Suppression of tryptophan synthase activates cotton immunity by triggering cell death via promoting SA synthesis
Summary
Primary metabolism plays an important role in plant growth and development, however the relationship between primary metabolism and the adaptive immune response is largely unknown. Here, we employed RNA interference (RNAi), virus-induced gene silencing (VIGS) technology, phytohormone profiling, genetic studies, and transcriptome and metabolome analysis to investigate the function of the tryptophan synthesis pathway in the resistance of cotton to V. dahliae. We found that knock-down of GbTSA1 (Tryptophan Synthase α) and GbTSB1 (tryptophan synthase β) induced a spontaneous cell death phenotype in a salicylic acid (SA)-dependent manner and enhanced resistance to V. dahliae in cotton plants. Metabolome analysis showed that indole and indolic metabolites were highly accumulated in GbTSA1- or GbTSB1-silenced plants. Transcriptomic analysis showed that exogenous indole promotes the expression levels of genes involved in SA synthesis and the defense response. Similarly, indole application strongly enhanced cotton resistance to V. dahliae. These results suggested that metabolic intermediates in the Trp synthesis pathway may be a signal to activate SA synthesis. These results also provided a strategy to elicit plant defense responses by the application of indole.
Introduction
Higher plants produce a huge array of low-molecular-weight compounds that protect plants against biotic and abiotic stresses. These natural products can be classified into two types according to their function in the plant defense response: One is the plant natural products with antimicrobial or toxic activities, called phytoanticipins and phytoalexins, that play important roles in the plant defense response (Bednarek and Osbourn, 2009; Ahuja et al., 2012). Most of these defensive compounds are derived from amino acid precursors. One example is the phenylpropanoid secondary metabolic pathway, with the precursor phenylalanine metabolized through the pivotal enzyme phenylalanine-ammonia lyase (PAL). Many secondary metabolites, such as lignins and flavonoids (Ferrer et al., 2008; Hu et al., 2018), have antimicrobial and anti-insect functions. Another example is the glucosinolates, which are commonly found in Brassicales and can protect plants from fungal, bacterial, and herbivore attacks (Barth and Jander, 2006; Bednarek et al., 2009). These secondary metabolites can be classified into three types based on their amino acid precursors: aliphatic glucosinolates derived from Ala, Leu, Ile, Val, and Met; benzenic glucosinolates derived from Phe or Tyr; and indolic glucosinolates derived from Trp (Sønderby et al., 2010). Moreover, some alkaloids with antimicrobial functions, such as camalexin, are also derived from Trp (Sanchez-Vallet et al., 2010).
Another group of plant metabolites has regulatory activities, including the defense hormones salicylic acid (SA) and jasmonic acid (JA) and functions as defensive signals by coordinating the expression of defense-related genes and promoting plant-pathogen resistance. Salicylic acid is one of the key defense-related hormones that activate the defense responses against biotrophic and hemi-biotrophic pathogens (Loake and Grant, 2007). The major role of SA is in the establishment of systemic acquired resistance (SAR), which is a long-lasting and broad-spectrum form of disease resistance (Durrant and Dong, 2004). In higher plants, two pathways have been reported in SA synthesis: one is the Phe pathway, with synthesis in the cytoplasm; another is the chorismate pathway, which produces the greater fraction (almost 90%) of SA in chloroplasts (Wildermuth et al., 2001). There are two key genes in SA synthesis and signaling pathways. One is isochorismate synthase (ICS), which is the critical gene for SA synthesis in the chorismate pathway (Wildermuth et al., 2001), another is non-expressor of pathogenesis-related gene 1 (NPR1), the master gene for SA signaling transduction (Cao et al., 1997). Generally, pathogen-triggered SA synthesis occurs in the chloroplast by activating ICS1, and higher expression of ICS1 is usually coupled with an increase in SA levels (Wildermuth et al., 2001). Several transcription factors (TFs), such as SARD1 (Systemic Acquired Resistance Deficient 1), CBP60g (Calmodulin- Binding Protein 60 g) (Zhang et al., 2010; Wang et al., 2011), WRKY28 (van Verk et al., 2011) and WRKY75 (Guo et al., 2017) have been identified for their direct binding activities to the promoter of ICS1 and effects on SA accumulation. Finally, the increased SA levels in pathogen-challenged tissues can activate the expression of pathogenesis-related (PR) genes and enhance resistance to a broad range of pathogens (Bari and Jones, 2009). The role of SA in cotton−V. dahliae interaction has also been investigated in several studies. Mo and colleagues (Mo et al., 2015) cloned a gene encoding polyamine oxidase (PAO), which catalyzes the conversion of spermine (Spm) to spermidine (Spd). Constitutive expression of GhPAO in Arabidopsis increased resistance to V. dahliae, while silencing of GhPAO in cotton enhanced the susceptibility to V. dahliae. Further analysis suggested that GhPAO contributes to resistance against V. dahliae through mediation of SA, Spm, and camalexin signaling. Moreover, the comparison between resistant (G. barbadense) and susceptible (G. hirsutum) species revealed that the SA level was higher in G. barbadense both pre- and post-inoculation. Silencing of GbEDS1, a key gene in the SA synthesis, led to decreased SA production and increased pathogen susceptibility (Zhang et al., 2017). Further strong evidence for the defense signaling role of SA in cotton response to V. dahliae was confirmed through the functional characterization of the isochorismatase effector VdIsc1 secreted by V. dahliae (Liu et al., 2014). This effector was found to be required for virulence and suppressed SA accumulation in the host. In addition to this finding, another secretory effector VdSCP41 could target SARD1 and CBP60g to control SA synthesis and inhibit immunity (Qin et al., 2018).
Aside from plant hormones, other low-molecular-weight compounds also have elicitor properties to fight pathogens (Noutoshi et al., 2012a,2012b). It has been reported that the secondary bile acid deoxycholic acid (DCA) elicits defense in Arabidopsis and reduces the proliferation of pathogens (Zarattini et al., 2016). In addition, acetic acid has been reported to promote de novo JA synthesis and enrichment of histone H4 acetylation, which represent novel survival strategies against drought in plants (Kim et al., 2017).
Tryptophan is an essential amino acid required by animals for the production of proteins and other compounds such as neurohormones, serotonin and the vitamin nicotinic acid. In plants, it is a precursor of auxin and a variety of secondary metabolites, such as camalexin and glucosinolates, which are commonly found in Brassicales and can protect plants from fungal, bacterial and herbivore attacks (Barth and Jander, 2006; Bednarek et al., 2009). Recently, researchers have found that tryptophan-derived serotonin plays an important role in insect resistance in rice (Lu et al., 2018). These essential functions of tryptophan emphasize the importance of studying its synthesis in plants. All seven genes involved in Trp synthesis were been identified and cloned in Arabidopsis in about the year 2009, but any functions of metabolites in tryptophan synthesis other than as Trp precursors remain largely unknown.
