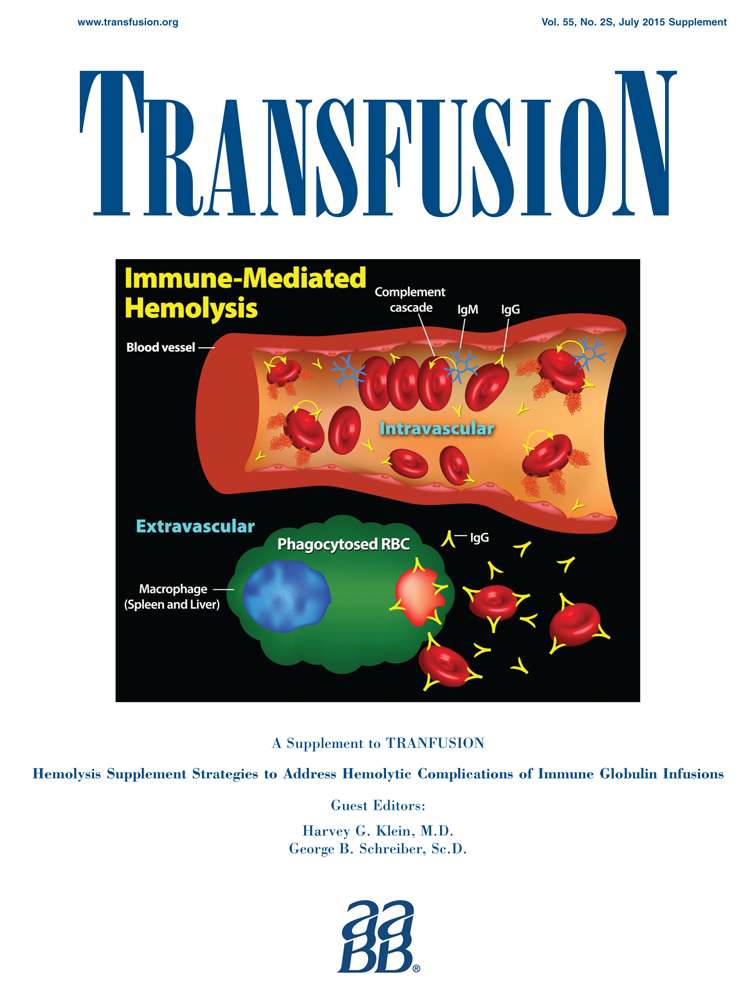
The role of isoagglutinins in intravenous immunoglobulin–related hemolysis
Abstract
BACKGROUND
Increased reporting of intravenous immunoglobulin (IVIG)-related hemolytic reactions (HRs) triggered an investigation by the German and Swiss health authorities to identify potential risk factors.
STUDY DESIGN AND METHODS
From the EudraVigilance database HRs reported between 2008 and 2013 were retrieved for seven IVIG preparations. HRs were classified as mild to moderate (hemoglobin [Hb] decline < 2 g/dL)] or severe (Hb decline > 2 g/dL) and separately analyzed for IVIG doses of less than 2 g/kg body weight and 2 g/kg body weight or more. It was assessed whether HR reporting rates correlate with the isoagglutinin content of the different preparations.
RESULTS
Of 569 HR cases retrieved, 103 cases were excluded due to insufficient data, leaving 466 for analysis. Ninety-three cases were classified as mild to moderate and 373 as severe. Approximately 80% of the severe HRs concerned patients with blood group A and only three patients with blood group O. Testing of isoagglutinin titers revealed substantial differences between the seven preparations. IVIG products with high anti-A/anti-B titers (≥32) had elevated HR reporting rates, particularly when cumulative doses at least 2 g/kg were administered.
CONCLUSION
The isoagglutinin content of IVIGs correlates with the risk for HRs. Exclusion of high-titer donations and manufacturing steps that deplete isoagglutinins should be considered for risk mitigation. In patients with blood groups A or AB receiving doses of at least 2 g/kg, the use of IVIG batches with low isoagglutinin titers should be considered to prevent HRs.