative transfusion is associated with less risk of postoperative SCD-related complications, compared to non-transfusion strategy in patients with Hb SS disease. This trial was discontinued prematurely due to high incidence of ACS. Although TAPS was a well-designed multicenter randomized controlled clinical trial, it included both adult and pediatric patients who underwent a variety of low- and moderate-risk surgeries not limited to adenotonsillectomy. Moreover, the authors have selected a lower cut-off Hb of 6.5 gm/dL as an inclusion criterion. These factors might explain the difference between our findings and the TAPS trial.
Recently, Estcourt et al21 and Alotaibi et al22 have conducted a systematic review and meta-analysis on preoperative transfusion in SCD. The authors found no difference between aggressive (defined as bringing Hb S level to less than 30%) and conservative (defined as increasing the total Hb to more than 10 gm/dL) transfusion strategies as regards mortality and postoperative complications. Despite paucity of clinical trials and low quality evidence, Estcourt et al21 stated that there was no difference in mortality and postoperative VOC between people receiving preoperative transfusions and those receiving no preoperative transfusions. There is a low quality evidence that preoperative transfusion may prevent development of acute chest syndrome, but it might increase the risk of circulatory overload.21 However, preoperative transfusion was not protective in the current study, as none of our patients in the non-transfusion arm developed ACS, compared to one case in the other arm. Of note, our enrolled patients represent a relatively low risk population overall. We did not encounter any cases of circulatory overload, mostly because of strict calculation of transfusion needs in pediatric age group and setting of a target Hb of 9.5-10 gm/dL.
Data from our geographical area are sparse. In a retrospective study, Al-Samak et al23 reported that exchange transfusion does not prevent perioperative complications of SCD patients. The authors considered a HBS of more than 40% as a risk factor of postoperative complications.
As far as adenotonsillectomy is particularly concerned, historical data had recommended a sort of preoperative transfusion therapy, either simple transfusion or exchange, for most patients with SCD.113) In 1999, Waldron et al10 found no difference between aggressive and conservative transfusion in reduction of postoperative SCD-related complications. Contrary to our results, a retrospective study done by Cavalier et al24 in 2009 reported a higher incidence of postoperative SCD-related complications (23%) in non-transfused subjects after adenotonsillectomy. A possible explanation is that the authors did not exclude patients with a previous history of ACS.
In addition to the medical complications of transfusion, the cost may be another burden. Minimizing preoperative transfusion is cost effective.25 We have calculated the cost of a single unit transfusion (excluding man power and the time needed to process the request) and it was around $52 per unit of blood and $135-285 for viral screening (the higher value is for PCR confirmation if needed).
The limitations of the current study are the relatively small sample size, and single institution experience that might cause a selection bias.
In conclusion, relatively low risk sickle cell disease patients without history of recent acute chest syndrome, or transfusion, and with a hemoglobin level above 7.5 g/dL might not need any transfusion prior to adenotonsillectomy. This approach is safe and does not increase the risk of postoperative surgical or SCD-related complications. We recommend performing more multicenter randomized controlled trials locally and regionally on different types of surgeries for better characterization of our findings and possible generation of local guidelines.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.