Cotton (Gossypium spp.) is an important economic crop around the world, providing a source of renewable textile fibers and oilseeds. However, Verticillium wilt, which is caused by the soil-borne vascular disease Verticillium dahliae, has become a major threat to cotton production, causing approximately 30% yield losses in severe outbreak years (Cai et al., 2009). Verticillium dahliae is a hemi-biotrophic pathogen (Thaler et al., 2004), and the diseased cotton is characterized by leaf chlorosis, wilt, or even defoliation, which reduces fiber production and quality. A shortage of resistant germplasms, deficient management and lack of effective fungicide or elicitor for controlling this disease has led it to be considered as the ‘cancer’ of cotton.
Although cotton is rich in secondary metabolites, few metabolites have been identified as playing a role in cotton resistance to V. dahliae. Tryptophan is not only an essential amino acid but a precursor for defensive antimicrobial compounds. Here, we investigated two tryptophan synthesis-related genes, GbTSA1 and GbTSB1. Down-regulation of these two genes led to a spontaneous cell death phenotype in the absence of pathogens. We show that the lesion-mimic phenotype was accompanied by the constitutive elevation of SA content and the corresponding signaling pathway. Furthermore, we proved evidence that the lesion-mimic phenotype acts in a SA-dependent manner through genetic complementation experiments. Metabolome and transcriptome analyses suggest that indole or indolic metabolites, the substrates of GbTSA1 and GbTSB1, play a vital role in the activation of SA synthesis and in the enhancement of cotton resistance to V. dahliae. Our results provide insights into the relationship between tryptophan metabolism and SA synthesis. Importantly, we show that indole has elicitor properties could be widely applied in plant protection programmes to trigger plant immunity.
Results
Knock-down of GbTSA1 triggers cell death and improves cotton resistance to V. dahliae
In our previous study, some genes putatively responsive to a broad range of pathogens in cotton were identified. Among these, tryptophan synthesis-related genes were up-regulated after 24 h post-inoculation with V. dahliae (Xu et al., 2014). Sequence analysis showed that one gene, designated as GbTSA1, shared high similarity with AtTSA1 (AT3G54640), one of the subunits comprising tryptophan synthase, which catalyzes the last steps of tryptophan synthesis (Figure S1a). Gene expression analysis showed that GbTSA1 was constitutively expressed in all tested tissues of G. barbadense (Figure S1b). The expression of GbTSA1 is greatly suppressed at 1 h and 6 h post V. dahliae infection, but highly activated at 24 h (Figure S1c). The protein sequence of GbTSA1 contains a chloroplast transit peptide (Tcp) in the N-terminus and was shown to be localized in the chloroplast through transient expression of a GbTSA1: GFP (green fluorescent protein) fusion construct in tobacco leaf cells (Figure S1d).
To explore the functions of GbTSA1, we first knocked down the expression of GbTSA1 by RNA interference (RNAi). However, only one transgenic line (si-1) was obtained because it is very difficult to obtain regenerated seedlings when the expression of GbTSA1 is knocked down. The seedlings showed lesion-mimics on the stem, cotyledon, and leaves (Figure 1a). Generally, the lesion-mimic phenotype is associated with a high expression level of PR genes (Sels et al., 2008) and the accumulation of SA (Alvarez, 2000). To investigate whether the lesion-mimic phenotype in si-1 seedlings was due to the activation of the SA pathway, we examined the expression levels of a number of PR genes and genes involved in the SA synthesis and signaling pathways. The results revealed that the transcripts of most PR genes, such as PR1, PR2, and PR5, which are considered as markers for the SA response (Van Loon and Van Strien, 1999), were highly elevated in si-1 seedlings compared with wild-type seedlings. Genes involved in SA biosynthesis or signal transduction, such as PAD4, SARD1, WRKY28, ICS1, and NPR1, also showed higher transcript levels (Figure 1b). It was difficult to get enough samples from transgenic plants for experiments, so virus-induced gene silencing (VIGS) was employed to knock-down its expression levels. GbTSA1-silenced seedlings were stunted, and spontaneous lesions appeared on leaves 16 days post VIGS infiltration, with none on TRV:00 leaves (Figure 2a). Genes involved in SA biosynthesis and signaling pathways were also significantly up-regulated in TRV: TSA1 seedlings (Figure 2b). In addition, the contents of hormones in TRV:TSA1 plants were measured using HPLC-MS, and the results showed that the content of SA in TRV:TSA1 plants was much higher than that in TRV:00 plants, while IAA content was significantly reduced (Figure 2c).
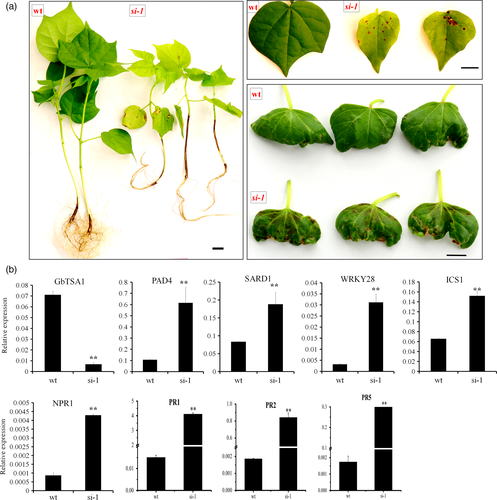
Morphological and molecular features of GbTSA1-RNAi (si-1) plants.
(a) T1 plants of GbTSA1-RNAi (si-1) show lesions on stem, cotyledon and leaves. Bars represent 1 cm.
(b) The transcript levels of GbTSA1, SA biosynthesis genes and PR genes in wild-type (WT) and si-1 plants at the 10-day-old plants before the lesions appeared on the plants. Values are the means ± SD for three biological replicates (**P < 0.01, Student's t-test).
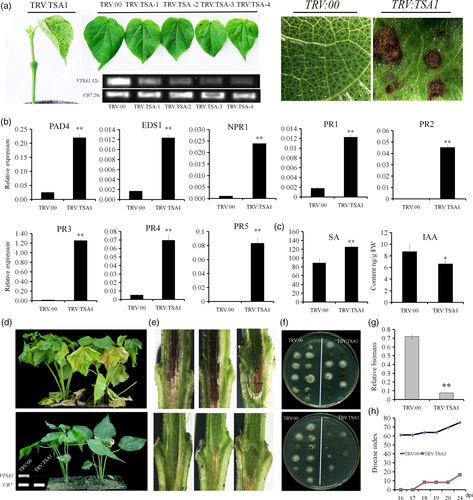
Knock-down of GbTSA1 triggers cell death, activates the SA biosynthesis and signaling pathways and enhances cotton resistance to V. dahliae.
(a) Spontaneous lesion formation on the leaves of TRV:TSA1 seedlings. RT-qPCR analysis indicated that the transcripts of GbTSA1 were reduced 14 days after infiltration. Photographs were obtained 21 days after VIGS.
