The tomato mutation nxd1 reveals a gene necessary for neoxanthin biosynthesis and demonstrates that violaxanthin is a sufficient precursor for abscisic acid biosynthesis
Summary
Carotenoid pigments are indispensable for plant life. They are synthesized within plastids where they provide essential functions in photosynthesis. Carotenoids serve as precursors for the synthesis of the strigolactone phytohormones, which are made from β-carotene, and of abscisic acid (ABA), which is produced from certain xanthophylls. Despite the significant progress that has been made in our understanding of the carotenoid biosynthesis pathway, the synthesis of the xanthophyll neoxanthin has remained unknown. We report here on the isolation of a tomato (Solanum lycopersicum) mutant, neoxanthin-deficient 1 (nxd1), which lacks neoxanthin, and on the cloning of a gene that is necessary for neoxanthin synthesis in both tomato and Arabidopsis. The locus nxd1 encodes a gene of unknown function that is conserved in all higher plants. The activity of NXD1 is essential but cannot solely support neoxanthin synthesis. Lack of neoxanthin does not significantly reduce the fitness of tomato plants in cultivated field conditions and does not impair the synthesis of ABA, suggesting that in tomato violaxanthin is a sufficient precursor for ABA production in vivo.
Introduction
Carotenoids are 40-carbon isoprenoid pigments which have various important roles in plant life (Cuttriss et al., 2011; Walter and Strack, 2011). In chloroplasts, carotenoids are indispensable for photosynthesis as components of light-harvesting complexes (LHCs) as well as the photosynthetic reaction centers. They contribute to plant reproduction by furnishing flowers and fruits with distinct pigmentation designed to attract animals for pollination and seed distribution and they serve as precursors for the plant hormones abscisic acid (ABA) and strigolactones (Walter et al., 2010). This article focuses on the biosynthesis of the xanthophyll neoxanthin.
Xanthophylls are oxygenated carotenes that function in LHCs both as antenna pigments and as protective molecules that prevent the formation of reactive oxygen species (ROS) under conditions of excess light by quenching excess excited state energy of chlorophylls (reviewed in Jahns and Holzwarth, 2012; Fuciman et al., 2012). Each LHC monomer protein binds four xanthophyll molecules – two luteins, one violaxanthin and one 9′-cis-neoxanthin (Croce et al., 1999b; Liu et al., 2004; Standfuss et al., 2005). Similar to lutein and violaxanthin, neoxanthin can transfer energy to chlorophylls (Frank et al., 2001). The specific roles of neoxanthin in the LHC have been described (Takaichi and Mimuro, 1998; Croce et al., 1999a,b; Hobe et al., 2006; Ruban et al., 2007; Fuciman et al., 2012). It was demonstrated that a light-induced change in the configuration of neoxanthin bound to LHCII leads to a conformational change, which activates energy dissipation from excited chlorophyll and thus facilitates photoprotection (Ruban et al., 2007). The conformational change was suggested to control the accessibility of molecular oxygen to the inner core of the complex and thus plays a role in protecting it (Mozzo et al., 2008). Studies of the Arabidopsis mutant aba4, which lacks 9′-cis-neoxanthin, demonstrated that neoxanthin protects the photosynthetic apparatus by scavenging ROS, especially superoxide anions formed in the Mehler reaction (Dall'Osto et al., 2007).
It is generally accepted that the hormone ABA is produced from the β-xanthophylls violaxanthin and neoxanthin (reviewed in Milborrow, 2001; Schwartz et al., 2003a; Finkelstein and Rock, 2002; Nambara and Marion-Poll, 2005). Biochemical assays in vitro indicated that both violaxanthin and neoxanthin were cleaved by the 9-cis-epoxy-carotenoid dioxygenase (NCED) to form ABA (Qin and Zeevaart, 1999; Chernys and Zeevaart, 2000) with a higher affinity to violaxanthin (Schwartz et al., 2003b). Yet, in all plants, 9′-cis-neoxanthin is far more abundant than 9-cis-violaxanthin. Therefore, the question about the direct precursor in vivo still remains unanswered. Two reports have underscored this quandary. A reduction in ABA production was recorded in the neoxanthin-deficient Arabidopsis mutant aba4, suggesting that ABA, especially under water stress, is derived from neoxanthin (North et al., 2007). However, ABA in the mutant was not completely abolished, indicating that it could also be synthesized from cis-violaxanthin. Moreover, the parasitic plant Cuscuta reflexa is capable of producing ABA even though this species lacks 9′-cis-neoxanthin (Qin et al., 2008).
Despite the significant progress that has been made in our understanding of the carotenoid biosynthesis pathway to date, the mechanism of formation of the xanthophyll neoxanthin remains unknown. Carotenoids are synthesized within plastids from the 2-C-methyl-d-erythritol 4-phosphate (MEP) pathway of isopentenyl diphosphate (IPP) synthesis. The carotenoid biosynthesis pathway in plants is essentially worked out (reviewed in Cunningham and Gantt, 1998; Hirschberg, 2001; DellaPenna and Pogson, 2006; Cazzonelli and Pogson, 2010; Ruiz-Sola and Rodriguez-Concepcion, 2012; Farre et al., 2010). It has been established that neoxanthin in plants is synthesized from violaxanthin (Li and Walton, 1990; Parry and Horgan, 1991; Rock and Zeevaart, 1991; Galpaz et al., 2008; Qin et al., 2008) by a presumed enzyme termed neoxanthin synthase (NSY). Two previous reports have suggested that NSY is encoded by a gene of the lycopene cyclase gene family (Al Babili et al., 2000; Bouvier et al., 2000). However, the ortholog of this gene in pepper codes for capsanthin–capsorubin synthase (CCS) (Bouvier et al., 1994) and in tomato for a chromoplast-specific lycopene β-cyclase (CYC-B) (Ronen et al., 2000). A null mutation in CycB in the tomato mutant ogc did not affect neoxanthin synthesis (Ronen et al., 2000). Moreover, no homologous gene has been identified in the genome of Arabidopsis. A gene involved in the conversion of violaxanthin to neoxanthin, aba4, was cloned in Arabidopsis (North et al., 2007). However, in vitro activity of the cloned gene has never been demonstrated. For these reasons, the identity of NSY is still not known.
We report here on the isolation of tomato mutants lacking neoxanthin and the cloning of a gene that is necessary for neoxanthin synthesis in plants. We further show that neoxanthin deficiency in tomato plants does not significantly reduce plants fitness in cultivated field conditions and does not impair the synthesis of ABA, suggesting that in tomato violaxanthin is a sufficient precursor for ABA production in vivo.
