Comparative analysis of the reactive oxygen species-producing enzymatic activity of Arabidopsis NADPH oxidases
Summary
Reactive oxygen species (ROS) produced by NADPH oxidases, called respiratory burst oxidase homologs (Rbohs), play crucial roles in development as well as biotic and abiotic stress responses in plants. Arabidopsis has 10 Rboh genes, AtRbohA to AtRbohJ. Five AtRbohs (AtRbohC, -D, -F, -H and -J) are synergistically activated by Ca2+-binding and protein phosphorylation to produce ROS that play various roles in planta, although the activities of the other Rbohs remain unknown. With a heterologous expression system, we found a range of ROS-producing activity among the AtRbohs with differences up to 100 times, indicating that the required amounts of ROS are different in each situation where AtRbohs act. To specify the functions of AtRbohs involved in cell growth, we focused on AtRbohC, -H and -J, which are involved in tip growth of root hairs or pollen tubes. Ectopic expression of the root hair factor AtRbohC/ROOT HAIR DEFECTIVE 2 (RHD2) in pollen tubes restored the atrbohH atrbohJ defects in tip growth of pollen tubes. However, expression of AtRbohH or -J in root hairs did not complement the tip growth defect in the atrbohC/rhd2 mutant. Our data indicate that Rbohs possess different ranges of enzymatic activity, and that some Rbohs have evolved to carry specific functions in cell growth.
Introduction
Aerobic organisms need to deal with the unavoidable generation of reactive oxygen species (ROS) by aerobic respiration, including the superoxide anion radical (O2−), hydrogen peroxide (H2O2) and the hydroxyl radical (•OH), because they can damage DNA, protein and lipids and thus are highly toxic in cells (Kehrer, 2000). This situation is particularly severe in plants due to the generation of ROS through photosynthesis. Recent findings have revealed that most organisms produce ROS by several kinds of enzymes and use them as signaling molecules for environmental responses and development (Kärkönen and Kuchitsu, 2015). In plants, ROS are involved in numerous processes such as disease resistance responses (Levine et al., 1994), salinity stress responses (Kurusu et al., 2015; Hossain and Dietz, 2016), cell growth by scission or cross-linking of cell walls (Everdeen et al., 1988; Fry, 1998; Schweikert et al., 2000; Schopfer, 2001; Rodríguez et al., 2002; Schopfer et al., 2002), root gravitropism (Joo et al., 2001), lateral root emergence (Orman-Ligeza et al., 2016), cell-fate transition in roots (Tsukagoshi et al., 2010), the development of male and female gametophytes (Jiménez-Quesada et al., 2016), mutualistic symbiosis with fungi (Tanaka et al., 2006), long-distance signal transduction (Miller et al., 2009), the development of Casparian strips (Lee et al., 2013) and organ abscission (Lee et al., 2018).
The NADPH oxidases (Nox) are ROS-producing enzymes that are conserved in many eukaryotes and play roles in signal transduction in defense and developmental processes. The Nox proteins carry six transmembrane domains and a conserved C-terminal region containing the NADPH-binding and FAD-binding motifs, which are crucial for transferring electrons to generate superoxide anions (Sumimoto, 2008). The mammalian gp91phox/Nox2, acting together with p22phox, requires other protein components such as p47phox, p67phox and Rac small GTPase for its activation, but plants do not appear to carry the clear homologs of p47phox or p67phox (Sumimoto, 2008). Generally, plant Nox proteins, called respiratory burst oxidase homologs (Rbohs), carry two EF-hand motifs and phosphorylation sites at the cytological N-terminal region, in addition to the transmembrane domains and C-terminal region (Figure 1a; Sumimoto, 2008; Oda et al., 2010; Kärkönen and Kuchitsu, 2015). This indicates that during evolution organisms have established their own regulatory system to activate the Nox enzymes.
