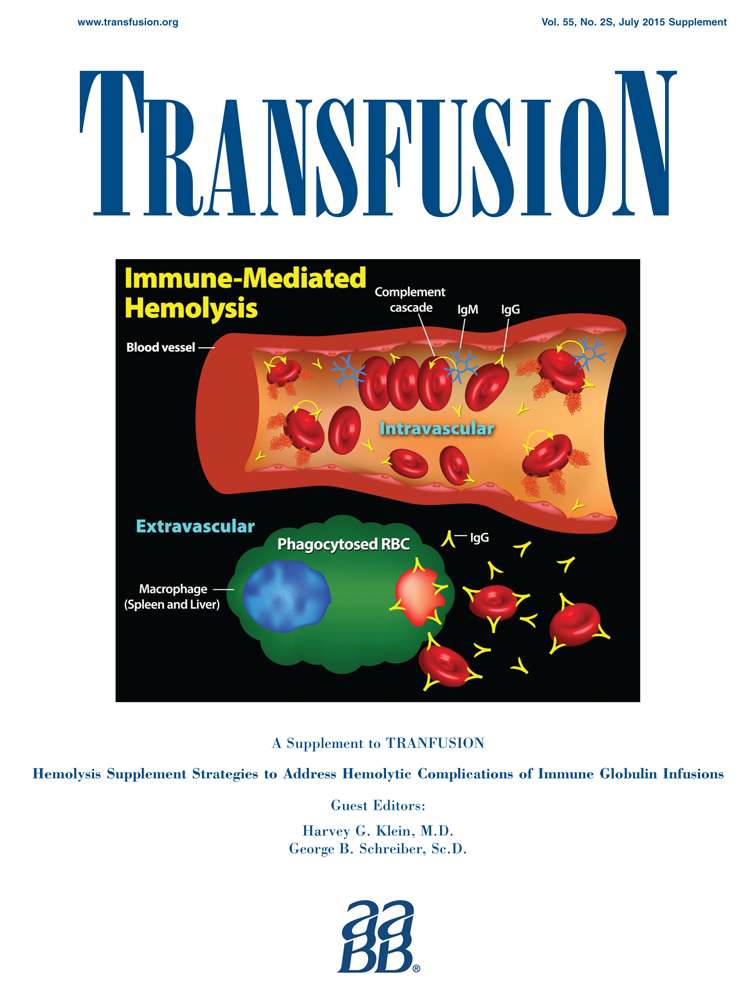
Pathogenesis and mechanisms of antibody-mediated hemolysis
Corresponding Author
Willy A. Flegel
Department of Transfusion Medicine, NIH Clinical Center, National Institutes of Health, Bethesda, Maryland
Address reprint requests to: Willy A. Flegel, MD, Department of Transfusion Medicine, NIH Clinical Center, National Institutes of Health, Bethesda, MD 20892; e-mail: [email protected].Search for more papers by this authorCorresponding Author
Willy A. Flegel
Department of Transfusion Medicine, NIH Clinical Center, National Institutes of Health, Bethesda, Maryland
Address reprint requests to: Willy A. Flegel, MD, Department of Transfusion Medicine, NIH Clinical Center, National Institutes of Health, Bethesda, MD 20892; e-mail: [email protected].Search for more papers by this authorThis work was supported by the Intramural Research Program of the NIH Clinical Center.
The views expressed do not necessarily represent the view of the National Institutes of Health, the Department of Health and Human Services, or the U.S. Federal Government.
This manuscript represents the author's thoughts on the subject and his interpretation of a set of slides prepared by Dr George Garratty (Slide Set S1, available as supporting information in the online version of this paper) as presented in the transcript of the Strategies to Address Hemolytic Complications of Immune Globulin Infusions Workshop organized by FDA/PPTA/NHLBI in Bethesda on January 28, 2014.
Abstract
BACKGROUND
The clinical consequences of antibodies to red blood cells (RBCs) have been studied for a century. Most clinically relevant antibodies can be detected by sensitive in vitro assays. Several mechanisms of antibody-mediated hemolysis are well understood. Such hemolysis after transfusion is reliably avoided in a donor-recipient pair, if one individual is negative for the cognate antigen to which the other has the antibody.