(b) Expression levels of PR genes and genes involved in the SA signaling pathway were detected 14 days after infiltration, before the appearance of lesions, in TRV:TSA1 plants. Values represent the means ± SD from at least three biological replicates (**P < 0.01, Student's t-test).
(c) Detection of IAA and SA contents in TRV:TSA1 plants 14 days after infiltration. Values represent the means ± SD from four biological replicates (**P < 0.01, Student's t-test).
(d) Disease symptoms of TRV:00, TRV:TSA1 plants 21 days after inoculation with ‘V991’. The upper line indicates TRV:00 plants with serious disease symptoms, and the lower lines indicate the disease symptoms of TRV:TSA1 plants, respectively. Photographs were obtained 21 days after inoculation. RT-PCR showed the expression of GbTSA1 in leaf samples from TRV:00 and TRV:TSA1 plants.
(e) Dark and necrotic vascular bundles of the dissected stems were photographed 16 days after V. dahliae inoculation. The upper and lower images are dissected stems from TRV:00 and TRV:TSA1 plants, respectively. Photographs were obtained under an Asana fluorescence microscope.
(f) Fungal recovery assay showed decreased fungal growth in TRV:TSA1 seedlings. The upper and lower images show two repeats of fungal recovery assay. Stem sections taken from inoculated seedlings were incubated at 25°C on potato dextrose agar, and photographs were obtained 4 days after inoculation.
(g) TRV:TSA1 seedlings showed enhanced resistance to V. dahliae, as evidenced by the decrease in fungal biomass. The relative biomass represents the transcript levels of the Verticillium internal transcribed spacer (ITS) compared with the transcript levels of cotton UB7. Values represent the means ± SD from three biological replicates (**P < 0.01, Student's t-test).
(h) Disease index statistics of TRV:00, TRV:TSA1 plants after inoculation, three biological replicates were performed.
Verticillium wilt is a type of severe vascular disease. Upon V. dahliae strain ‘V991’ inoculation, the GbTSA1-silenced seedlings showed less leaf chlorosis, less wilting (Figure 2d), and fewer necrotic vascular bundles with less disease index (DI) than did TRV:00 plants (Figure 2e,h). Furthermore, less fungal colonization was found in GbTSA1-silenced plants in fungal recovery assays (Figure 2f). Fungal biomass detection showed similar results (Figure 2g). These results indicated that knock-down of GbTSA1 activates the plant immune system and improves cotton resistance to V. dahliae.
Improved resistance in GbTSA1-silenced seedlings is SA-dependent
To explore the function of the SA synthesis and signaling pathways in activating plant immunity in GbTSA1-silenced seedlings, VIGS constructs of GbICS1, the key gene in SA synthesis, and GbNPR1, the key regulator in the SA signaling pathway were built, and infiltrated into plants using Agrobacterium tumefaciens strain GV3101 as vector. Two weeks after the infiltration of combinations with GbICS1 (TRV:SI) or with GbNPR1 (TRV:SN), the lesion-mimic phenotypes of GbTSA1-silenced plants were recovered (Figure 3a). Small differences in plant height could be found between the co-suppressed seedlings and TRV:ICS1 and TRV:NPR1 seedlings (Figure 3b). More importantly, the expression levels of the marker genes, PR1, PR2, and PR5 in TRV:SI and TRV:SN plants were similar to those in TRV:00 plants due to the high efficiency in down-regulation of single and double genes in our experiment (Figure 3c). Furthermore, the SA content decreased to normal levels or even lower in TRV:SI and TRV:SN seedlings (Figure 3d). The IAA content was also restored to normal levels in most combinations, while the content of JA-Ile was not altered in any treatment (Figure 3d).

Co-suppression of GbICS1 and GbNPR1 rescues the phenotypes of GbTSA1-silenced plants.
(a) Morphological features of single-gene- or double-gene-silenced plants by VIGS. Co-suppressed plants had similar phenotypes to TRV:ICS1 and TRV:NPR1 plants, and the lesions were recovered in co-suppressed plants. TRV:SI (TRV:TSA1&ICS) and TRV:SN (TRV: TSA1&NPR1) represent co-suppressed plants with different combinations. Photographs were obtained 3 weeks after infiltration.
(b) Comparison of plant heights 21 days after VIGS injection. Values represent the means ± SD from three biological replicates (LSD < 0.05).
(c) Expression patterns of marker genes involved in the SA synthesis and signaling pathways in single- or double-gene-silenced plants assessed by RT-qPCR. Values represent the means ± SD from three biological replicates (LSD < 0.05).
(d) Detection of SA, IAA, and JA-Ile contents in seedlings of TRV:00, TRV:TSA1, and co-suppression combinations with GbICS1 and GbNPR1 14 days after infiltration. Values represent the means ± SD from four biological replicates (LSD < 0.05).
To investigate the role of SA signaling in the cotton response to V. dahliae, VIGS-silenced seedlings were inoculated with V. dahliae strain ‘V991’. Compared with TRV:00 plants, GbTSA1-silenced plants showed more resistance to V. dahliae, while the knock-down of GbICS1 or GbNPR1 made the plants more susceptible (Figure S2a). Plants with double-silenced genes, such as TRV:SI and TRV:SN, showed similar phenotypes as GbICS1- and GbNPR1-silenced plants to V. dahliae. The DI and fungal recovery assays also supported these results (Figure S2b,c).
To elucidate the biological significance of the elevated SA signaling pathways in GbTSA1-silenced plants, exogenous SA was applied to cotton plants at different concentrations (200 μm, 500 μm and 1 mm). The inoculation and DI assay results revealed that pre-treatment with SA could reduce the virulence of V. dahliae by elevating the expression of PR genes (Figure S3). Our results therefore demonstrate that the activated plant immune system in GbTSA1-silenced plants is dependent on SA synthesis, and the SA signaling pathway positively regulates cotton resistance to V. dahliae.
The lesion-mimic phenotype is putatively gene specific and Trp independent
The syntheses of Trp and SA share the same precursor chorismate (Wildermuth et al., 2001), therefore the lesion-mimic phenotype and the elevated SA contents in GbTSA1-silenced plants were hypothesized to represent metabolic flux redirection. To examine this possibility, the other five genes involved in the Trp synthesis pathway, namely GbASA, GbASB, GbTRP1, GbPAI, and GbTSB1, were subjected to functional analysis. VIGS constructs for each gene were completed and analysed, respectively, and also used together to generate the six-gene-silenced plants, TRV:Hex.