Results
The tomato mutation nxd1 lacks neoxanthin
Two recessive mutations with pale yellow flowers were identified in the tomato (Solanum lycopersicum) mutant collection generated by ethyl methanesulfonate (EMS) in the variety M82 (Menda et al., 2004; Galpaz et al., 2008) (Figure 1). The mutant lines e1692 and de0778 were back-crossed with the ‘wild-type’ cultivar M82 in order to obtain F2 mutant plants which are isogenic to M82. The two mutants were crossed and found to be allelic, as F1 plants exhibited the mutant phenotype.

The yellow color of flowers in tomato is determined by the accumulation of xanthophylls, which comprise 97% of all carotenoids, with neoxanthin being the predominant species (Table 1). Carotenoid analysis in petals of the mutants revealed a reduction of about 50% in the concentration of xanthophylls due to the absence of neoxanthin. Therefore, we named the mutation locus neoxanthin-deficient 1 (nxd1) and the alleles e1692 and de0778 nxd1-1 and nxd1-2, respectively. The leaf and fruit color of the mutants appeared normal. However, pigment analysis indicated a lack of neoxanthin in leaves as well (Table 1). The small amounts of neoxanthin in leaves of nxd1-1 may be attributed to leakiness of the mutation in this allele. All other carotenoids were present in these tissues, suggesting that the mutation nxd1 specifically impairs the conversion of violaxanthin to neoxanthin.
| Line | Phytoene + phytofluene | Lycopene | β-Carotene | Zeaxanthin | Antheraxanthin | trans-Violaxanthin | 9-cis-Violaxanthin | 9,13-di-cis-Violaxanthin | trans-Neoxanthin | 9′-cis-Neoxanthin | 9′,13′-di-cis-Neoxanthin | Lutein | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flowers | wt | 0 | 0 | 0 | 0 | 1.7 ± 0.3 | 111.4 ± 2 | 52.3 ± 2 | 25.6 ± 3 | 331.6 ± 5 | 99.0 ± 8 | 82.3 ± 6 | 2.8 ± 0.3 | 707 ± 17 |
| nxd1-1 | 0 | 0 | 0 | 0 | 1.5 ± 1 | 181 ± 4.6 | 168.3 ± 5 | 34.6 ± 3 | 5.7 ± 0.5 | 0 | 0 | 22 ± 0.2 | 413 ± 8 | |
| Leaves | Wt | 0 | 0 | 61.5 ± 1 | 12.1 ± 4 | 12.7 ± 2 | 27.6 ± 5 | 1.2 ± 0.6 | 1.8 ± 0.3 | 2 ± 0.1 | 30.1 ± 0.2 | 3.7 ± 0.2 | 103.1 ± 1 | 256 ± 25 |
| nxd1-1 | 0 | 0 | 73.8 ± 3 | 53 ± 1 | 9.9 ± 2 | 68.6 ± 3 | 13.2 ± 0.5 | 3.1 ± 0.3 | 2 ± 0.1 | 4.6 ± 0.1 | 0 | 122.6 ± 5 | 351 ± 10 | |
| nxd1-2 | 0 | 0 | 173.0 ± 58.9 | 0 | 15.5 ± 2.3 | 43.4 ± 21.6 | 1.8 ± 1.0 | 5.5 ± 2.2 | 0 | 0 | 0 | 105.8 ± 29 | 251 ± 70 | |
| Fruit | Wt | 9 ± 2.2 | 67.3 ± 4.7 | 1.2 ± 0.2 | 0.5 ± 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.2 ± 0.2 | 78 ± 5 |
| nxd1-1 | 10 ± 3.5 | 70.5 ± 6.4 | 1.5 ± 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.3 ± 0.7 | 83 ± 5 |
- Concentrations given in μg g−1 fresh weight tissues ± standard deviation (n ≥ 3).
- wt, wild type.
Neoxanthin and violaxanthin both occur in three geometric isomers: all-trans, 9-cis and di-cis, most probably 9,13-di-cis (Molnar et al., 1986; Grudzinski et al., 2001; Zubik et al., 2011) (Figure 2 and Figure S1). All neoxanthin isomers were eliminated in nxd1 flowers while levels of all three violaxanthin isomers were elevated (Table 1). In wild-type (wt, M82) leaves, the prevalent epoxy-xanthophylls are 9′-cis-neoxanthin and all-trans-violaxanthin (Croce et al., 1999a; Liu et al., 2004; Caffarri et al., 2007). In the leaves of nxd1 mutants, 9′-cis-neoxanthin was drastically reduced (in nxd1-1) or abolished (in nxd1-2) and di-cis-neoxanthin was completely eliminated, whereas the concentrations of all three isomers of violaxanthin were elevated. Due to compensation by violaxanthin and lutein, total carotenoid levels in nxd1 leaves were not lower than in the wt. Carotenogenesis in fruits of nxd1 appears normal, as they accumulate mainly the red pigment lycopene, a carotenoid intermediate in the biosynthetic pathway upstream to the xanthophylls (Table 1).
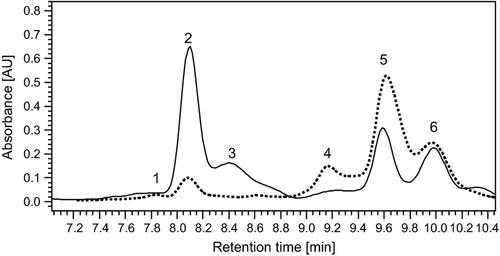
Carotenoid analysis by HPLC.
Chromatogram of leaf β-xanthophylls extracted from the wild type (solid line) and the mutant nxd1-1 (dashed line) at an absorbance of 450 nm: peak 1, 9,13-di-cis-neoxanthin; peak 2, 9′-cis-neoxanthin; peak 3, all-trans-neoxanthin; peak 4, 9,13-di-cis-violaxanthin; peak 5, 9-cis-violaxanthin; peak 6, all-trans-violaxanthin. The absorbance spectra of the peaks are presented in Figure S1.
Effect of neoxanthin deficiency on plant growth
The rate of photosynthesis as measured by chlorophyll fluorescence in young leaves was similar in wt and nxd1-1. The maximum quantum yield of photosystem II (PSII) (Fv/Fm) was 0.837 ± 0.005 and 0.817 ± 0.014 in wt and nxd1-1, respectively, and PSII maximum efficiency (Fv′/Fm′) was 0.48 ± 0.02 and 0.45 ± 0.02 in wt and nxd1-1, respectively.
Likewise, non-photochemical quenching (NPQ) kinetics following exposure of young leaves to 1250 μmol photons m−2 sec−1 for 10 min indicated a similar capacity for dissipation of excess energy in mutants nxd1-1 and nxd1-2 as in the wt (Figure 3). However, recovery of NPQ in the dark was slightly slower in the mutants.