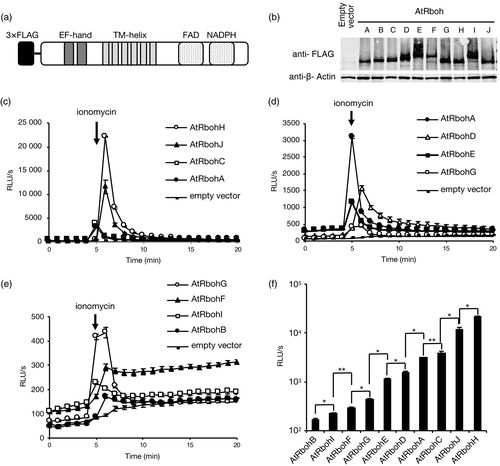
Comparison of ionomycin-induced reactive oxygen species (ROS)-producing activities among Arabidopsis respiratory burst oxidase homologs (AtRbohs).(a) Schematic representation of the construct for AtRboh expression. The AtRbohs carry N-terminal EF-hand motifs and C-terminal FAD- and NADPH-binding domains, connected by six transmembrane (TM) helices. (b) Western blotting analysis of the expression levels of N-terminal 3×FLAG-tagged AtRbohA–AtRbohJ proteins in transfected human embryonic kidney (HEK) 293T cells. β-actin was used as a loading control. (c)–(e) Ionomycin-induced production of reactive oxygen species (ROS) in AtRbohA–AtRbohJ transfected HEK293T cells. Empty vector-transfected cells were used to measure an internal ROS-producing activity in HEK293T. After baseline measurements of ROS production for 5 min, 1 μm ionomycin was added to the culture medium. The ROS production was measured by chemiluminescence and is shown as relative luminescence units (RLU) per second. Results given are means ± standard error (n = 3). (f) Comparison of maximum ROS-producing activities between AtRbohA–AtRbohJ transfected HEK293T cells. The P-values were determined using Student's t-test (*P < 0.05; **P > 0.05).
The Rboh genes were first isolated from rice and then from Arabidopsis thaliana as homologs of mammalian gp91phox/Nox2 (Groom et al., 1996; Torres et al., 1998). So far nine and ten Rbohs have been identified in rice and Arabidopsis, respectively (Kaur et al., 2014; Kaur and Pati, 2016). They have also been identified in other plants including tobacco, potato, tomato, Medicago and maize, where they regulate pathogen defense, abiotic stress responses, symbiosis or cell growth (Yoshioka et al., 2001, 2003; Simon-Plas et al., 2002; Liszkay et al., 2004; Sagi et al., 2004; Marino et al., 2011; Nestler et al., 2014). The 10 Rboh genes in Arabidopsis are referred to as AtRbohA to AtRbohJ, and genetic studies have suggested that they have distinct roles (Table 1).
| Clade | AGI | Molecular weighta | Functions | |
|---|---|---|---|---|
| Subfamilies I and II | ||||
| AtRbohA | ACG | At5g07390 | 102934.6 | |
| AtRbohB | B | At1g09090 | 96389.3 | Seed after-ripening1 |
| AtRbohC | ACG | At5g51060 | 102517.4 | Root hair tip growth2,3 , root hydrotropism4, mechanosensing5 |
| AtRbohD | D | At5g47910 | 103907.7 | Pathogen defense6,7, stomatal closure8 , osmosensitive metabolic changes induced by cellulose biosynthesis inhibitor9, rapid systemic signaling in response to exogenous stimuli10 , lignin deposition caused by cell wall biosynthesis inhibitor11,12, salinity stress response13 , systemic acquired acclimation by abiotic stress14, jasmonic acid-induced ROS production15, abscission of floral organs16 |
| AtRbohG | ACG | At4g25090 | 96861.7 | |
| Subfamily III & IV | ||||
| AtRbohE | E | At1g19230 | 107701.6 | Programmed cell death in tapetum16 |
| AtRbohF | F | At1g64060 | 108417.3 | Pathogen defense6,7, stomatal closure8,osmosensitive metabolic changes induced by cellulose biosynthesis inhibitor9, lignin deposition caused by cell wall biosynthesis inhibitor11,12, salinity-stress response13, jasmonic acid-induced ROS production15, Casparian strip formation16, abscission of floral organs17 |
| AtRbohI | I | At4g11230 | 106951.0 | Drought stress response in seeds and roots17 |
| Subfamily V | ||||
| AtRbohH | HJ | At5g60010 | 100626.7 | Pollen tube tip growth18,19,20, Polar root hair growth21 |
| AtRbohJ | HJ | At3g45810 | 102936.2 | Pollen tube tip growth18, Polar root hair growth21 |
- aMolecular weight information from the TAIR website.
