Intravenous immune globulin–related hemolysis: comparing two different methods for case assessment
Abstract
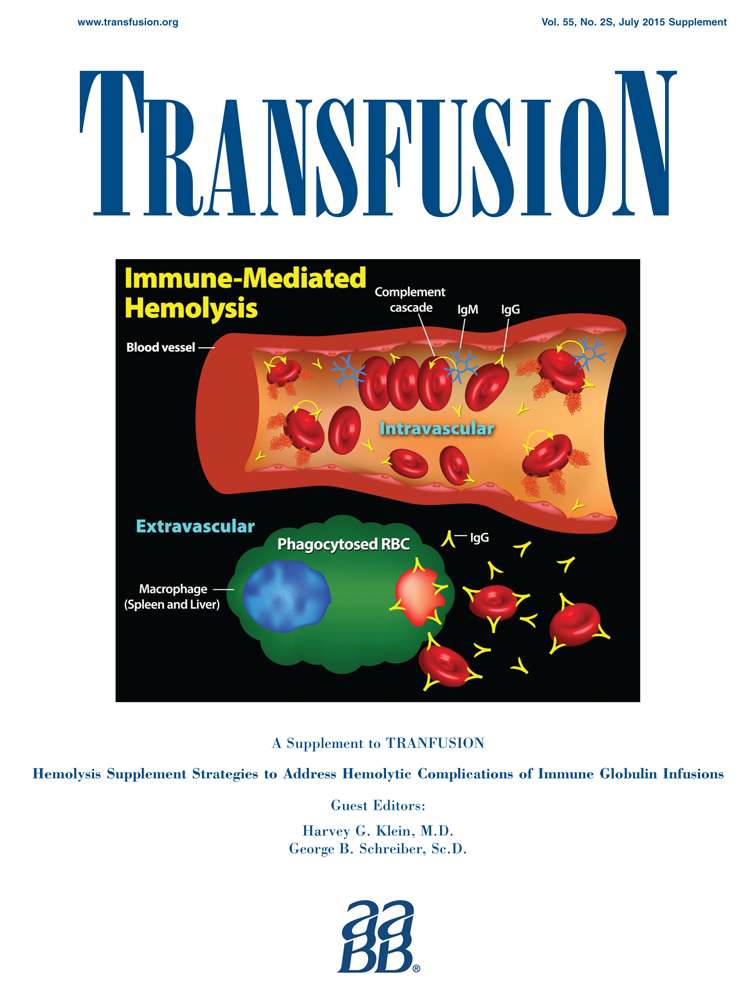
BACKGROUND
Hemolysis is a rare adverse event associated with the use of intravenous immune globulin (IVIG). Using the Canadian experience, this article compares two methods used for case ascertainment of hemolysis after IVIG.
STUDY DESIGN AND METHODS
This is a retrospective, descriptive analysis of case reports of hemolytic reaction after exposure to IVIG. The cases were ascertained from the Canada Vigilance database using the standardized MedDRA query for hemolytic disorders between the years 2006 and 2012 inclusively. The presence of a causal relationship was determined by using the Transfusion Transmitted Injuries Surveillance System (TTISS) algorithm. Cases were then reviewed against the standardized case definition of IVIG-associated hemolysis proposed by the IVIG Hemolysis Pharmacovigilance Group.
RESULTS
A total of 942 adverse event reports after exposure to IVIG had been received by Canada Vigilance during the study period. A search for the adverse event of hemolytic disorder retrieved 313 reports (33%). Using a modified TTISS definition of hemolysis and the TTISS causality assessment algorithm, 226 cases were found to be at least possibly related to administration of IVIG, whereas 69 cases met the definition of the IVIG Hemolysis Pharmacovigilance Group. The amount of IVIG distributed increased over the study period; this trend was not reflected in the number of adverse reaction reports for “hemolytic disorder” received.
CONCLUSION
The number of case reports per year received for the adverse event “hemolytic disorder” after exposure to IVIG fluctuated. The number of confirmed case reports varied depending on the case definition being used.