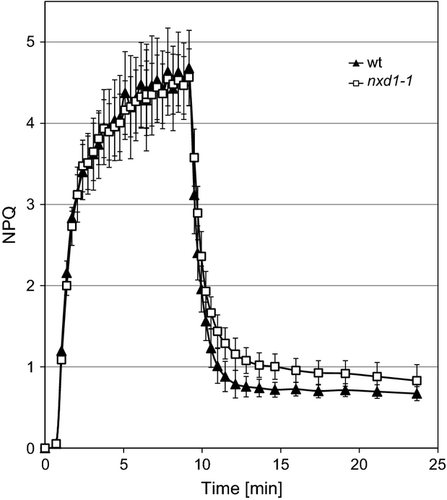
Induction of non-photochemical quenching (NPQ).
Induction of NPQ in dark-adapted leaves exposed to actinic light at 610 mmol photons m−2 sec−1. Exposure to light is indicated as a white horizontal bar. NPQ values were calculated as NPQ = (Fm – Fm′)/Fm′ (mean values ±SD, n = 3). wt, wild type.
Another consequence of the effect of neoxanthin deficiency on the overall process of photosynthesis is the performance of mutant plants in the field. The tomato variety M82 has determinate branches which allow quantitative measurements of yield. Field-grown plants of nxd1 exhibited slightly enhanced senescence in old leaves. Nevertheless, the effect of neoxanthin deficiency on the yield of nxd1-1 plants grown in the field was minimal, if any, since vegetative biomass and fruit yield were not significantly different from wt plants even under non-irrigated, i.e. water-stressed, conditions (Figure 4).
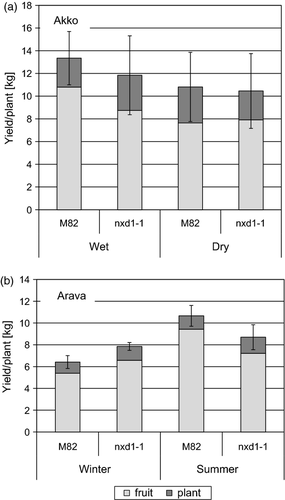
Total yield of wild type (M82) and nxd1-1 tomato plants grown in the field.
(a) Plants grown in summer at Akko with or without irrigation.
(b) Plants grown at Arava.
Light gray, fruit; dark gray, vegetative organs.
Map-based cloning of nxd1
A neoxanthin-deficient mutant in Arabidopsis thaliana, aba4, was previously reported by North et al. (2007). Two orthologs of the Aba4 gene exist in tomato, termed here SlAba4a (gene Solyc02g086050, cDNA SGN-U563158, http://solgenomics.net/) and SlAba4b (Solyc02g063170, cDNA SGN-U582083) (Figure S2A), which are located in two different arms of chromosome 2. We have sequenced SlAba4-1 and SlAba4-2 from nxd1 and wt plants and found no alterations in their genomic DNA sequences or reduction in their transcript levels in nxd1-1 (Figure S2B). Moreover, as described here, the nxd1 locus in tomato was mapped to chromosome 12. These results reinforce the characterization of Nxd1 as a gene whose function is necessary for the synthesis of neoxanthin in tomato.
To elucidate the genetic basis of nxd1, the mutation locus was mapped using segregating F2 plants of a cross between nxd1-1 with the tomato wild species Solanum pennellii. Screening of 800 F2 plants with 83 polymorphic PCR markers between S. pennellii and S. lycopersicum indicated co-segregation at the resolution of about 1.5 cM between the mutation and marker C2_At3g24490 on chromosome 12, corresponding to a region represented in the introgression line IL 12-3 (Eshed and Zamir, 1995) (Figure 5). For fine mapping, a population of 1720 F2 plants from a cross of nxd1-1 × IL12-3 was created and screened with the markers C2_At3g24490 and SGN-U603146, revealing 27 informative recombination events between them. The small number of recombinants was probably due to the location of the gene in a heterochromatic chromosomal region in proximity to the centromere. Additional markers were then designed using genomic sequence information which became available from the tomato genome project (http://solgenomics.net/). These markers further defined the genomic location of the locus nxd1 to a region near the centromere of chromosome 12, between the markers 12-Cntg26372 and SGN-U567375 in which no recombinants were found (Figure 5).
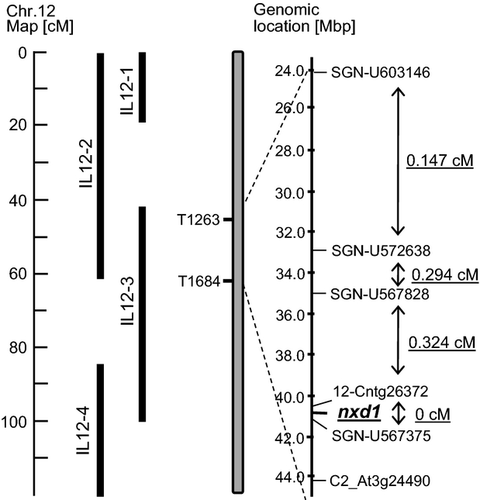
Map-based cloning scheme of the nxd1 locus.
Figure shows the markers used in screening of 1700 F2 plants from a cross nxd1-1 × IL12-2 are indicated as well as recombination frequencies in centimorgans (cM).
We hypothesized that the mutation in nxd1 might affect the transcript level of this gene either due to a direct effect on its transcription or as a result of a nonsense-mediated degradation effect in case of an early stop codon. To find genes that exhibit lower expression in the mutant nxd1-1, we carried out transcriptomic analyses in leaves and flowers from nxd1-1 and M82 plants using the Affymetrix GeneChip EU-TOM3. One of the transcripts whose level in nxd1-1 was reduced by 62% in leaves and by 42% in flowers was Unigene SGN-U573074, which is encoded by the predicted gene Solyc12g041880 found to be located on chromosome 12, 9.8 kb from marker 12-03179. According to the genome data (The Tomato Genome Consortium, 2012) (http://solgenomics.net/), SGN_Solyc12g041880 contains nine introns and encodes a transcript of 1147 nucleotides (Figure S3). In agreement with the GeneChip data, the mRNA levels of Unigene SGN-U573074 measured in leaves of nxd1-1 and nxd1-2 by quantitative RT-PCR were 44 and 31%, respectively, compared with the wt (Figure 6).
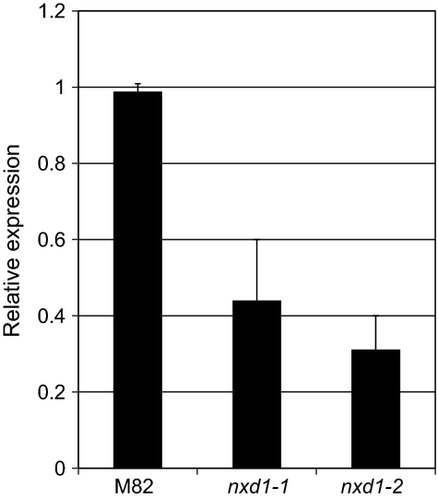
Expression of the gene Nxd1 in the wild type (M82) and mutants nxd1-1 and nxd1-2.