STUDY DESIGN AND RESULTS
Mechanisms of antibody-mediated hemolysis were reviewed based on a presentation at the Strategies to Address Hemolytic Complications of Immune Globulin Infusions Workshop addressing intravenous immunoglobulin (IVIG) and ABO antibodies. The presented topics included the rates of intravascular and extravascular hemolysis; immunoglobulin (Ig)M and IgG isoagglutinins; auto- and alloantibodies; antibody specificity; A, B, A,B, and A1 antigens; A1 versus A2 phenotypes; monocytes-macrophages, other immune cells, and complement; monocyte monolayer assay; antibody-dependent cell-mediated cytotoxicity; and transfusion reactions due to ABO and other antibodies.
CONCLUSION
Several clinically relevant questions remained unresolved, and diagnostic tools were lacking to routinely and reliably predict the clinical consequences of RBC antibodies. Most hemolytic transfusion reactions associated with IVIG were due to ABO antibodies. Reducing the titers of such antibodies in IVIG may lower the frequency of this kind of adverse event. The only way to stop these events is to have no anti-A or anti-B in the IVIG products.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
| Filename | Description |
|---|---|
| trf13147-sup-0001-suppinfo.pdf4.7 MB |
Slide Set S1. George Garratty: Pathogenesis and mechanisms of immune hemolytic anemia. January 2014. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1 Garratty G. Erythrocyte-bound complement components in health and disease. Prog Clin Biol Res 1980; 43: 133-55.
- 2 Garratty G. The James Blundell Award Lecture 2007: do we really understand immune red cell destruction? Transfus Med 2008; 18: 321-34.
- 3 Garratty G. The significance of IgG on the red cell surface. Transfus Med Rev 1987; 1: 47-57.
- 4 Freedman J. The significance of complement on the red cell surface. Transfus Med Rev 1987; 1: 58-70.
- 5
Petz LD,
Garratty G. Immune hemolytic anemias. Philadelphia: Churchill Livingstone/Elsevier Science; 2004. p. 133-4.
10.1016/B978-0-443-08559-8.50008-X Google Scholar
- 6 Flegel WA, Natanson C, Klein HG. Does prolonged storage of red blood cells cause harm? Br J Haematol 2014; 165: 3-16.
- 7 Quinti I, Pulvirenti F, Milito C, et al. Hemolysis in patients with antibody deficiencies on immunoglobulin replacement treatment. Transfusion 2015;55:1067-74.
- 8 Michelis FV, Branch DR, Scovell I, et al. Acute hemolysis after intravenous immunoglobulin amid host factors of ABO-mismatched bone marrow transplantation, inflammation, and activated mononuclear phagocytes. Transfusion 2014; 54: 681-90.
- 9 Desborough MJ, Miller J, Thorpe SJ, et al. Intravenous immunoglobulin-induced haemolysis: a case report and review of the literature. Transfus Med 2014; 24: 219-26.
- 10 Fontaine MJ, Mills AM, Weiss S, et al. How we treat: risk mitigation for ABO-incompatible plasma in plateletpheresis products. Transfusion 2012; 52: 2081-5.
- 11 Pendergrast JM, Pavenski K, Hannach B. Does deficiency of plasma A/B substances increase the risk of IVIG-mediated hemolysis? Transfusion 2007; 47: 197A.
- 12 Daniel-Johnson J, Leitman S, Klein H, et al. Probiotic-associated high-titer anti-B in a group A platelet donor as a cause of severe hemolytic transfusion reactions. Transfusion 2009; 49: 1845-9.
- 13 Kahwaji J, Barker E, Pepkowitz S, et al. Acute hemolysis after high-dose intravenous immunoglobulin therapy in highly HLA sensitized patients. Clin J Am Soc Nephrol 2009; 4: 1993-7.
- 14 Sakem B, Matozan K, Nydegger UE, et al. Anti-red blood cell antibodies, free light chains, and antiphospholipid antibodies in intravenous immunoglobulin preparations. Isr Med Assoc J 2013; 15: 617-21.
- 15 Rosse WF, Hillmen P, Schreiber AD. Immune-mediated hemolytic anemia. Hematology Am Soc Hematol Educ Program 2004: 48-62.
- 16 Perlmann P, Holm G. Cytotoxic effects of lymphoid cells in vitro. Adv Immunol 1969; 11: 117-93.