- 1Müller et al. (2009); 2Foreman et al. (2003); 3Takeda et al. (2008); 4Krieger et al. (2016); 5Monshausen et al., 2009; 6Torres et al., 2002; 7Torres et al., 2005; 8Kwak et al., 2003; 9Wormit et al., 2012; 10Miller et al., 2009; 11Hamann et al., 2009; 12Denness et al., 2011; 13Ma et al., 2012; 14Suzuki et al., 2013; 15Maruta et al. 2011; 16Lee et al., 2018; 17Lee et al., 2018; He et al., 2017; 18Kaya et al., 2014; 19Boisson-Dernier et al., 2013; 20Lassig et al., 2014; 21Mangano et al., 2017.
While the spatio-temporal control of AtRboh expression is important for their functions (Morales et al., 2016; Orman-Ligeza et al., 2016), control of the enzymatic activity of AtRboh proteins is also crucial at a cellular level, as high levels of ROS can be toxic for cells (Yun et al., 2011; Kadota et al., 2014). Their activities can be successfully analyzed by heterologous expression in the human embryonic kidney (HEK) 293T cell line (Ogasawara et al., 2008), which lacks expression of endogenous NADPH oxidases Nox1 and Nox2 and does not produce significant amounts of extracellular ROS (Shiose et al., 2001). Transient expression in HEK293T cells has shown that Ca2+ binding to the EF-hand and protein phosphorylation synergistically activates the ROS-producing activity of AtRbohC/ROOT HAIR DEFECTIVE 2 (RHD2), AtRbohD, AtRbohF, AtRbohH and AtRbohJ (Ogasawara et al., 2008; Takeda et al., 2008; Kimura et al., 2012; Kaya et al., 2014). Co-expression analyses of AtRbohs and their putative regulators such as AtCIPK26 or AtSRC2 have also suggested regulatory mechanisms for the activity of AtRboh proteins (Kawarazaki et al., 2013; Kimura et al., 2013). However, the ROS-producing activity of other AtRbohs remains unclear.
Here we examined the enzymatic activity of all 10 AtRbohs in the HEK293T cell line to compare their ROS-producing ability. Our data revealed striking differences in the ROS-producing activity of these enzymes. Moreover, we performed complementary analysis of AtRbohs involved in tip growth of pollen tubes or root hairs, indicating the functional diversification of AtRbohs during evolution.
Results and Discussion
Comparison of the ROS-producing enzymatic activity of AtRbohs
To compare the ROS-producing enzymatic activity of AtRbohs we used a heterologous expression system with HEK293T cells. Coding sequences of AtRboh were fused to a 3×FLAG epitope tag (Figure 1a) to detect expressed proteins by Western blot analysis, confirming comparable expression in HEK293T cells (Figure 1b). After baseline measurements of ROS production for 5 min, 1 μm of ionomycin, a Ca2+ ionophore that induces influx of Ca2+ into the cells, was added to the medium. Ionomycin triggered rapid and transient production of ROS in all AtRboh-transfected cells, but not in empty vector-transfected controls (Figure 1c–e), suggesting that Ca2+ influx induces the ROS-producing enzymatic activity of AtRbohs. The highest activity was carried by AtRbohH, followed by AtRbohJ, -C and -A (Figure 1c). AtRbohA, -D, -E and -G produced around 10 times less ROS, and AtRbohG, -F, -I and -B produced even less than this (Figure 1d,e). The specific ROS-producing activity varied 100-fold between different AtRbohs in HEK293T cells (Figure 1f).