The relative concentration of Nxd1 mRNA extracted from young leaves was determined by real-time quantitative RT-PCR. Data shown are means ± SD (n = 3).
The DNA sequencing of the genomic region of Solyc12g041880 in mutant nxd1-1 revealed a single point mutation, a C to T transition, in nucleotide 966 (Figures 7 and S3). However, sequencing of cDNA from nxd1-1 plants indicated a deletion of four nucleotides, 140–143, compared with the wt sequence (Figure 7). Since these four nucleotides are located at the 3′ end of the first exon, it appears that the C to T transition created a new splicing site four nucleotides upstream of the one in the wt. Consequently, a frameshift mutation was created in nxd1-1, leading to an early stop codon. The DNA sequencing of Solyc12g041880 in mutant nxd1-2 revealed an A to T transition in nucleotide 1195 in the second intron (Figures 7 and S3). This mutation did not change the mRNA as determined by cDNA sequencing. But the location of the mutation in the 3′ splicing site of the second intron can explain the three-fold decrease in the mRNA level of Nxd1. These results strongly suggest that Solyc12g041880 is the same as the gene Nxd1 in tomato.

Mutations in Nxd1 found in the mutants nxd1-1 and nxd1-2.
Genomic DNA (top lines) and cDNA sequences indicated a splicing mutation in nxd1-1. The deletion of four nucleotides in nxd1-1 mRNA was detected in all cDNA sequenced. Intron sequences are underlined.
The gene Nxd1
Sequence of the full-length cDNA of Nxd1 in tomato predicts a polypeptide of 295 amino acids with a calculated molecular weight of 32.4 kDa (Figure S4). The predicted amino acid sequence of NXD1 from S. lycopersicum is identical in the wild tomato species Solanum chilense, Solanum habrochaites, S. pennellii, Solanum cheesmaniae, Solanum pimpinellifolium, Solanum peruvianum, Solanum chemielewskii and Solanum neorickii and highly conserved in other plants (Figure S4). Bioinformatics searching in genomic databases indicated that Nxd1 is exclusively found in all higher plants and some green algae, e.g. Chlamydomonas reinhardtii. The amino acid sequence of the gene product is unique, with no apparent chloroplast localization sequence or other known functional motifs.
The genome of A. thaliana contains a gene of unknown function, At1g28100, encoding a polypeptide whose amino acid sequence is 59% identical (67% similar) to the tomato NXD1 (Figure S4). We have obtained an Arabidopsis line in Col-0 background, SALK_020928 (Alonso et al., 2003), which carries a T-DNA insertion in the first intron of At1G28100 in the 5′ untranslated region (Figure S5). Homozygous plants to this T-DNA insertion grow normally without any visible phenotype. However, analysis of leaf carotenoids from these plants revealed that, similarly to the nxd1 phenotype in tomato, they lack neoxanthin (Table 2). Thus, we concluded that the gene At1G28100 is the Arabidopsis ortholog of Nxd1 (At_Nxd1). Taken together, these data establish that the Nxd1 gene product is necessary for the synthesis of neoxanthin in plants.
| β-Carotene | Antheraxanthin | trans-Violaxanthin | 9-cis-Violaxanthin | 13-cis-Violaxanthin | trans-Neoxanthin | 9′-cis-Neoxanthin | 9′,13′-cis-Neoxanthin | Lutein | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| wt (col) | 65.8 ± 23.2 | 16.0 ± 0.95 | 14.1 ± 4.2 | 23.6 ± 1.5 | 5.5 ± 0.9 | 34.1 ± 5.5 | 40.7 ± 12.5 | 6.6 ± 2.0 | 246.0 ± 101.7 | 452.6 ± 101.7 |
| At-nxd1 | 97.0 ± 70.7 | 23.6 ± 3.4 | 26.5 ± 13.2 | 43.0 ± 23.2 | 19.8 ± 7.7 | 0 | 0 | 0 | 299.1 ± 75.2 | 509.0 ± 157.3 |
| aba4 | 106.4 ± 35.2 | 19.4 ± 7.0 | 37.5 ± 13.3 | 50.5 ± 31.6 | 15.3 ± 1.9 | 0 | 0 | 0 | 190.0 ± 10.7 | 419 ± 130.9 |
- Concentrations given in μg g−1 fresh weight tissues ± standard deviation (n ≥ 3).
- wt, wild type.
NXD1 is necessary but not sufficient for neoxanthin synthesis
To test for enzymatic activity of NXD1, we expressed the full-length cDNA of Nxd1 from Arabidopsis in Escherichia coli cells (strain XL1-Blue) carrying the plasmid pAC-VIOL. Bacterial cells with pAC-VIOL accumulate mainly all-trans violaxanthin (>85% of total carotenoids), as well as low levels of zeaxanthin, antheraxanthin and other carotenoids (Table 3). Expression of the Arabidopsis cDNA of At_Nxd1 carried by plasmid pBS-NXD1 in these E. coli cells did not alter the carotenoid composition. Likewise, no change in carotenoid composition was observed when At_Nxd1 cDNA was co-expressed with the Arabidopsis cDNA of Aba4 (carried in the expression plasmid pBS-ABA4) (Table 3). These negative results may well be due to lack of functionality of the NXD1 polypeptide specifically in E. coli. Alternatively, these data suggest that NXD1 is necessary but not sufficient for neoxanthin synthesis from violaxanthin in vivo.
| Plasmids | Carotenoid composition (percentage of total) | ||||
|---|---|---|---|---|---|
| trans-Violaxanthin | cis-Violaxanthin | Antheraxanthin | Zeaxanthin | β-Cryptoxanthin | |
| pAC-Viol | 89.21 ± 1.83 | 4.24 ± 0.17 | 1.09 ± 0.68 | 1.13 ± 0.8 | 4.32 ± 0.3 |
| pAC-Viol + pGEM-At_Nxd1 | 84.63 ± 4.92 | 5.68 ± 1.08 | 3.39 ± 2.22 | 2.96 ± 2.49 | 3.34 ± 0.85 |
| pAC-Viol + pGEM-At_Aba4 | 86.09 ± 4.43 | 4.41 ± 0.96 | 3.77 ± 2.27 | 3.50 ± 2.75 | 2.23 ± 0.67 |
| pAC-Viol + pGEM-At_Nxd1 + At_Aba4 | 89.26 ± 3.16 | 3.79 ± 1.0 | 1.87 ± 1.15 | 1.76 ± 1.44 | 3.32 ± 0.64 |
Biosynthesis of ABA in nxd1 plants
It is acknowledged that ABA is synthesized from 9-cis isomers of both neoxanthin and violaxanthin (reviewed in Finkelstein and Rock, 2002; Nambara and Marion-Poll, 2005; Taylor et al., 2005; Schwartz et al., 2003a). Although nxd1 mutants contain little or no neoxanthin, they do not display any visible phenotype typical of ABA deficiency. To determine the effects of neoxanthin deficiency on physiological processes regulated by ABA, we analyzed rapid response to drought stress by measuring the rate of water loss in detached leaves. Leaflets of the tomato mutants nxd1-1, nxd1-2 and high-pigment 3 (hp3), which is deficient in ABA due to a mutation in the gene for zeaxanthin epoxidase (Zep) (Galpaz et al., 2008), and of the wild-type (M82) were removed from 6-week old plants and placed on open plates at room temperature. The kinetics of water loss was essentially identical in nxd1 mutants and the wt, in contrast to the fast rate in the ABA-deficient mutant hp3 (Figure 8).