Our results show that the TRV:ASA, TRV:ASB, and TRV:PAI plants grew normally, while the TRV:TRP1- and GbTSB1-silenced plants showed an extremely stunted and severely necrotic phenotype, which is similar to TRV:TSA1 plants (Figure 4a,b). Expression analysis showed that all the corresponding genes were successfully down-regulated in the specific or Hex plants after Agrobacterium infiltration (Figure S4a,b). Importantly, the content of chorismate accumulated to high levels only in TRV:ASA, TRV:ASB, and TRV:Hex but not in TRV:TRP1, TRV: PAI, TRV:TSA1, or TRV:TSB1 plants (Figure 4c). To determine whether the phenotypes of the TRV:TRP1, TRV:TSA1, and TRV:TSB1 plants were associated with a shortage of Trp, we measured the Trp contents. The results show that Trp levels were extremely reduced in TRV:ASA and TRV:ASB, and slightly increased in TRV:TRP1, TRV:PAI, and TRV:TSA1 plants (Figure 4c). The content of SA was significantly increased only in TRV:TSA1 and TRV:TSB1 and was dramatically reduced in TRV:TRP1 plants (Figure 4d). These results suggest that the elevated SA content in TRV:TSA1 and TRV:TSB1 plants is not due to the metabolic flux redirection, but a putatively gene-specific phenotype.
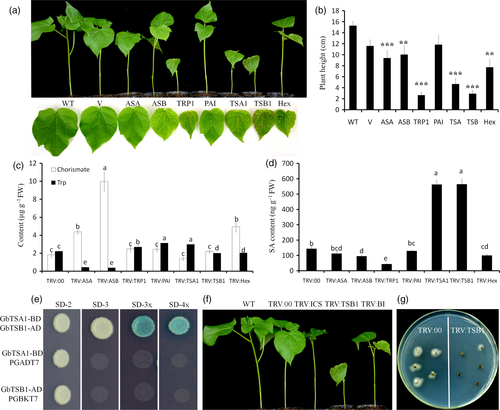
Phenotypes of the genes involved in tryptophan synthesis after VIGS.
(a) Phenotypes of plant height and the lesion-mimic of leaves. The samples from left to right are: WT, V (TRV: 00), ASA (TRV:GbASA), ASB (TRV:GbASB), TRP1 (TRV:GbTRP1), PAI (TRV:GbPAI), TSA1 (TRV:TSA1), TSB1 (TRV:GbTSB1) and TRV:Hex plants. ‘Hex’ indicates GbASA-, GbASB-, GbTRP1-, GbPAI-, GbTSA1-, and GbTSB11-silencing in one plant. Photographs were obtained 16 days after VIGS.
(b) Plant heights of WT, V (TRV: 00), ASA (TRV:GbASA), ASB (TRV:GbASB), TRP1 (TRV:GbTRP1), PAI (TRV:GbPAI), TSA1 (TRV:TSA1), TSB1 (TRV:GbTSB1), and TRV:Hex plants. For each treatment, at least 16 plants were measured (**P < 0.01, Student's t-test).
(c, d) Chorismate, Trp, and SA contents measured by LC-MS. Values represent the means ± SD from three biological replicates (LSD < 0.05).
(e) Y2H assays confirmed the interactions between GbTSA1 and GbTSB1. Transformed yeast cells were grown on SD-2 (SD−Trp−Leu), SD-3 (SD−Trp−Leu−His), SD-3X (SD−Trp−Leu−His with X-α-gal), SD-4X (SD−Trp−Leu−His−Ade with X-α-gal) media, and the blue colonies on SD-3X and SD-4X media indicate positive interactions. Transformed yeast cells with GbTSA1-BD and empty vector PGADT7, GbTSB1-AD, and empty vector PGBKT7 were used as negative control.
(f) Cell death phenotypes of GbTSB1 knock-down plants were dependent on the SA pathway. From left to right the plants represent WT, TRV:00, TRV:ICS1, TRV:TSB1, and TRV:BI (TRV: TSB1& ICS1). Photographs were obtained 16 days after VIGS.
(g) Fungal recovery assay showed that TRV:TSB1 seedlings were more resistant to V. dahliae. Photographs were obtained 4 days after fungal recovery assay.
TSB1 is the enzyme catalyzing the final step of Trp synthesis, and TSA1 and TSB1 can form a hetero-complex in plants (Radwanski et al., 1995). Yeast two-hybrid assays demonstrated that GbTSA1 and GbTSB1 can interact with each other (Figure 4e). Importantly, the lesion-mimic phenotype of TRV:TSB1 plants could also be recovered by knock-down of GbICS1 (Figures 4f, S5a). Furthermore, TRV:TSB1 seedlings showed an even more severe necrotic phenotype than TRV:TSA1 seedlings (Figure S5b), higher PR gene expression levels and elevated SA contents (Figure S5c,d), along with increased resistance to V. dahliae (Figures 4g, S5e).
The transcript levels of each gene in the Trp pathway were evaluated in silenced plants by reverse transcription quantitative polymerase chain reaction (RT-qPCR). The results revealed that the expression levels of GbASA, GbASB, and GbPAI were up-regulated in GbTRP1-, GbTSA1-, and GbTSB1-silenced plants (Figure S6a), and the transcripts of PR family, WRKY40 and WRKY70 genes were elevated only in TRV: TRP1, TRV: TSA1, and TRV: TSB1 plants (Figure S6b). It is very interesting that all six genes were knocked down in the ‘TRV:Hex’ plants by VIGS, but the transcripts of PR genes in ‘TRV:Hex’ were not expressed at high levels. Tryptophan was exogenously applied to investigate whether Trp could rescue the lesion-mimic phenotype. The results showed that no obvious differences could be found in the phenotypes of TRV:TRP1, TRV:TSA1, and TRV:TSB1 plants after Trp exogenous application (Figure S7a). To our surprise, more free Trp contents were observed in the leaves of TRV:TRP1, TRV:TSA1, and TRV:TSB1 plants compared with TRV:00 plants without tryptophan treatment, although the synthesis of Trp was inhibited when the expression of GbTRP1, GbTSA1, and GbTSB1 was knocked down. For plants treated with tryptophan, significant increases in the contents of Trp could be found in the roots of all plants and in leaves of TRV:TSA1 and TRV:TSB1 plants (Figure S7b), which suggests that exogenous tryptophan has been successfully absorbed into the plants. Therefore, it is concluded that the lesion-mimic phenotype of TRV:TSA1 and TRV:TSB1 plants is Trp content-independent.