Ca2+ regulation in ROS production is conserved among AtRbohs
The cytosolic N-terminal region of AtRbohs contains two EF-hand motifs, where the conformation changes upon Ca2+-binding, leading to the activation of ROS production (Ogasawara et al., 2008; Oda et al., 2010). Point mutation in the conserved glutamic acid (E at the position –Z) of the first EF-hand Ca2+-binding loop reduces Ca2+ affinity and significantly abolishes the ROS-producing activity of AtRbohC/RHD2, AtRbohD, -F, -H and -J (Ogasawara et al., 2008; Takeda et al., 2008; Kimura et al., 2012; Kaya et al., 2014). To examine whether the importance of this amino acid residue is conserved among AtRbohs, we analyzed the ROS-producing activity of mutated AtRbohs where the E at the position –Z was converted to glutamine. Mutated proteins of AtRbohA, -B, -E and -G showed reduced ionomycin-triggered ROS-producing activity without affecting the level of expression (Figure 2a–d). The E residue at the –Z position of the first EF-hand is predicted to bind Ca2+, inducing a conformational change in the EF-hand region (Ogasawara et al., 2008; Oda et al., 2010). Together with the previous data, our results suggest that binding of Ca2+ to the EF-hand motifs is a common regulatory mechanism for production of ROS by AtRbohs.
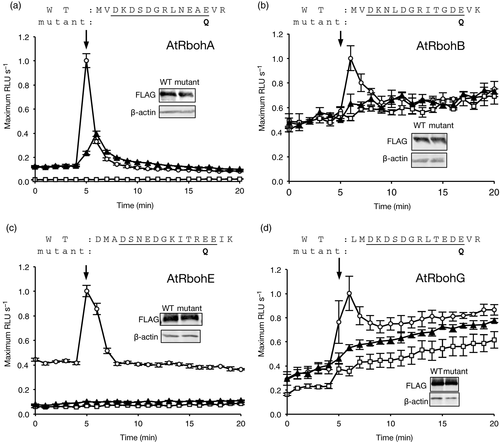
Effect of mutation in the canonical EF-hand motif of AtRbohA, AtRbohB, AtRbohE and AtRbohG on ionomycin-induced production of reactive oxygen species (ROS).(a)–(d) Ionomycin-induced production of ROS in AtRbohA-, AtRbohB-, AtRbohE- and AtRbohG- and their EF-hand mutant-transfected human embryonic kidney (HEK) 293T cells, respectively. The underlines on the amino acid sequences indicate the Ca2+-binding loop in the first EF-hand motif in each AtRboh. The bold letters indicates the substitution of the bidentate (E) to the non-bidentate Asn (Q) in each EF-hand mutant. Open circle, wild-type Rboh; closed triangle, EF-hand mutant; open square, empty vector-transfected cells. The maximum value of the luminescence unit in wild-type transfected cells was set at 1.0. Results given are means ± standard error (n = 3). The protein expression level of the wild type and EF-hand mutant was checked with anti-FLAG antibody. β-actin was used as a loading control.
Alignment of the two EF-hand motifs of AtRbohs revealed that AtRbohI carries unique amino acid residues and lacks the conserved aspartic acid (D) in the first EF-hand motif (Figure 3a), which is involved in Ca2+-binding (Oda et al., 2010). This may cause the low ionomycin-induced ROS production activity of AtRbohI in HEK293T cells (Figure 1e). Point mutation of this conserved aspartic acid (D) residue in the other AtRbohs resulted in a decrease in Ca2+ affinity and ROS-producing activity, indicating the importance of this residue in Ca2+-binding and Ca2+-triggered activation (Ogasawara et al., 2008; Kimura et al., 2012; Kaya et al., 2014). We generated the AtRbohI_ins268D mutant, which has an additional D to mimic the sequence of the conserved EF-hand motif. The insertional mutation did not affect the level of expression (Figure 3b) and, contrary to our expectation, the mutated protein showed lower enzymatic activity rather than improving ionomycin-triggered ROS production (Figure 3c).