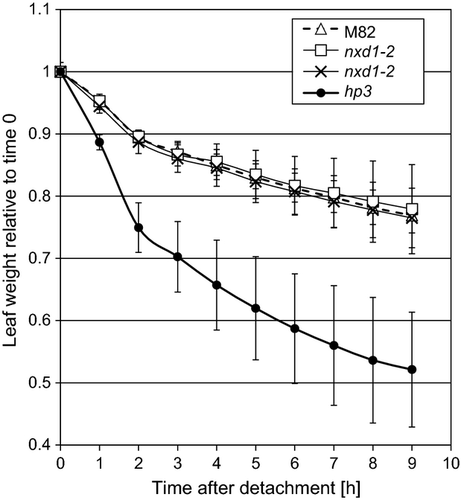
Kinetics of water loss in detached leaves of wild type (M82) and mutants nxd1-1, nxd1-2 and hp3 (Galpaz et al., 2008).
Detached leaves were placed abaxial side up in open Petri dishes at room temperature (23 ± 1 °C). Water loss is expressed as relative leaf weight at a given time compared with time zero. Values are means ± SD (n = 5).
Tolerance to long-term drought stress was estimated in whole plants grown in the greenhouse by the number of days to wilting after watering was stopped. The average time to wilting was 12 days in hp3 plants, 17 days in wt (M82) and 21 days in mutant nxd1-1 (Figure S6). This observation, together with the yield performance under dry field conditions, indicates increased tolerance of nxd1 plants to progressive drought stress.
The concentration of ABA was measured in detached leaves of well-watered greenhouse plants of nxd1-1, hp3 and the wt. At the time of detachment of the leaves, ABA levels were similar in both mutants and the wt. However, 5 h after detachment, a massive increase in the steady-state level of ABA was detected in both wt and nxd1 leaves, but not in hp3. The observed ABA levels in nxd1 were slightly higher than in wt, although not statistically significant (Figure 9).
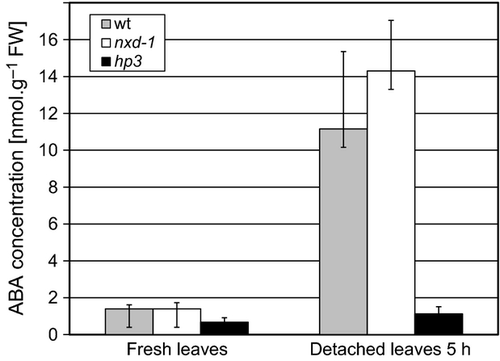
Abscisic acid (ABA) concentration in leaves of wild type (wt, M82) and mutants nxd1-1 and hp3.
Abscisic acid was determined immunologically in fresh leaves and in detached leaves after 5 h. Data shown are means ± SD (n = 5).
Taken together, these results indicate that neoxanthin deficiency in nxd1 does not affect the level of ABA and suggest that violaxanthin may be a sufficient precursor for ABA synthesis in tomato.
Discussion
Nxd1 encodes a function necessary for neoxanthin biosynthesis
This work targeted the elucidation of neoxanthin synthase, the last unknown enzyme in the central pathway of carotenoid biosynthesis in plants. Genetic and molecular analyses of two alleles of nxd1, a recessive neoxanthin-deficient mutation in tomato, have identified a gene of unknown function which is necessary for neoxanthin synthesis. Two unrelated mutations in the gene Nxd1 were identified in nxd1-1 and nxd1-2 alleles in tomato. A loss-of-function mutation in the orthologous Nxd1 gene of Arabidopsis showed an identical phenotype of lack of neoxanthin in leaves. Nxd1 occurs exclusively in plants and some green algae and in tomato it is expressed in leaves, green fruit and flowers. Based on these findings, we conclude that Nxd1 encodes a function which is essential for neoxanthin synthesis in plants. The low level of neoxanthin in allele nxd1-1 (about 1.2 and 18% of the concentration in wt in flowers and leaves, respectively) is attributed to leakiness, possibly due to a low incidence of normal splicing that may produce minute amounts of full-length and properly spliced Nxd1 mRNA.
The accumulation and composition of xanthophyll isomers differs between tomato tissues. In petals, the isomers which mainly accumulate are all-trans-neoxanthin and all-trans-violaxanthin, whereas in leaves the main isomers are 9-cis-neoxanthin and all-trans-violaxanthin (Table 1). These differences are most likely due to tissue-specific regulation of xanthophyll biosynthesis at the biochemical level. In both tomato and Arabidopsis, the trans to cis isomerization of violaxanthin was not prevented by mutations in Nxd1 since violaxanthin that is accumulated in mutant plants retains a high ratio of 9-cis and di-cis to all-trans isomers (Tables 1 and 2). Similarly, cis-violaxanthin isomers also appear in the Arabidopsis mutant aba4 (Table 2). These results imply that the synthesis of 9′-cis-neoxanthin is composed of two independent steps that may proceed in inverse order: (i) the opening of the epoxide ring of violaxanthin to generate a chiral allene; (ii) trans to cis isomerization of C9, which may occur in violaxanthin or neoxanthin (Figure 10). NXD1 and ABA4 are only required for the first step.
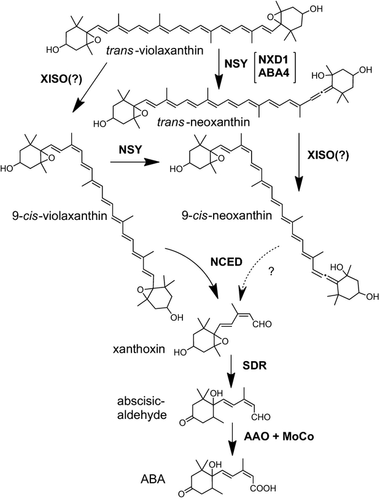
Proposed pathway of 9′-cis-neoxanthin biosynthesis in plants.
