Top down thinking: how uncrossmatched RBCs confounded ABO typing
ABBREVIATIONS
-
- ED
-
- emergency department
-
- MF
-
- mixed field
-
- WBIT
-
- wrong blood in tube
Obtaining a valid type and screen is an essential step in ensuring recipient safety. When a patient receives uncrossmatched group O red blood cells (RBCs) because of an urgent need for transfusion, it is important that the sample for the type and screen is drawn early in the resuscitation to prevent these cells from interfering with the determination the recipient's native ABO type in the forward typing. A recent study demonstrated that the ABO type of 665/695 (95.7%) non–group O recipients could be accurately determined on the first type and screen sample obtained by the blood bank after the transfusion of uncrossmatched type O RBC–containing products (i.e., RBCs and whole blood units). However, the likelihood of obtaining a valid ABO type decreased as the number of uncrossmatched group O RBC–containing products increased such that 15% of patients who received more than 10 units did not have a valid ABO type on the first sample.1 The following is a case report of a patient who received uncrossmatched group O RBCs and whose ABO group was discrepant on a subsequent sample received by the blood bank.
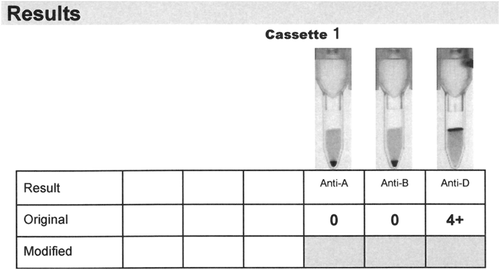
The patient was an 81-year-old male who presented to the emergency department (ED) with a hemoglobin concentration of 2.0 mmol/L (3.22 g/dL) due to persistent upper gastrointestinal bleeding caused in part by dysregulated warfarin treatment. His admission vital signs were as follows: heart rate, 128 bpm; respiratory rate, 100 bpm; blood pressure, 60/33 mmHg; and oxygen saturation, 80% on room air. His ABO type had been established as A RhD positive on earlier admissions to this hospital. As the patient was unstable, the decision to transfuse him with uncrossmatched RBC units was made. He received 2 O RhD-negative units from the ED refrigerator (both stored for 4 days) and then 4 additional O RhD-positive units from the blood bank (all stored for 33 days). All 6 units were leucoreduced and stored in SAGM (maximum shelf life, 35 days). The transfusions were tolerated well. The sample for the type and screen was drawn after the patient received these 6 units. When the sample arrived at the blood bank, it was properly labeled with all the necessary patient identification, and it was accepted for testing. The sample was centrifuged per protocol for 300 seconds at 1800 × g with a centrifuge (Rotanta 460, Hettich). Within minutes, the sample was transferred to a blood bank analyzer (Ortho Vision Analyzer, Ortho Clinical Diagnostics) on which the forward and D typing was performed with cassettes (Ortho BioVue Anti-A/Anti-B/Anti-D cassettes, Ortho Clinical Diagnostics). The forward type was unequivocally blood group O RhD positive and mixed field (MF) agglutination was not detected in either the anti-A or anti-D column (Fig. 1). As he had historically typed group A RhD positive, some level of MF agglutination had been expected, and the initial explanation for the discrepant ABO group was that a sampling error had occurred. Therefore, a second sample was drawn from the patient. The forward type on this second sample was serologically identical to that of the first sample; that is, the patient was group O RhD positive, with no MF.
The reverse typing (Ortho BioVue System, Anti-A/Anti-B/Anti-D Control/Reverse Diluent, Ortho Clinical Diagnostics) performed on the second sample demonstrated the complete absence of anti-A and a strong anti-B was detected, suggesting that the patient was group A, as he had been found to be on earlier admissions (data not shown).
To further elucidate the typing discrepancy, a manual forward type was performed using gel card (DiaClon ABD-Confirmation for Patients ID gel card, BioRad Laboratories) with 1) RBCs aspirated from close to the bottom of the centrifuged tube, thus imitating the aspiration mode of the Ortho Vision Analyzer; and 2) RBCs aspirated from close to the top of the tube.
When the RBCs were aspirated from the bottom of the sample tube, a significant population of unagglutinated group O RBCs was observed along with a minor population of strongly agglutinated A cells (Fig. 2, right-hand side). The B typing was uncomplicated, and the D typing was strongly positive, with a small amount of MF agglutination detected. When the RBCs were aspirated from the top of the sample tube, the majority of the RBCs typed as group A, with a minor population of group O RBCs. The B and D testing results were identical to the results seen when the cells were aspirated from the bottom of the tube.

The small difference in ABO typing the RBC from the bottom of the tube with the manual method as compared to the Ortho Vision is most likely due to the manual pipetting being slightly more unprecise, but differences in formulation of the BioRad gel card and the Ortho cassettes cannot be ruled out. With regard to top-bottom typing discrepancy, it would have been tempting to postulate that the donor RBCs settled to the bottom of the test tube due to having been close maximum shelf life. However, it is known that the mean corpuscular volume of stored RBCs tends to increase2 and the density of stored RBCs either does not change3 or decreases during storage4 which would not explain why the donor RBCs were found at the bottom of the tube.
In contrast, the comparatively fresh O RhD-negative RBCs did not influence typing insofar as the reactivity with anti-D did not change according to whether the RBCs were aspirated from the top or the bottom of the tube.
It may be concluded that in the evaluation of a sample that appears to contain a wrong blood in tube (WBIT) error in a patient who has been transfused with ABO and or Rh-nonidentical RBCs, the RBCs from the top and the bottom of the tube could be tested to determine if a true WBIT error had occurred during sample collection or if the same phenomenon as described in this report was occurring.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.