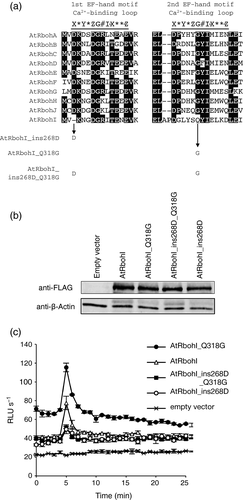
Effect of mutation in the non-canonical EF-hand motif of AtRbohI on ionomycin-induced production of reactive oxygen species (ROS).(a) The alignment of amino acid sequences of the Ca2+-binding loop in the first and second EF-hand motif of 10 Arabidopsis respiratory burst oxidase homologs (AtRbohs). The Ca2+-binding loops in the EF-hand motifs are underlined. The consensus Ca2+-binding site is shown: first (X), third (Y), fifth (Z), seventh (#), ninth (-X) = Ca2+ ligand; twelfth (-Z) = Ca2+ ligand, a bidentate Glu (E); fifth (G) = Gly; eighth (I) = Ile or other aliphatic residues are found at this position; *, any residue.(b) Western blotting analysis of the expression levels of N-terminal 3×FLAG-tagged wild-type AtRbohI and the EF-hand mutant proteins in transfected human embryonic kidney (HEK) 293T cells. β-actin was used as a loading control.(c) Ionomycin-induced ROS production in wild-type AtRbohI or the EF-hand mutant transfected HEK293T cells. Empty vector-transfected cells were used to measure the internal ROS-producing activity in HEK293T. Results given are means ± standard error (n = 3). RLU, relative luminescence units.
In the second EF-hand motif of AtRbohI, the conserved glycine (G318) is converted to glutamine, and two additional amino acids (alanine and proline) are inserted (Figure 3a). To examine whether these variations in EF-hand motifs of AtRbohI contribute to the ionomycin-triggered ROS-producing activity, we generated AtRbohI_Q318G and AtRbohI_ins268D_Q318G mutated proteins (Figure 3a). These mutations did not affect the level of protein expression (Figure 3b). AtRbohI_Q318G mutation increased the overall activity and did not affect the response to ionomycin (Figure 3c), suggesting that the conserved G318 is not critical in the Ca2+ response. The AtRbohI_ins268D_Q318G mutants showed reduced ROS-producing activity similar to AtRbohI_ins268D (Figure 3c), indicating that the insertion of the conserved D to mimic the conserved EF-hand motif is rather inhibitory to the ROS-producing activity of AtRbohI. Taken together, AtRbohI has unique EF-hand structure and carries lower ionomycin-triggered ROS-producing activity.
Protein phosphorylation-induced activation is conserved among all AtRbohs
Previous studies have shown that protein phosphorylation of several AtRbohs stimulates their ROS-producing activity. Calyculin A (CA), a protein phosphatase inhibitor, induces ROS production in HEK293T cells transfected with AtRbohC/RHD2, AtRbohD, -F, -H or -J (Ogasawara et al., 2008; Takeda et al., 2008; Kimura et al., 2012; Kaya et al., 2014), suggesting that AtRbohC, -D, -F, -H and -J are activated by protein phosphorylation. Several putative phosphorylation sites, including serine residues in the N-terminal cytosolic region, have been proposed to be critical for activation (Takeda et al., 2008; Dubiella et al., 2013; Kadota et al., 2014; Li et al., 2014; Chen et al., 2017). To investigate whether phosphorylation-dependent activation is a common regulatory mechanism in all AtRbohs, we compared the CA-induced production of ROS in HEK293T cells transfected with each AtRboh. As shown in Figure 4, all AtRbohs showed CA-induced ROS-producing activity but with striking differences, although the protein expression level did not differ much in the HEK293T cells (Figure 1b). AtRbohA showed the highest activity and AtRbohB the lowest. Calyculin A induced rapid and transient production of ROS that decreased within a couple of minutes in AtRbohA, whereas the other AtRbohs showed sustained production of ROS for at least 30 min after application of CA (Figure 4). Together with the results in response to ionomycin, our data suggest that each AtRboh possesses a different activity, indicating different responses to Ca2+ and protein phosphorylation.