Indole activates the plant defense response and SA synthesis
To explore the possible reason of lesion-mimic phenotype and elevation of SA synthesis in GbTSA1- and GbTSB1-silenced plants, we measured the metabolites in TRV:TSA1, TRV:TSB1 seedlings by gas chromatography-mass spectrometry (GC-MS). The results show that indole and indolic metabolites were highly accumulated in TRV:TSA1 and TRV:TSB1 plants compared with TRV:00 plants (Figures 5a, S8). It has been reported that Trp-derived metabolites have an effect on programmed cell death in MAMP-triggered immunity (Fukunaga et al., 2016). To identify whether indolic metabolites also regulate plant immunity, we first investigated whether indole enhances cotton resistance to V. dahliae. Cotton plants were pre-treated with different concentrations of indole (0, 10 μm, 50 μm, 100 μm, 200 μm, 500 μm, 1 mm) for 24 h (Figure S9a) and then were inoculated with ‘V991’. The results showed that 200 μm indole treatment could strongly enhance cotton resistance to V. dahliae (Figure S9b,c). To further understand the function of indole in cotton resistance to V. dahliae, genome-wide gene expression profiles were compared between 500 μm indole- and water-treated wild-type plants. The results showed that 1817 genes were up-regulated and 3180 genes were down-regulated in the indole-treated plants (Figure S10; Table S3). Gene Ontology (GO) enrichment analysis showed that categories including ‘protein kinase activity’, ‘regulation of transcription’ and ‘response to stress’ were enriched among the up-regulated genes, and the most represented categories among the down-regulated genes were ‘amino acid metabolic process’, ‘nitrogen compound metabolic process’, and ‘organic substance biosynthetic process’ (Figure S10). KEGG analysis showed that the pathways of plant−pathogen interaction were enriched among the up-regulated genes (Figure 5b) and the pathways of phenylalanine metabolism were enriched among the down-regulated genes (Figure S11). Specifically, 63 genes involved in plant−pathogen interaction and 11 SA synthesis-related genes were activated upon indole treatment. Although WRKY TFs play roles in many plant processes, the major role of this family is in plant immune response (Rushton et al., 2010). Our data showed that indole induced the expression of 39 WRKY-encoding genes, and many have been reported to be involved in the plant defense response, including WRKY18, WRKY28, WRKY33, WRKY40, and WRKY 48 (Pandey and Somssich, 2009) (Figure 5c and Table S1). In addition, 500 μm indole- and water-treated wild-type plants were inoculated with ‘V991’ again and the same results were obtained, in that the plants showed strongly enhanced resistance to V. dahliae when pre-treated with 0.5 mm indole (Figure 5d–f).
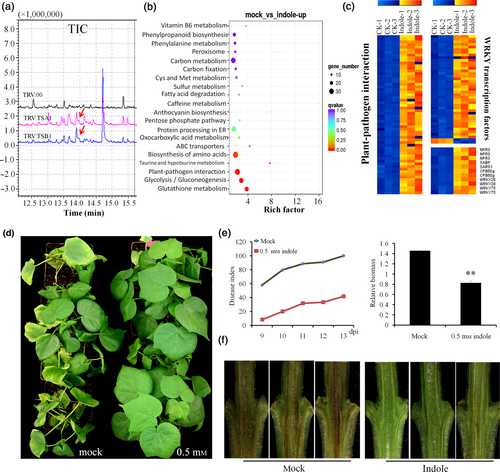
Indole promotes defense and SA synthesis-related genes and enhances the resistance of cotton to V. dahliae.
(a) Total ion chromatograph (TIC) for the measurement of indole contents by GC-MS in TRV:00, TRV:TSA1, and TRV:TSB1 leaves. The red arrows indicate the peaks of indole in TRV:TSA1 and TRV:TSB1, respectively.
(b) KEGG pathway analysis of the up-regulated genes in indole treatment plants. Rich factor: percentage of enriched genes comparing with background in corresponding KEGG pathway. The q value is the P-value after multiple hypothesis test correction.
(c) Genes associated with defense, plant hormone (SA) signal transduction, and WRKY transcription factors were selected for heat map analysis, values are log2 signal ratios between indole-treated and mock-treated control plants.
(d) Cotton plants were pre-treated with 0.5 mμ indole by nutrient solution culture for 48 h, plants were then inoculated with ‘V991’ by the root dip method and transplanted into the soil. Photographs were obtained 12 days after inoculation.
(e) The disease index and relative fungal biomass assay showed that indole treatment could enhance the resistance of cotton to V. dahliae. Values represent the means ± SD from three biological replicates (**P < 0.01, Student's t-test).
(f) Dark and necrotic vascular bundles of the dissected stems of mock and indole-treated plants were photographed 12 days after V. dahliae inoculation. Photographss were obtained under an Asana fluorescence microscope.
Our transcriptome data and RT-qPCR verification showed that the transcripts of SARD1, CBP60g, WRKY28, and WRKY75 were highly induced by indole treatment (Table S1; Figure 6a,b). The expression levels of GbSARD and GbWRKY28 were also highly increased in TRV:TSA1 and TRV:TSB1 plants (Figure 6c). Furthermore, the SARD1- and WRKY28-binding motifs could be found in the promoter sequence of GbICS1 (Figure S12). A dual-luciferase reporter assay confirmed that GbSARD and GbWRKY28 could bind the promoter of GbICS1 and activate the expression of GbICS1 (Figure 6d). To further validate whether the higher expression of GbSARD and GbWRKY28 is the reason for the activation of the SA pathway in TRV:TSA1 and TRV:TSB1 seedlings, we knocked down the expression of GbSARD in TRV:TSA1- and TRV:TSB1-silenced plants, the stunted and lesion-mimic phenotypes of TRV:TSA1 and TRV:TSB1 plants were almost rescued (Figure 7a,b). The expression of GbICS1 and PRs in double mutant plants also returned to normal levels (Figure 7c).
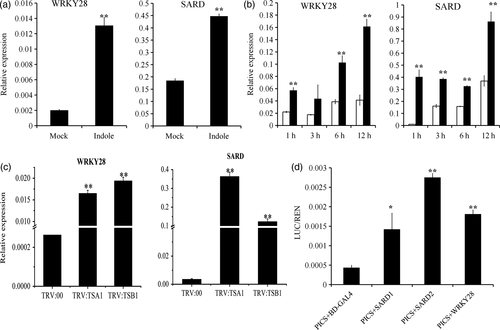
Indole promotes the expression of GbSARD and GbWRKY28, the transcription factors binding the promoter of GbICS1.
(a) Expression levels of GbSARD and GbWRKY28 were activated in cotton roots after 0.5 mm indole application evaluated by RT-qPCR. Values represent the means ± SD from three biological replicates (**P < 0.01, Student's t-test).
(b) Expression levels of GbSARD and GbWRKY28 were activated in cotton leaves after 1 mm indole application evaluated by RT-qPCR. Values represent the means ± SD from three biological replicates (**P < 0.01, Student's t-test).
(c) Expression levels of GbSARD and GbWRKY28 in TRV:00, TRV:TSA1 and TRV:TSB1 plants evaluated by RT-qPCR. Values represent the means ± SD from three biological replicates (**P < 0.01, Student's t-test).
(d) Transcriptional activation of GbSARD and GbWRKY28 on the promoter of GbICS1 in vivo by DLR assay. BD-GAL4 served as a negative control. Values represent the means ± SD from three biological replicates (**P < 0.01, Student's t-test).