Abbreviations: AAO-MoCo, abscisic aldehyde oxygenase-molybdenum cofactor; NCED, 9-cis-epoxycarotenoid dioxygenase; NSY, neoxanthin synthase; SDR, short-chain dehydrogenase/reductase; XISO, xanthophyll 9-cis isomerase.
We were unable to demonstrate neoxanthin synthesis activity by NXD1 in the functional expression assay in E. coli (Table 3). Since ABA4 was also shown to be essential for neoxanthin synthesis in Arabidopsis (North et al., 2007), it is possible that ABA4 and NXD1 jointly carry out neoxanthin synthesis. However, E. coli cells co-expressing cDNAs of both At_NXD1 and At_ABA4 did not synthesize neoxanthin. One explanation for this result is that NXD1, alone or together with ABA4, might not be active in bacterial cells. Another possibility is that the presumed NSY enzyme is a multi-polypeptide complex of which NXD1 is only one component, and additional as yet unknown polypeptide(s) are required for its activity. An alternative explanation is that the NXD1 polypeptide is not neoxanthin synthase nor is it directly involved in NSY activity. This hypothesis is supported by three characteristics of NXD1. (i) Unlike all other known enzymes involved in carotenoid biosynthesis, including desaturases, isomerases, hydroxylases and epoxidases, the presumed amino acid sequence of NXD1 has no recognized sequence motif of any kind. (ii) Neoxanthin synthase must be localized within plastids. Yet, the amino acid sequence of NXD1 does not have chloroplast targeting signals which occur in about 86% of nuclear-encoded chloroplast proteins (Zybailov et al., 2008). (iii) NXD1 has not been detected in the published proteomes of any plastids (Kleffmann et al., 2004; Barsan et al., 2010, 2012; Ferro et al., 2010; Joyard et al., 2010; Demartini et al., 2011; van Wijk and Baginsky, 2011; Bruley et al., 2012; Huang et al., 2013) or in databases such as AT_CHLORO (www.grenoble.prabi.fr/at_chloro) and plprot (http://www.plprot.ethz.ch/).
In the genomes of only three species – Fragaria vesca subsp. Vesca, Vitis vinifera and Ricinus communis – a single Nxd1 sequence is found to be fused to the carboxyl terminus of a pentatricopeptide repeat (PPR) protein that is highly conserved with the Arabidopsis gene At5g13270. This gene encodes RARE1, a trans-factor essential for C-to-U editing of the chloroplast transcript of accD, which catalyzes the first step in fatty acid biosynthesis in chloroplasts (Robbins et al., 2009). In Arabidopsis, RARE1 is found within chloroplasts in a complex of >200 kDa, interacting with the RNA-editing factor interacting protein 1 (RIP1, At3g15000), which was detected in both chloroplasts and mitochondria (Bentolila et al., 2012). The predicted RARE1-NXD1 fused proteins in F. vesca (accession XM_004310193), V. vinifera (accession XM_002266990) and R. communis (accession XM_002512611), contain 835–837 amino acids and their PPR domains are about 60% identical to the Arabidopsis RARE1. It is tempting to speculate that NXD1 interacts with RARE1 and is possibly associated with its biological functions. Although the precise biochemical function of NXD1 in neoxanthin formation remains unknown, we postulate that it is involved in an auxiliary process which provides a necessary function for NSY activity.
How critical is neoxanthin to plants?
The xanthophyll neoxanthin which is present in the LHCs of essentially all higher plants plays roles in photosynthesis, metabolism and pigmentation. The functions of neoxanthin in photosynthesis have been documented at the biochemical and biophysical levels (Takaichi and Mimuro, 1998; Croce et al., 1999a,b; Hobe et al., 2006; Ruban et al., 2007; Fuciman et al., 2012). Our data show that lack of neoxanthin in the nxd1-1 mutant did not affect electron transfer through PSII and did not reduce the photoprotective capacity of the LHC, as reflected by non-photochemical quenching kinetics. Neoxanthin in the 9′-cis isomeric configuration is exclusively bound to the N1 niche of protein LHCII, the major LHC of photosystem II (Liu et al., 2004; Standfuss et al., 2005). However, in the absence of neoxanthin, the N1 site in LHCII can also be occupied by 9-cis-violaxanthin (Snyder et al., 2004; Dall'Osto et al., 2007). It has been proposed that the N1 site in LHCII binds neoxanthin and violaxanthin at a ratio of 0.75:0.30 (Fuciman et al., 2012). In the mutant aba4 of Arabidopsis, violaxanthin can substitute neoxanthin in LHCII, causing a small reduction in the capacity for photoprotection and increased susceptibility of membrane lipids to photo-oxidation by reactive oxygen species (Dall'Osto et al., 2007). A powerful indication of the comprehensive contribution of neoxanthin to photosynthesis and to overall plant fitness can be inferred from the performance of field-grown plants which lack this xanthophyll. Since neoxanthin-deficient plants did not exhibit a significant reduction in yield, we postulate that neoxanthin can be functionally replaced by the increased 9-cis-violaxanthin observed in nxd1 leaves. Since the tomato plants were grown in a cultivated field one cannot rule out other functions of neoxanthin that enhance evolutionary fitness in the wild. Nevertheless, this result is a remarkable manifestation of the adaptive flexibility that exists in plants to cope with biochemical and physiological deficiencies.
Abscisic acid biosynthesis
The first committed step in ABA biosynthesis is the oxidative cleavage of 9-cis epoxycarotenoids, carried out by the enzyme NCED. It is assumed that both 9-cis-violaxanthin and 9′-cis-neoxanthin serve as substrates in this reaction (Schwartz et al., 1997) (reviewed in Nambara and Marion-Poll, 2005). Unlike the phenotype of the aba4 Arabidopsis mutant, we have not observed ABA deficiency in nxd1 tomato plants. Furthermore, nxd1-1 plants were more resistant to drought conditions, an opposite phenotype to ABA deficiency (Figure S5). This is also manifested in dry field conditions where nxd1-1 plants had comparable yields and biomass to the wt. Based on these results, together with the fact that mutant hp3, which lacks both violaxanthin and neoxanthin, is ABA deficient (Galpaz et al., 2008), we suggest that 9-cis-violaxanthin is not only a bona fide substrate for NCED but also a sufficient source and possibly the major precursor for ABA biosynthesis in vivo. This hypothesis is supported by the discovery that the parasitic plant Cuscuta reflexa is capable of producing ABA even though it lacks neoxanthin (Qin et al., 2008).