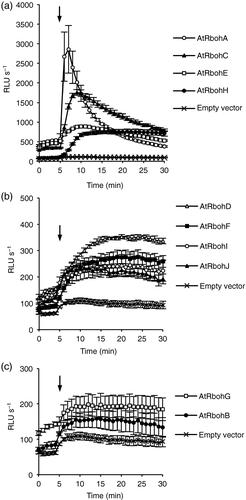
Comparison of calyculin A-induced reactive oxygen species (ROS)-producing activities among Arabidopsis respiratory burst oxidase homologs (AtRbohs).Calyculin A-induced ROS production in AtRbohA–AtRbohJ transfected human embryonic kidney (HEK) 293T cells. Empty vector-transfected cells were used to measure the internal ROS-producing activity in HEK293T. After baseline measurements of ROS production for 5 min, 0.1 μm calyculin A was added to culture medium without Ca2+. Results given are means ± standard error (n = 3).
Functional conservation of AtRbohs involved in tip growth
To investigate the functional conservation of AtRbohs in plant development, we compared AtRbohC, -H and -J, which are involved in tip growth of plant cells. AtRbohC/RHD2 locates to the root hair tip, where it maintains tip growth (Foreman et al., 2003; Takeda et al., 2008), and AtRbohH and -J, localized to the tip of pollen tubes, regulate their tip growth in a similar manner (Kaya et al., 2014). Root hairs and pollen tubes develop into long tubular structures, allowing them to play critical roles for plants: root hairs absorb water and nutrients from soil and are also involved in plant–microbe interactions, while pollen tubes deliver sperm nuclei to the female gametophyte. Tip growth in these two types of cells involves numerous common factors including Rboh proteins and ROS, Ca2+ and ROP GTPase (Mangano et al., 2016).
We expressed AtRbohC, -H and -J genes under the control of cis-regulatory sequences (promoter and terminator) of AtRbohH or AtRbohJ in the atrbohH-3 atrbohJ-2 double mutant, where the tip growth of pollen tubes is impaired resulting in reduced seed production (Figure 5) (Kaya et al., 2014). Expression of either AtRbohH or AtRbohJ under the control of the AtRbohH or AtRbohJ cis-regulatory sequences complemented the defects of the double mutant in pollen tube elongation and seed number (Figure 5). Intriguingly the expression of AtRbohC/RHD2 under the control of the AtRbohH or AtRbohJ cis-regulatory sequences restored pollen tube growth and seed number in the atrbohH-3 atrbohJ-2 double mutant (Figure 5). This indicates that AtRbohC/RHD2 can compensate for the AtRbohH/-J function.
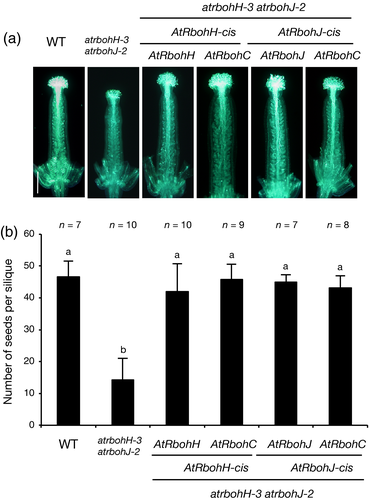
Complementation analysis of the atrbohH atrbohJ double mutant phenotype with AtRbohC.In the atrbohH-3 atrbohJ-2 double mutant, AtRbohC was ectopically expressed under the control of the AtRbohH and AtRbohJ cis-regulatory sequences, respectively. Each AtRbohH and AtRbohJ was expressed by its own cis-regulatory sequences as control experiments. Pollen grains were from the wild type (WT; Col), the atrbohH-3 atrbohJ-2 double mutant and the AtRbohC-, AtRbohH- and AtRbohJ-expressing atrbohH-3 atrbohJ-2 double mutant. Pistils were from the WT.(a) Pistils were harvested 12 h after hand pollination and stained with aniline blue. Scale bar = 250 μm.(b) Each bar represents the mean number of seeds in a silique ± standard error (n = 7–10). Siliques were harvested about 10 days after hand pollination. There is no statistically significant difference with the same letter (Tukey's test, P > 0.05).