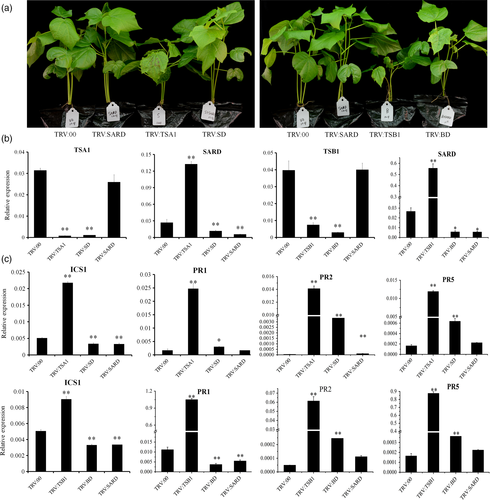
Knock-down of GbSARD greatly rescues the phenotypes of TRV:TSA1 and TRV: TSB1 seedlings.
(a) Morphological features of single-gene and double-gene VIGS plants. Photographs were obtained 21 days after injection. The explanation of abbreviations is as follows: TRV:SD, (TRV:TSA1&SARD); TRV:BD, (TRV:TSB1&SARD).
(b) Gene expression in silenced plants evaluated by RT-qPCR. This experiment was performed in three biological repeats (**P < 0.01, Student's t-test).
(c) PR gene expression in plants silenced by single or double genes as evaluated by RT-qPCR. Values represent the means ± SD from three biological replicates (**P < 0.01, Student's t-test).
These results showed that knock-down of GbTSA1 or GbTSB1 leads to the accumulation of indole and indolic metabolites, which up-regulate the expression of GbSARD and GbWRKY28. As a sequence, the constitutively activated SA synthesis and signaling pathways enhanced the resistance of cotton to V. dahliae (Figure S13).
Discussion
Tryptophan synthetic pathways may confer broad-spectrum resistance in plants by activating the SA pathway in cotton
In Arabidopsis, derivatives of tryptophan metabolism include auxin, glucosinolates, phytoalexins, alkaloids, serotonin, and other indolic compounds (Iven et al., 2012; Dubouzet et al., 2013). Secondary metabolites derived from tryptophan play vital roles in plant development and defense responses. Some of these derivatives are involved in systemic acquired resistance and programmed cell death. For instance, the tryptophan-derived indolic metabolites indole-3-ylmethylamine (I3A), indole-3-carboxylic acid (ICA), and indole-3-carbaldehyde (ICC) are activated in both P. syringae-inoculated and distant non-inoculated leaves, and genetic analyses have revealed that systemically elevated indoles are associated with systemic increases in SA (Stahl et al., 2016). Recently, it was reported that necrotic spotted lesion1 (nsl1) mutants activate cell death phenotypes when treated with non-adapted fungal pathogens and bacterial MAMPs, such as flg22 in accession Col-0 (Fukunaga et al., 2016). The cell death response was SA-dependent because the depletion of ICS1 repressed flg22-inducible lesion formation. Importantly, loss-of-function mutations in PEN2, which is involved in the metabolism of tryptophan (Trp)-derived indole glucosinolates, suppressed the accumulation of SA and cell death in flg22-induced nsl1 mutants, thereby revealing a previously unsuspected role of Trp-derived metabolites in the regulation of the SA defense phytohormone pathway and PCD.
In our previous study, GbTSA1 and GbTSB1, which are involved in tryptophan synthesis, were identified by bioinformatics analysis as responding to a broad range of pathogens in cotton (Xu et al., 2014). We show here that knock-down of GbTSA1 and GbTSB1 genes makes the silenced plants develop a spontaneous cell death phenotype in a SA-dependent manner (Figures 2, 3). Further study identified that knock-down of these Trp synthesis genes leads to cell death by constitutively activating the expression of GbSARD and GbWRKY28, key TFs that are able to up-regulate the expression of GbICS1 and activate SA synthesis and systemic programmed cell death (Figures 6, 7). These results are consistent with the phenotypes of over-expression lines of SARD1 in Arabidopsis (Zhang et al., 2010).
Furthermore, GbTSA1 and GbTSB1 knock-down plants increased resistance to V. dahliae, and the resistance phenotype was repressed when the SA synthesis and signaling pathways were blocked (Figures 2, S2). It has been reported that tryptophan-derived metabolites such as camalexin act as antifungal compound, limiting Verticillium proliferation (Iven et al., 2012); however, no antifungal properties of indole could be observed in vitro in our study (Figure S14). We therefore speculate that the enhanced resistance in GbTSA1- and GbTSB1-silenced plants is dependent on the elevation of SA pathways and the SA signaling pathway that positively regulate cotton resistance to V. dahliae with indole as an elicitor. Both JA and SA have been reported to be involved in cotton resistance to V. dahliae. In this study, silencing of GbTSA1 did not affect the content of JA (Figure 3d), while the expression of JA synthesis and signal pathway-related genes were even slightly increased in TRV:TSA1 plants (Figure S15). However, another lesion-mimic mutants ssi2 simultaneously up-regulates SA-mediated responses and inhibits JA-inducible defenses, finally resulting in increased susceptibility to V. dahliae (Gao et al., 2013). These results indicate that the crosstalk between JA and SA pathways is complicated, which remains to be elucidated using GbTSA1 and GbSSI2.
Disease index fluctuates greatly with the temporal research conditions
Verticillium dahliae is a soil-borne fungus that infects from the roots of the host. Disease index was used to illustrate the disease susceptibility of infected plants, but the DI, higher or lower, is dependent on a range of conditions such as pathogenicity and concentration of V. dahliae, the growth stage and status of plants, temperature, and soil water content. Although Gossypium barbadense cv. Hai7124 is a tolerant (not resistant) variety in production practices, this variety can be fully infected, when the roots were wounded, by inoculation with highly pathogenic V. dahliae strain at the seedling stage under indoor conditions. There are many key factors that influence the disease incidence and it is impossible to achieve consistency of all the conditions in different batches of experiments. This explains why we saw very high DIs in this research, although the DI was less high in our previous study (Gao et al., 2013). In any case, the proper use of controls allows the accurate evaluation of disease status.
VIGS is efficient in functional screen for candidate genes
Amino acid auxotrophs or mutants of other primary metabolic pathway genes are often difficult to obtain because of lethality. However, such mutants can provide invaluable information in furthering our understanding of fundamental biosynthetic processes. Knock-down of such genes by using VIGS technology is an efficient method to study metabolic processes and flux. VIGS is a relatively simple and efficient approach to achieve the down-regulation of target genes, which can help elucidate mechanisms in non-model plant species for which mutants and stable genetic transformation systems are not readily available. Many improvements have been made in VIGS-derived microRNA and protein over-expression (Gu et al., 2014; Sha et al., 2014), which expands the applications of the VIGS method. However, most studies only focused on the functional characterization of single genes. Genome-wide association studies (GWAS) are widely used to mine favorable natural alleles associated with important traits in crops (Shang et al., 2014; Li et al., 2017; Xiao et al., 2017). A single QTL or gene locus may cover 400 kb to 1 Mb of the genome and generally contains more than 10 genes. For example, Wang et al. (2017) identified 93 domestication sweeps related to plant height, resistance, and fiber-quality traits in cotton through GWAS. Identifying which key gene or allele contributes to the trait is a huge task. In our study, we successfully knocked down the expression of up to six genes simultaneously, which illustrates the value of the VIGS method for the study of metabolic pathways, and the characterization of multiple genes simultaneously.