- 17 Holm G. Lysis of antibody-treated human erythrocytes by human leukocytes and macrophages in tissue culture. Int Arch Allergy Appl Immunol 1972; 43: 671-82.
- 18 Hunt JS, Beck ML, Hardman JT, et al. Characterization of human erythrocyte alloantibodies by IgG subclass and monocyte interaction. Am J Clin Pathol 1980; 74: 259-64.
- 19 Schanfield MS, Stevens JO, Bauman D. The detection of clinically significant erythrocyte alloantibodies using a human mononuclear phagocyte assay. Transfusion 1981; 21: 571-6.
- 20 Conley CL, Lippman SM, Ness PM, et al. Autoimmune hemolytic anemia with reticulocytopenia and erythroid marrow. N Engl J Med 1982; 306: 281-6.
- 21 Gallagher MT, Branch DR, Mison A, et al. Evaluation of reticuloendothelial function in autoimmune hemolytic anemia using an in vitro assay of monocyte-macrophage interaction with erythrocytes. Exp Hematol 1983; 11: 82-9.
- 22 Garratty G. Predicting the clinical significance of red cell antibodies with in vitro cellular assays. Transfus Med Rev 1990; 4: 297-312.
- 23 van der Meulen FW, van der Hart M, Fleer A, et al. The role of adherence to human mononuclear phagocytes in the destruction of red cells sensitized with non-complement binding IgG antibodies. Br J Haematol 1978; 38: 541-9.
- 24 Hernandez JA, Steane SM. Erythrophagocytosis by segmented neutrophils in paroxysmal cold hemoglobinuria. Am J Clin Pathol 1984; 81: 787-9.
- 25 Wagner C, Iking-Konert C, Denefleh B, et al. Granzyme B and perforin: constitutive expression in human polymorphonuclear neutrophils. Blood 2004; 103: 1099-104.
- 26 Fanger NA, Voigtlaender D, Liu C, et al. Characterization of expression, cytokine regulation, and effector function of the high affinity IgG receptor Fc gamma RI (CD64) expressed on human blood dendritic cells. J Immunol 1997; 158: 3090-8.
- 27
Roos D,
Spits H,
Hack CE. Innate immunity — phagocytes, natural killer cells and the complement system. In: FP Nijkamp, MJ Parnham, editors. Principles of immunopharmacology. Basel: Birkhäuser Verlag; 2005. p. 63-80.
10.1007/3-7643-7408-X_5 Google Scholar
- 28 Urbaniak SJ. Lymphoid cell dependent (K-cell) lysis of human erythrocytes sensitized with Rhesus alloantibodies. Br J Haematol 1976; 33: 409-13.
- 29 Northoff H, Grätz W, Schultze D. Anti-D vermittelte zelluläre Zytotoxizität. Ärztl Lab 1977; 23: 481-6.
- 30 Northoff H, Kluge A, Resch K. Antibody dependent cellular cytotoxicity (ADCC) against human erythrocytes, mediated by human blood group alloantibodies: a model for the role of antigen density in target cell lysis. Z Immunitätsforsch 1978; 154: 15-33.
- 31 Groscurth P, Filgueira L. Killing mechanisms of cytotoxic T lymphocytes. News Physiol Sci 1998; 13: 17-21.
- 32 Handwerger BS, Kay NE, Douglas SD. Lymphocyte-mediated antibody-dependent cytolysis: role in immune hemolysis. Vox Sang 1978; 34: 276-80.
- 33 Olovnikova NI, Belkina EV, Nikolaeva TL, et al. Lymphocyte antibody-dependent cytotoxicity test for evaluation of clinical role of monoclonal anti-D-antibodies for prevention of Rhesus sensitization. Bull Exp Biol Med 2006; 141: 57-61.
- 34 Kurlander RJ, Rosse WF, Ferreira E. Quantitative evaluation of antibody-dependent lymphocyte-mediated lysis of human red cells. Am J Hematol 1979; 6: 295-311.
- 35 Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011; 11: 723-37.
- 36 Ohgimoto K, Ohgimoto S, Ihara T, et al. Difference in production of infectious wild-type measles and vaccine viruses in monocyte-derived dendritic cells. Virus Res 2007; 123: 1-8.