Next we examined functional replacement in root hairs. In the atrbohC/rhd2-5 mutant, root hairs are initiated but elongation by tip growth is impaired (Figure 6 and Figure S1 in the online Supporting Information). Expression of AtRbohC/RHD2 under the control of the AtRbohC/RHD2 cis-regulatory sequences restored tip growth (Figures 6 and S1). However, the root hair defects were not fully complemented with the expression of AtRbohH or -J under the control of the same AtRbohC/RHD2 cis-regulatory sequences (Figures 6 and S1). We examined the accumulation of ROS at the root hair tip. Nitro blue tetrazolium staining was observed in the wild type and AtRbohC-expressing line but not in the lines expressing AtRbohH and -J (Figures S2 and S3). Alignment of AtRbohC, AtRbohH and AtRbohJ shows some specific insertions/deletions (Figure S4) that may be involved in the different activation mechanisms in root hairs and pollen tubes.
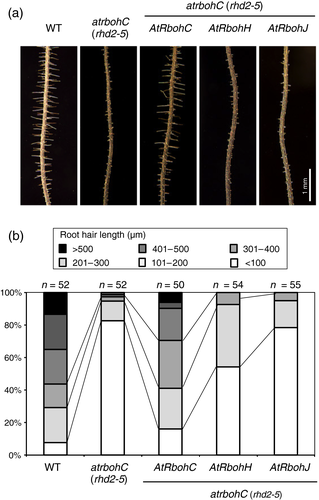
Complementation analysis of the atrbohC/rhd2 mutant with AtRbohH and -J.In the atrbohC/rhd2-5 mutant, each AtRbohC, AtRbohH or AtRbohJ was ectopically expressed under the control of the AtRbohC cis-regulatory sequences. AtRbohC was expressed by the same promoter region as control experiments.(a) Rescue of root hair defects of atrbohC/rhd2 by expression of AtRbohH and -J.(b) Root hairs located within in 5 mm of the root tip were measured in different genetic backgrounds and represented by percentage of hair length. The number of root hairs examined is shown above the graph.
Pollen tubes deliver male nuclei to the female gametophyte, so tip growth of pollen tubes is critical for plant reproduction, while root hairs are more primitive somatic cells that absorb water and nutrients from soil. Phylogenetic analysis shows that AtRbohC and AtRbohH/-J belong to different subfamilies (Figure S5). AtRbohC/RHD2 may be a primitive type so that it can function in the other cell types, while AtRbohH and AtRbohJ cannot function in root hairs because they have acquired specific functions in later-arisen reproductive cells. This can be explained by different regulators of AtRboh protein, such as ROP (Rho in plants) GTPases, Ca2+-dependent protein kinases or other unknown factors, but this needs to be analyzed further in future.
AtRbohC, -H and -J possessed higher ROS-producing activity, while AtRbohD and AtRbohF showed rather lower enzymatic activity. AtRbohC is a root-specific AtRboh and AtRbohH and -J are pollen specific AtRbohs. AtRbohC, -H and -J are required continuously at the tip of growing root hair or pollen tube, where they may be involved in Ca2+-dependent positive feedback regulation and/or cell-wall regulation for tubular morphogenesis. AtRbohD and AtRbohF are expressed in many tissues throughout the plant and are important for for responses to abiotic and biotic stresses (e.g. AtRbohD is the main component of pathogen-associated molecular pattern-induced transient ROS production) (Torres et al., 2005; Kadota et al., 2015). We suppose that the amount of ROS required for endogenous development and stress responses may be different, so that the activities of AtRbohs are different depending on their biological function.
Conclusions and perspectives
The Rbohs are involved in the production of ROS in numerous aspects of plant life. Our results show that ROS-producing activity among ten AtRbohs is dramatically different in their regulation of Ca2+ in HEK293T cells, and suggest that all AtRbohs are activated by protein phosphorylation. We propose that each AtRboh is involved in tuning of ROS production at different sites. Point mutation in the regulatory motifs may at least partially account for the functional differentiation of AtRbohs during evolution. Complementation analysis revealed the functional conservation and distinction of the AtRbohs, suggesting that they have been modified to have specific roles in different cell types. Our data propose the functional diversification of Rboh proteins, and thus it would be interesting to compare the activity and functions in other plants. Heterologous expression in HEK293T cells would be a powerful tool for these comparative studies.