Experimental Procedures
Plant material
The following tomato (S. lycopersicum) cv. M82 lines were used in this work: M82 (referred to as wt), EMS- generated mutants e1692 (nxd1-1), de778 (nxd1-2), and e1472 (hp3) from the tomato mutant collection (Menda et al., 2004) http://zamir.sgn.cornell.edu/mutants/). Additional wild tomato species used were: S. pennellii (LA0716), S. pimpinellifolium (LA1589) LA1245, S. cheesmanii (LA0166), S. peruvianum (LA1944) LA0361, S. chemielewskii (LA1840), S. parviflorum LA1329 and S. chilenese. Additional S. lycopersicum lines used were: Rutgers, Ailsa Craig (AC) and Heinz.
An F2 segregating mapping population was created by crossing the tomato mutant nxd1-1 with the wild species S. pennellii, and later with the introgression line IL12-3 (Eshed and Zamir, 1995; Liu et al., 2003). Plants were grown in the greenhouse of the Alexander Silberman Institute of Life Sciences, at the Hebrew University of Jerusalem, year-round in 4-L pots with commercial soil (Shacham, Givat Ada, Israel, http://www.shacham-g-a.com/index.htm). Between April and August plants were also grown in the experimental field in Akko and from September to December in Arava.
The following Arabidopsis (A. thaliana) lines were used in this work: Col-0 (referred to as wt), the T-DNA insertion mutant SALK_137455.52.40.x (At_aba4) and the T-DNA insertion mutant SALK_020928.56.00.x (At_nxd1), all obtained from the Arabidopsis Biological Resource Center (http://abrc.osu.edu/).
DNA extraction and mapping with DNA markers
A sample of approximately 15 mg from young tomato leaves was used for DNA extraction as described previously (Eshed and Zamir, 1995). Amplifications of specific sequences from total genomic DNA were done with PCR using a Readymix kit (PCR-Ready® High Yield, Syntezza, http://www.syntezza.com/). To analyze restriction fragment length polymorphisms (RFLPs), genomic DNA was digested with restriction enzymes according to previously described protocols (Tanksley et al., 1992). The primers used for DNA markers are given in Table S1.
RNA purification, cDNA synthesis and qRT-PCR
Total RNA was extracted from plant tissues by GeneJET Plant RNA Purification Mini Kit #K0801 (Thermo Scientific, http://www.thermofisher.com/). RNA was treated with DNase I (#M0303L, New England BioLabs, https://www.neb.com/) and then reverse transcribed by Moloney Murine Leukemia Virus Reverse Transcriptase (#M0253L, New England BioLabs). Complementary DNA (cDNA) was amplified by 7900HT Fast Real-Time PCR System (Applied Biosystems, http://www.appliedbiosystems.com/). Quantitative PCR reactions were performed using the KAPA SYBR® FAST qPCR kit (Kapa Biosystems, http://www.kapabiosystems.com/) according to the manufacturer's protocol on an ABI Prism 7900HT (Applied Biosystems). Thermocycling conditions were 95°C for 1 min followed by 40 cycles of 95°C for 20 sec, 60°C for 30 sec and fluorescence acquisition at 60°C. To rule out the possibility of contamination with genomic DNA, the primers were designed to span intron sequences. Melting curve analysis and sequencing verified PCR product specificity.
Relative quantification was done according to the 2-ΔΔCt method (Livak and Schmittgen, 2001) using the gene for actin 4 (Solyc04g011500) as a reference for normalization in tomato, and the for gene actin 2 (At3G18780) for a reference in Arabidopsis (Lovdal and Lillo, 2009).
The following primers were used for PCR amplification in tomato: for Actin 4, 5′-AGATTAAGGTGGTCGCTCCA-3′ (forward) and 5′-AGAAGCACTTCCTGTGGACAA-3′ (reverse); for ABA4-1 (Solyc02g063170), 5′-CAGACTGCCTCTCCTGCTCT-3′ (forward) and 5′-TCTGAACTTGGCACCCA-3′ (reverse); for ABA4-2 (Solyc02g086050), 5′-TGTGCTCGGACTTCTGTACG-3′ (forward) and 5′-ACTTGCCTTGCAGCAAAAAG-3′ (reverse); for NXD1, 5′-CGATGAGCTTGTGGTGATTG-3′ (forward) and 5′-CTTTCCGGTTTCTGTGGAAG-3′ (reverse); for PSY1, 5′-CCGAGAGAAGAAGGGCTATC-3′ (forward) and 5′-TCCATACGCATTCCTTCAAT-3′ (reverse); for PSY2, 5′-TGCAGCTTTATCCGACACTG-3′ (forward) and 5′-GCAATGCCCATAATTGGAAC-3′ (reverse); for PP2C8, 5′-AACTCCGGACAAAATCATCG-3′ (forward) and 5′-TTGCTAAAACGCCGAGAACT-3′ (reverse); for PYL4, 5′-TCACTTACATTACCACACCCACGCT-3′ (forward) and 5′-TTACCTGAACCTCCCTTAATGTGCC-3′ (reverse).
The following primers were used for PCR amplification in Arabidopsis: for At_Actin2, 5′-CCGGTATTGTGCTGGATTCT-3′ (forward) and 5′-TTACAATTTCCCGCTCTGCT-3′ (reverse); for At_NXD1, 5′-GGGACAGAGCTTTTGGTTTC-3′ (forward) and 5′-AGCCATTTTGATTGCAGGTC-3′ (reverse).
Sequence analysis
For analysis of the DNA sequence of the gene Nxd1, we amplified genomic sequences by PCR using the following primers: fragment 1, 5′-AACATTGGTGGCCAAGAAAA-3′ (forward) and 5′-GGATTCCACACTAATCCTGC-3′ (reverse); fragment 2, 5′-TGGGTTATGGAAAACCTCCA-3′ (forward) and 5′-ATTTCCAAGGCTTGAACACG-3′ (reverse); fragment 3, 5′-TTGAAGCCCCGCATAGTAAC-3′ (forward) and 5′-ACTCCCTCCCTTGGTTAGGA-3′ (reverse); fragment 4, 5′-GGCGTGAAAACCGATAAAGA-3′ (forward) and 5′-CCTCTGGGCACTCTGCTAAC-3′ (reverse); fragment 5, 5′-GGATTCTAAGGGATGGATGG-3′ (forward) and 5′-CCTCTGGGCACTCTGCTAAC-3′ (reverse).
For analysis of the cDNA sequence of the gene Nxd1, we amplified fragments by PCR using the following primers: 5′-TTGGTATTTTGGAGTTCATGGA-3′ (forward) and 5′-ACCAACAATCTTATGAAGGCG-3′ (reverse).
Phusion High Fidelity DNA polymerase (New England Biolabs) was used for PCR under the following conditions: 98°C for 30 sec., followed by 35 cycles of 10 sec at 98°C, 30 sec at 61°C and 30 sec at 72°C. The PCR products were cleaned using Exonuclease I and FastAP® thermosensitive alkaline phosphatase (Thermo Scientific, http://www.thermoscientificbio.com/).