Lesion-mimic can be SA-dependent and SA-independent
In plants, mutants with hypersensitive response (HR)-like lesions and activated defense responses in the absence of pathogens have been designated as ‘lesion-mimic mutants’ (Heath, 2000; Brodersen et al., 2002). Identification and characterization of these mutants has been a powerful approach to unravel the signaling components involved in the HR response and their connection to disease resistance. Many lesion-mimic mutants have been reported, and about 40 associated genes have been isolated (Bruggeman et al., 2015). Those genes fall into several functional groups, including ‘membrane-associated protein’ (Büschges et al., 1997), ‘ion channel’ (Balague, 2003), ‘zinc-finger protein’ (Dietrich et al., 1997), ‘porphyrin metabolism’ (Chai et al., 2017), and ‘fatty acid metabolism’ (Sun et al., 2014). In our study, we found a group of lesion-mimic mutants that participate in tryptophan synthesis. The transgenic plants with knock-down of GbTSA1 and GbTSB1 developed spontaneous necrotic lesions in the absence of pathogens, and these lesions usually occurred randomly on the leaf (Figures 2a, 4a). These lines also show other hallmarks of the HR, such as the constitutive expression of PR genes, accumulation of SA and enhanced resistance to pathogen infection (Figures 2, S5). Many of the lesion-mimic mutants are SA dependent (Weymann et al., 1995; Greenberg et al., 2000), similar to the GbTSA1 and GbTSB1 knock-down mutants. However, some lesion-mimic mutants are SA independent. For example, in our previously study, the ssn lesion-mimic mutant is SA independent (Sun et al., 2014). We also found that knock-down of GbTRP1 triggered a lesion-mimic phenotype and constitutive expression of PR genes without SA accumulation (Figures 4a,d , S16). The reason for the lesion-mimic in the GbTRP1 knock-down seedlings needs to be explored further.
Indolic metabolites activate the plant immunity system via triggering SA synthesis
The regulatory activities of plant metabolites have intrigued researchers, and the roles of metabolites derived from amino acid metabolism in plant-pathogen resistance go far beyond those in phytoanticipin and phytoalexin production. For example, pipecolic acid, produced by the amino acid lysine, contributes to SAR establishment and plant defense amplification and priming (Návarová et al., 2012). In prokaryotes, such as bacteria, indole is synthesized from tryptophan by tryptophanase (TnaA) and modulates spore formation (Kim et al., 2011), antibiotic tolerance (Vega et al., 2012), and biofilm formation (Martino et al., 2003). Maize is able to produce indole using indole-3-glycerol phosphate lyases, and releases indole as a rapid and potent aerial priming agent in response to herbivore attack (Frey et al., 2000; Erb et al., 2015). In Arabidopsis, indolic metabolism is broadly activated in SAR establishment (Stahl et al., 2016).
In our study, indole and indolic metabolites were accumulated to high levels in TRV:TSA1 and TRV:TSB1 plants compared with TRV:00 plants (Figure 5a and Figure S8). Transcriptome analysis showed that exogenous indole promotes the expression of defense response genes and SA synthesis-related genes (Figure 5b,c). Furthermore, exogenous application of indole on cotton roots and leaves strongly induced the expression of GbSARD and GbWRKY28 (Figure 6a,b), implying that the higher expression of GbSARD and GbWRKY28 in GbTSA1- and GbTSB1-silenced plants may be due to the accumulation of indolic metabolites. Furthermore, application of indole to cotton plants strongly enhances cotton resistance to V. dahliae (Figures 5d–f, S9). We therefore propose that indole or indolic derivatives may be useful as ‘plant immune activators’ to trigger plant immunity response and might be valuable tools in plant protection strategies. How plants perceive indole and transduce the signal to trigger plant immunity response remains to be discovered.
Experimental procedures
Plant materials and growth conditions
Cotton plants, G. barbadense cv. Hai7124 and G. hirsutum cv. J668 were grown in a controlled environment chamber under a 14 h light/10 h dark cycle at 25°C for treatment and sampling. In total, 30 cotton seedlings cv. J668 were cultivated in Hoagland solution for 3 weeks, and then 0.5 mm indole was applied to the solution of 15 plants, the other 15 plants were used as control, with three biological repeats. Root samples were collected at 24 h after treatment and immediately frozen in liquid nitrogen. For leaf treatments, leaf discs (1 cm) of 3-week-old plants were sampled and floated overnight on sterile water. Half of the disc was then placed in a 1 mm indole solution, and the other half was used as a control. Leaf samples were collected for analysis at 1, 3, 6 and 12 h after treatment, at least 50 discs were sampled for each time points, with three biological repeats. For Trp treatment, plants were injected with TRV vectors, and then the plants were cultured in a nutrient solution containing 0.05 g L−1 Trp; the solution was changed every 5 days. Two weeks later, leaves and roots were sampled for Trp measurement.
Gene cloning, sequence analysis and expression pattern analysis
Homologous sequences of cotton were identified through BLAST with the cotton genome sequence (www.cottongen.org/). The signal peptide was predicted using ChloroP (www.cbs.dtu.dk/services/ChloroP/). The analysis of expression patterns in response to V. dahliae was performed according to Xu et al. (2014).
RT-qPCR and RT-PCR
Total RNA was extracted from samples according to Zhu et al. (2005) and was then reverse-transcribed to cDNA for gene expression analysis.
Quantitative real-time PCR (RT-qPCR) was performed using the ABI Prism 7500 system (Applied Biosystems, Foster City, CA, USA). A 20 μl reaction mixture containing diluted cDNA and Green Super-mix was used for RT-qPCR following the manufacturer's protocol. The procedure was as follows: 95°C for 1 min, followed by 40 cycles of 95°C for 5 sec and 60°C for 40 sec. The RT-qPCR primers were designed using Primer 5.0. The RT-PCR procedure was as follows: one cycle of 5 min at 94°C as an initial denaturation steps followed by denaturation for 30 sec at 94°C, annealing for 30 sec at 58°C, extension for 30 sec at 72°C and a final step at 72°C for 6 min.
VIGS procedure
The conserved regions of GbASA, GbASB, GbPAI, GbTRP1, GbTSA1, GbTSB1, GbICS1, GbNPR1, GbSARD and GbWRKY28 were individually selected as targets for VIGS and then amplified from the root samples of cv. Hai7124. PCR products were digested by two restriction endonucleases, BamHI and KpnI, and ligated to the TRV vector as reported previously (Gao et al., 2013). The primer sequences are listed in Table S2. Finally, the TRV vectors were transformed into Agrobacterium tumefaciens GV3101 through electroporation. A. tumefaciens samples with different TRV vectors were mixed in equal volumes for the co-suppression of two or more genes. A. tumefaciens harboring TRV vectors were infiltrated into two fully expanded cotyledons of 10-day-old seedlings as described by Gao et al. (2013). Seedlings were grown at 25°C in an incubator (ATC26, Conviron, http://www.conviron.com/) with a 14 h/10 h light/dark photoperiod. The primers listed in Table S2 were used to evaluate the silencing effect based on the expression levels of the target genes in leaf tissue.