- 37 Voigt J, Hunniger K, Bouzani M, et al. Human natural killer cells acting as phagocytes against Candida albicans and mounting an inflammatory response that modulates neutrophil antifungal activity. J Infect Dis 2014; 209: 616-26.
- 38 Zupańska B. Assays to predict the clinical significance of blood group antibodies. Curr Opin Hematol 1998; 5: 412-6.
- 39 Salama A, Mueller-Eckhardt C. Delayed hemolytic transfusion reactions; evidence for complement activation involving allogeneic and autologous red cells. Transfusion 1984; 24: 188-93.
- 40 Arndt P, Garratty G. Evaluation of the optimal incubation temperature for detecting certain IgG antibodies with potential clinical significance. Transfusion 1988; 28: 210-3.
- 41 Pruss A, Salama A, Ahrens N, et al. Immune hemolysis—serological and clinical aspects. Clin Exp Med 2003; 3: 55-64.
- 42 Swiecicki PL, Hegerova LT, Gertz MA. Cold agglutinin disease. Blood 2013; 122: 1114-21.
- 43 Kelton JG, Hamid C, Aker S, et al. The amount of blood group A substance on platelets is proportional to the amount in the plasma. Blood 1982; 59: 980-5.
- 44 Nydegger UE, Tevaearai H, Berdat P, et al. Histo-blood group antigens as allo- and autoantigens. Ann N Y Acad Sci 2005; 1050: 40-51.
- 45 Rachkewich RA, Crookston MC, Tilley CA, et al. Evidence that blood group A antigen on lymphocytes is derived from the plasma. J Immunogenet 1978; 5: 25-9.
- 46 Branch DR. Anti-A and anti-B: what are they and where do they come from? Transfusion 2015; 55 (Suppl 2): S74-S79.
- 47 Siani B, Willimann K, Wymann S, et al. Donor screening reduces the isoagglutinin titer in immunoglobulin products. Transfusion 2015; 55 (Suppl 2): S95-S97.
- 48 Landsteiner K. Zur Kenntnis der antifermentativen, lytischen und agglutinierenden Wirkungen des Blutserums und der Lymphe. Zbl Bakt Hyg I Abt Orig A 1900; 27: 357-62.
- 49 Ford WW, Halsey JT. Contributions to the study of hemagglutinins and hemolysins. J Med Res 1904; 11: 403-25.
- 50 Daufi L, Rondell P. Universality of anti-A and anti-B human isolysins. Vox Sang 1971; 21: 81-5.
- 51 Anliker M, von Zabern I, Höchsmann B, et al. A new blood group antigen is defined by anti-CD59, detected in a CD59-deficient patient. Transfusion 2014; 54: 1817-22.
- 52 Telen MJ, Hall SE, Green AM, et al. Identification of human erythrocyte blood group antigens on decay-accelerating factor (DAF) and an erythrocyte phenotype negative for DAF. J Exp Med 1988; 167: 1993-8.
- 53 Motoyama N, Okada N, Yamashina M, et al. Paroxysmal nocturnal hemoglobinuria due to hereditary nucleotide deletion in the HRF20 (CD59) gene. Eur J Immunol 1992; 22: 2669-73.
- 54 Höchsmann B, Dohna-Schwake C, Kyrieleis HA, et al. Targeted therapy with eculizumab for inherited CD59 deficiency. N Engl J Med 2014; 370: 90-2.
- 55 Telen MJ. Glycosyl phosphatidylinositol-linked blood group antigens and paroxysmal nocturnal hemoglobinuria. Transfus Clin Biol 1995; 2: 277-90.
- 56 Cooling L. ABO and platelet transfusion therapy. Immunohematology 2007; 23: 20-33.
- 57 Perry H, Henry S. Training students in serologic reaction grading increased perception of self-efficacy and ability to recognize serologic reactions but decreased grading accuracy. Transfusion 2015 [Epub ahead of print].
- 58 Hill EA, Bryant BJ. Comparison of antibody titers in donor specimens and associated AS-1 leukoreduced donor units. Transfusion 2014; 54: 1580-4.
- 59 Quillen K, Sheldon SL, Daniel-Johnson JA, et al. A practical strategy to reduce the risk of passive hemolysis by screening plateletpheresis donors for high-titer ABO antibodies. Transfusion 2011; 51: 92-6.