Experimental Procedures
Plasmid construction and transgenic lines
To express AtRboh in HEK293T cells with a 3×FLAG epitope tag, a synthetic DNA fragment of the Kozak (GCCGCCACC)-3×FLAG epitope tag (Sigma-Aldrich, http://www.sigmaaldrich.com/), -BamHI-EcoRV-stop(TAATAG), was inserted into NheI and KpnI sites in the pcDNA3.1(-) vector (Invitrogen, http://www.invitrogen.com/), resulting in a pcDNA3.1-Kozak-3×FLAG vector. The coding sequence (CDS) of AtRboh genes (except for AtRbohG) was PCR-amplified from a cDNA library and cloned into the BamHI or EcoRV site of pcDNA3.1-kozak-3×FLAG vector. For AtRbohG, a synthetic gene for Homo sapiens was used. EF-hand mutants were generated by point-mutation primers and the mega-primer PCR method. Oligonucleotide primer sequences for all constructions are shown in Table S1. AtRbohH-pro::AtRbohC::AtRbohH-ter and AtRbohJ-pro::AtRbohC::AtRbohJ-ter constructs were generated by replacing the CDS of AtRbohH-pro::AtRbohH::AtRbohH-ter or AtRbohJ-pro::AtRbohJ::AtRbohJ-ter (Kaya et al., 2014) with the CDS of AtRbohC, and were expressed in the atrbohH3 atrbohJ-2 double mutant. AtRbohC-pro::AtRbohC/AtRbohH/AtRbohJ::AtRbohC-ter constructs were generated by cloning the promoter, terminator of AtbohC and CDS (AtRbohC, AtRbohH or AtRbohH) into the pZP2H-lac binary vector for expression in atrbohC/rhd2-5 mutant. These plasmids were introduced into plants by Agrobacterium (GV3101::pMP90)-mediated transformation.
Cell culture and transfection
HEK293T cells were maintained at 37°C in 5% CO2 in Dulbecco's Modified Eagle's Medium nutrient mixture Ham's F-12 (WAKO, http://ffwk.fujifilm.co.jp) supplemented with 10% fetal bovine serum (HyClone, www.gelifesciences.com). HEK293T cells were transiently transfected with pcDNA3.1-3×FLAG-AtRbohs or empty vector by GeneJuice transfection regent (Novagen, http://www.merckmillipore.com) according to the manufacturer's instructions.
Measurement of ROS production in HEK293T cells
The ROS-producing activity of AtRbohs was assayed as described previously (Ogasawara et al., 2008). Detection of ROS production was by a peroxidase-dependent luminol-amplified chemiluminescence technique. Chemiluminescence was measured for 1 sec every 60 sec at 37°C using a microplate luminometer Centro LB960 (Berthold Technologies, https://www.berthold.com/). Production of ROS was expressed in relative luminescence units (RLU) per second. Data are presented as the average of three samples in a representative experiment. We independently replicated this experiment more than five times with similar results.
Aniline blue staining
Aniline blue staining was performed as described previously (Kaya et al., 2014). Briefly, pistils were collected 12 h after hand pollination and fixed in acetic acid:ethanol solution (25:75). The fixed pistils were softened in 1 N NaOH for 30 min at 60°C and then stained with 0.01% aniline blue in 2% potassium phosphate buffer (K3PO4).
Author contributions
HK designed the experiments. KK supervised this study. AI, AI, HH and TK performed the measurement of ROS production. TK, KM, YY, YM and AN contributed to the functional analysis of AtRbohs in plants. MK performed the phylogenetic tree analysis. HK, ST, MK, SK, MA and KK wrote the manuscript.
Acknowledgements
We thank Dr Michael Wrzaczek and Ms Kerri Hunter for critical reading and commenting on the manuscript. This work was supported in part by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan [Grants-in-Aid for Young Scientist (B) to HK (no. 21770054), for Scientific Research on Innovative Areas to HK (no. 21200068) and to K.K. (Nos. 21117516 and 23117718) and for Scientific Research B to KK (nos 19370023 and 23380027)].
Conflict of interest
The authors declare no conflict of interest.