Pathogen inoculation, recovery cultivation, infected plant dissection, and disease index calculation
Hormone determination
Extraction and measurement of the endogenous hormones SA and IAA were as described by Gao et al. (2013). In brief, four replicates, each of 100 mg (fresh weight) of leaf samples, were harvested 14 days after injection, ground to a fine powder in liquid nitrogen, mixed with 900 μl of ice-cold 80% methanol containing NAA (1-Naphthaleneacetic acid, CAS 86-87-3) (100 ng ml−1) and 2H5-IAA (10 ng ml−1) as an internal standard, and placed on a shaker for 16 h at 4°C in the dark. The samples were then centrifuged at 13 523 g (4°C) for 15 min, and the supernatant was collected and transferred into a new tube. The supernatant was then evaporated to dryness by nitrogen gas, and the residue was reconstituted in 400 μl of 80% methanol. Finally, the extracts were used for analyses of hormone contents using an Agilent 4000Q-TRAP HPLC-MS system. Quantification was achieved using a five-point calibration curve ranging from 3.125 to 50 ng ml−1 SA and IAA containing 100 ng NAA and 10 ng 2H5-IAA as internal standards.
Trp measurement
Freeze-dried samples were ground into powder, mixed with 1.5 ml of ice-cold 80% methanol, and placed in a shaker for 16 h at 4°C in the dark. Samples were then centrifuged for 15 min at 12 000 rpm (4°C), and the supernatant was collected and transferred to a new tube. Extracts were used for analyses of Trp content using the Agilent 4000Q-TRAP HPLC-MS system with the methods described by Chen et al. (2014).
Indole measurement
The determination of indole levels in leaves was performed by a modified vapor-phase extraction method (Schmelz et al., 2004). Briefly, 200 mg of frozen leaf samples were homogenized in 600 μl of extraction buffer (water:1-propanol:HCl = 1:2:0.005) and 1 ml of methylene chloride. The mixture was shaken thoroughly at 4°C for 30 min and centrifuged at 12 000 rpm for phase separation, and the lower, organic phase was then carefully transferred to a new tube. The extracts were ready to be analyzed by GC-MS. For GC separation, the injector temperature was set to 270°C with a constant flow of helium (1.2 ml min−1), and the following temperature program was used: 50°C/3 min with 8°C/min to 240°C, with 20°C/min to 320°C/10 min.
Transcriptome studies
For RNA-seq, three independent biological replicates were produced. Cotton seedlings cv. J668 were cultivated in Hoagland solution for 3 weeks, and then 0.5 mm indole was applied to the solution. Root samples were collected at 24 h after treatment and immediately frozen in liquid nitrogen. Total RNA was extracted using the Qiagen RNeasy kit according to the manufacturer's instructions. Sequencing was performed on the Illumina HiSeq™ 2000 system in the Beijing mega genomics company. The differentially expressed genes were identified using the DESeq package with the negative binomial distribution (FDR < 0.05).
Sub-cellular localization
To localize the GbTSA1 proteins, GbTSA1 cDNA sequences without stop codons were inserted into the C-terminal GFP-fusion expression vector PMDC84 (Curtis and Grossniklaus, 2003), with CaMV35s:GFP used as the control. Both vectors were introduced into the A. tumefaciens strain GV3101 for the transformation of 3-week-old tobacco leaves to determine the subcellular localization of GbTSA1:GFP. Green fluorescent protein expression was observed 48 h after transformation under a microscope (LeicaMicrosystems TCS SP2 AOBS, Germany).
Yeast two-hybrid assay
A cDNA library of cotton roots 12 and 24 h after V. dahliae infection was constructed for Y2H screening using the Matchmaker Gold Yeast Two-Hybrid System (Clontech, Cat. No. 630489). The GbTSA1 gene was fused to the GAL4 DNA-binding domain in pGBKT7 to ensure that there was no auto-activation or toxicity due to the X-α-Gal assay in yeast, and the GbTSA1 fusion protein was used as bait to identify interacting proteins. Only GbTSB1 was identified. Then full-length GbTSB1 ORF was fused to the GAL4 DNA activation domain in PGADT7 for further verification. The protein−protein detection assay was performed according to Hu et al. (2016).
Dual-luciferase reporter (DLR) assay
The dual-luciferase reporter assay was performed as described previously (Hao et al., 2012). The promoter sequence of GbICS1 (from −100 to −1665) was amplified by PCR using Gossypium barbadense cv. Hai7124 genomic DNA. The PCR products were then separately ligated into the GAL4-LUC vectors to generate ProGbICS1-GAL-LUC as reporter constructs. Full-length GbSARD1, GbSARD2 and GbWRKY28, the genes with the greatest homology to Arabidopsis genes, were cloned using Gossypium barbadense cv. Hai7124 root cDNA and then fused into the 35S-pBDGAL4 vectors, respectively. The 35S-pBDGAL4 vectors were used as a negative control and AtUbiquitin3-Renilla-LUC as an internal control to analyze the interactions between ProGbICS1 and GbSARD1, GbSARD2 and GbWRKY28. Protoplasts were isolated from cotton embryogenic callus according to Yang et al. (2008), and the transformation and detection methods were according to Min et al. (2015).
Accession numbers
Sequence data from this article can be found in the CottonGen database (http://www.cottongen.org) or GenBank databases under the following accession numbers: AtINS, AT4G02610; AtTSA1, AT3G54640; GbTSA1, GOBAR_AA14756.1; GbTSB1, GOBAR_DD33483.1; GbICS1, GOBAR_AA19816.1; GbNPR1, GOBAR_DD10427.1; GhUB7, Gh_A11G0969.
Acknowledgements
The vectors used for VIGS were kindly provided by Prof. Bart Thomma (Laboratory of Phytopathology, Wageningen University and Research Center, The Netherlands). We would like to thank Prof. Guiliang Jian (Institute of Plant Protection, Chinese Academy of Agricultural Sciences, China) for providing V. dahliae strain ‘V991’. We are grateful to Dongqin Li (Huazhong Agricultural University, China) for assistance with LC/MS and to Fengfeng Li (Huazhong Agricultural University, China) for assistance with GC-MS. Funding support from National Natural Science Foundation of China (U1703231) and the China Agricultural Research System (CARS-18-09) is appreciated.
Author contributions
X.Z. and L.Z. designed the experiment. L.X. isolated the TSA1 genes. Y.M. performed most of the research and drafted the manuscript. M.S., X.H. and L.Z. helped perform the experiments. Y.M. analyzed the data. X.Z. and L.Z. revised the manuscript.
Conflict of Interest
The authors declare no conflict of interest.