- 60 Henry S, Clark P, Woodfield G. High titre IgG ABO antibodies in group O Polynesian and European blood donors: incidence, variability, racial and gender differences. N Z J Med Lab Sci 1995; 49: 164-5.
- 61 Pierce RN, Reich LM, Mayer K. Hemolysis following platelet transfusions from ABO-incompatible donors. Transfusion 1985; 25: 60-2.
- 62 Stainsby D. ABO incompatible transfusions—experience from the UK Serious Hazards of Transfusion (SHOT) scheme Transfusions ABO incompatible. Transfus Clin Biol 2005; 12: 385-8.
- 63 Thorpe SJ, Fox BJ, Dolman CD, et al. Batches of intravenous immunoglobulin associated with adverse reactions in recipients contain atypically high anti-Rh D activity. Vox Sang 2003; 85: 80-4.
- 64 Garratty G. How concerned should we be about missing antibodies to low incidence antigens? Transfusion 2003; 43: 844-7.
- 65 Cherian G, Search S, Thomas E, et al. An acute haemolytic transfusion reaction caused by anti-Wr. Transfus Med 2007; 17: 312-4.
- 66 Boctor FN. Overt immediate hemolytic transfusion reaction attributable to anti-Wr(a). Immunohematology 2008; 24: 113-5.
- 67 Oberman HA, Beck ML. Red blood cell sensitization due to unexpected Rh antibodies in immune serum globulin. Transfusion 1971; 11: 382-4.
- 68 Rushin J, Rumsey DH, Ewing CA, et al. Detection of multiple passively acquired alloantibodies following infusions of IV Rh immune globulin. Transfusion 2000; 40: 551-4.
- 69 Narra S, Al-Kawas F, Carroll JE, et al. Biliary sludge and obstruction: another sign of a delayed hemolytic transfusion reaction. Transfusion 2011; 51: 686-7.
- 70 Novaretti MC, Gallucci AO, Dorlhiac-Llacer PE, et al. Rapid detection of high titer anti-A/anti-B in blood donors using an Olympus PK 7200 equipment. Transfusion 2009; 49: 116A.
- 71
Flegel WA,
Wagner FF. Blutgruppen: Alloantigene auf Erythrozyten. In: V Kiefel, editor. Transfusionsmedizin und Immunhamatologie. Berlin: Springer; 2010. p. 133-68.
10.1007/978-3-642-12765-6_11 Google Scholar
- 72 Svensson L, Rydberg L, de Mattos LC, et al. Blood group A and A revisited: an immunochemical analysis. Vox Sang 2009; 96: 56-61.
- 73 Northoff H, Wölpl A, Sugg U, et al. An unusual sample of irregular anti-A1, probably causing an early delayed transfusion reaction. Blut 1986; 52: 317-21.
- 74 Obukhova P, Korchagina E, Henry S, et al. Natural anti-A and anti-B of the ABO system: allo- and autoantibodies have different epitope specificity. Transfusion 2012; 52: 860-9.
- 75 Salama A, Hugo F, Heinrich D, et al. Deposition of terminal C5b-9 complement complexes on erythrocytes and leukocytes during cardiopulmonary bypass. N Engl J Med 1988; 318: 408-14.
- 76 Gaither TA, Vargas I, Inada S, et al. The complement fragment C3d facilitates phagocytosis by monocytes. Immunology 1987; 62: 405-11.
- 77 Bajic G, Yatime L, Sim RB, et al. Structural insight on the recognition of surface-bound opsonins by the integrin I domain of complement receptor 3. Proc Natl Acad Sci U S A 2013; 110: 16426-31.
- 78 von Zabern I, Ehlers M, Grunwald U, et al. Release of mediators of systemic inflammatory response syndrome in the course of a severe delayed hemolytic transfusion reaction caused by anti-D. Transfusion 1998; 38: 459-68.
- 79 Pendergrast J, Willie-Ramharack K, Riden SL, et al. The role of inflammation in intravenous immune globulin (IVIG)-mediated hemolysis. Transfusion 2015; 55 (Suppl 2): S65-S73.
- 80 Branch DR, Gallagher MT, Mison AP, et al. In vitro determination of red cell alloantibody significance using an assay of monocyte-macrophage interaction with sensitized erythrocytes. Br J Haematol 1984; 56: 19-29.
- 81 Arndt PA, Garratty G. A retrospective analysis of the value of monocyte monolayer assay results for predicting the clinical significance of blood group alloantibodies. Transfusion 2004; 44: 1273-81.
- 82 Ness PM, Shirey RS, Thoman SK, et al. The differentiation of delayed serologic and delayed hemolytic transfusion reactions: incidence, long-term serologic findings, and clinical significance. Transfusion 1990; 30: 688-93.
- 83 Pineda AA, Vamvakas EC, Gorden LD, et al. Trends in the incidence of delayed hemolytic and delayed serologic transfusion reactions. Transfusion 1999; 39: 1097-103.
- 84 Flegel WA, Johnson ST, Keller MA, et al. Molecular immunohematology round table discussions at the AABB Annual Meeting, Boston 2012. Blood Transfus 2014; 12: 280-6.
- 85 Keller-Stanislawski B, Lohmann A, Günay S, et al. The German Haemovigilance System—reports of serious adverse transfusion reactions between 1997 and 2007. Transfus Med 2009; 19: 340-9.
- 86 Stainsby D, Jones H, Asher D, et al. Serious hazards of transfusion: a decade of hemovigilance in the UK. Transfus Med Rev 2006; 20: 273-82.
- 87 Robillard P, Nawej KI, Jochem K. The Quebec hemovigilance system: description and results from the first two years. Transfus Apher Sci 2004; 31: 111-22.
- 88 Vamvakas EC, Blajchman MA. Blood still kills: six strategies to further reduce allogeneic blood transfusion-related mortality. Transfus Med Rev 2010; 24: 77-124.
- 89 Reddy DR, Guru PK, Blessing MM, et al. Transfusion-related acute lung injury after IVIG for myasthenic crisis. Neurocrit Care 2015 Feb 13 [Epub ahead of print].
- 90 Linden JV, Wagner K, Voytovich AE, et al. Transfusion errors in New York State: an analysis of 10 years’ experience. Transfusion 2000; 40: 1207-13.
- 91 Janatpour KA, Kalmin ND, Jensen HM, et al. Clinical outcomes of ABO-incompatible RBC transfusions. Am J Clin Pathol 2008; 129: 276-81.
- 92 Robillard P, Nawej KI, Garneau N. Trends in red cell-associated ABO mistransfusion, acute and delayed hemolytic and delayed serologic transfusion reactions in the Quebec Hemovigilance System. Transfus Apher Sci 2004; 31: 111-22.
- 93 Rieben R, Buchs JP, Flückiger E, et al. Antibodies to histo-blood group substances A and B: agglutination titers, Ig class, and IgG subclasses in healthy persons of different age categories. Transfusion 1991; 31: 607-15.
- 94 Holburn AM, Masters CA. The radioimmunoassay of serum and salivary blood group A and Lea glycoproteins. Br J Haematol 1974; 28: 157-67.
- 95 Gaensslen RE, Bell SC, Lee HC. Distributions of genetic markers in United States populations: I. Blood group and secretor systems. J Forensic Sci 1987; 32: 1016-58.
- 96 Holburn AM, Masters CA. The reactions of IgG and IgM anti-A and anti-B blood group antibodies with 125I-labelled blood group glycoproteins. Vox Sang 1974; 27: 115-23.
- 97 Zimring JC. Possible product risks related to presence of immune complexes, complement and immunoglobulin association, and other factors. Transfusion 2015; 55: S86-9.
- 98 O'Donghaile D, Kelley W, Klein HG, et al. Recommendations for transfusion in ABO-incompatible hematopoietic stem cell transplantation. Transfusion 2012; 52: 456-8.
- 99 Henry S. The Se(w) FUT2 mutation A385T does not result in a nonsecretor allele. Transfusion 2014; 54: 3255.
- 100 Ferrer-Admetlla A, Sikora M, Laayouni H, et al. A natural history of FUT2 polymorphism in humans. Mol Biol Evol 2009; 26: 1993-2003.
- 101 Tilley CA, Crookston MC, Brown BL, et al. A and B and A1Leb substances in glycosphingolipid fractions of human serum. Vox Sang 1975; 28: 25-33.
- 102 Castilho L, Rios M, Bianco C Jr, et al. DNA-based typing of blood groups for the management of multiply-transfused sickle cell disease patients. Transfusion 2002; 42: 232-8.