Sequence determination was done in ABI Prism 377 DNA sequencer (Perkin Elmer; http://www.perkinelmer.com/). Vector NTI sequence analysis software was used for sequence analyses.
Global gene expression analysis
Total RNA was extracted from flowers and young leaves of M82 and nxd1-1 (three samples from each tissue) and cDNA was synthesized as described above. Hybridization to the Affymetrix GeneChip EU-TOM3 (Affymetrix Inc., http://www.affymetrix.com/) was done in three biological replicates at The Center for Genomic Technologies, The Hebrew University of Jerusalem, Israel. Isolated total RNA samples (250 ng) were processed according to the recommended protocol by Affymetrix: the Ambion® WT Expression Kit for Affymetrix® GeneChip®; Whole Transcript (WT) Expression Arrays; and the GeneChip® WT Terminal Labeling and Hybridization User Manual for use with the Ambion® WT Expression Kit. The hybridization volume was 200 μl. Hybridizations were conducted on an Affymetrix GeneChip® Hybridization Oven 640. Standard post-hybridization wash and double-stain protocols were applied on an Affymetrix GeneChip® Fluidics Station 450. Arrays were scanned on an Affymetrix Genechip® scanner 3000 7G with an Autoloader.
Data were statistically analyzed using Partek software (http://www.partek.com/), employing cutoffs of differential expression (1.6) and significance (P < 0.05).
Carotenoid analysis
Carotenoids were extracted and analyzed by HPLC for identification and quantification. To minimize carotenoid degradation and isomerization, pigment extraction and analysis were carried out in dim light and the carotenoid samples were kept under anoxygenic conditions at −70°C. Flower pigments were extracted from 0.2 to 0.4 g of petal tissue. The tissue was ground in 1 ml of acetone and filtered. The tissue debris was ground again and extracted in 1 ml of dichloromethane and the solvent was filtered and pooled with the acetone filtrate. The grinding and collection of solvents was repeated until the tissue lost all of its color. The colored organic phase was collected and dried under a stream of N2. The dry lipid extract was saponified by dissolving it in 450 μl of 100% ethanol and 50 μl of 60% KOH and incubated overnight at 4°C. An equal volume (500 μl) of diethyl ether and 30% volume (150 μl) of NaCl were added for phase separation. The colored organic fraction (upper phase) was collected, dried under a stream of N2 and the dry lipid extract was re-dissolved in 450 μl of acetone for carotenoid analysis. The purpose of the saponification process was to hydrolyze esterified carotenoids. Fruit pigments were extracted from 0.2 to 0.6 g fresh tomato pericarp tissue. The tissue was ground in 1 ml of acetone and filtered. The tissue debris was ground again in 1 ml of dichloromethane, filtered and pooled with the acetone filtrate. The grinding and collection of solvents was repeated until the tissue lost all of its color. Pigments were extracted by partitioning the solvent mixture against an equal volume of diethyl ether and a 0.2 volume of 12% w/v NaCl/H2O. The colored organic fraction (upper phase) was collected and dried under a stream of N2 and the dry lipid extract was re-dissolved in 500 μl acetone for further analysis. Leaf pigments were extracted from 0.3 to 1 g young leaves. Tissue was ground in 700 μl acetone and filtered. This process was repeated until the tissue lost its color. The green solvent was collected, dried under a stream of N2 and re-dissolved in 500 μl acetone and saponified as described above.
The concentrations of total carotenoids and chlorophyll were determined by spectroscopy (Lichtenthaler and Wellburn, 1983). Carotenoids were separated by HPLC using a Waters system consisting of a Waters 600 pump, Waters 996 photodiode array detector and Waters 717 plus Autosampler (Waters, http://www.waters.com/). The static phase consisted of a Spherisorb® ODS2 C18 reversed-phase column from Phenomenex (silica 5 μm × 3.2 mm × 250 mm) (Phenomenex®, http://www.phenomenex.com/) and the mobile phase consisted of the following solvent gradient at a constant flow of 1.6 ml min−1 according to Table 4.
| Time (min) | Acetonitrile:H2O (9:1) | Ethyl acetate (%) |
|---|---|---|
| 0–8 | 100% | 0 |
| 8–12 | 80% | 20 |
| 12–26 | 65% | 35 |
| 26–26.1 | 45% | 55 |
| 26.1–33 | 0 | 100 |
The column was equilibrated for at least 5 min between injections with 100% acetonitrile:H2O (9:1).The spectra between 200 and 700 nm were recorded at a rate of one full spectrum per second. Analysis of the data was done with the chromatography software Millennium (Waters). The carotenoids were identified according to their typical retention time (certified by standards) and characteristic absorption spectra. The amounts of individual carotenoids were calculated from the HPLC chromatogram according to a calibration curve performed for known amounts of all-trans-lycopene standard (kindly provided by LycoRed, Yavneh, Israel, http://www.lycored.com/), considering differences in values of extinction coefficient of different carotenoids. The amount of total carotenoid in each sample is simply calculated as the sum of the amounts of the different carotenoids in the sample.
The ABA analysis
Abscisic acid was determined immunologically using the Phytodetek_ABA test kit of Agdia Inc. (http://www.agdia.com). The concentration of ABA was measured according to the manufacturer's protocol in leaves from plants grown in the greenhouse, either fresh or 5 h after detachment.
Functional expression of genes in E. coli
The plasmid pAC-VIOL was first constructed. This 12 814-bp plasmid was made by inserting the Arabidopsis cDNA encoding zeaxanthin epoxidase (ZEP) into the plasmid pAC-ZEAXipi (Cunningham and Gantt, 2007). It includes also the carotenoid biosynthesis genes crtE, for geranylgeranyl diphosphate synthase, crtY for lycopene β-cyclase, crtI for phytoene desaturase, crtB for phytoene synthase, crtZ for β-carotene hydroxylase and ipi2 for isopentenyl isomerase from Pantoea agglomerans in the plasmid vector pACYC184 (Figure S7).
The cDNAs of Nxd1 from tomato and At_Nxd1 and At_Aba4 from Arabidopsis were expressed under the T7 promoter in the following plasmids: pGEM-Nxd1 (about 3900 bp), pGEM-At_Nxd1 (about 3900 bp), pGEM-At_Aba4 (about 3700 bp) and pGEM-At_Nxd1 + At_Aba4 (about 4500 bp) (Figure S8). pGEM-At_Nxd1 and the pGEM-At_Aba4 plasmids were created by amplification with PCR of cDNA from Col-0 Arabidopsis using the following primers: for At_Nxd1, 5′-TCCATGGACGTTGAAGAAAAG-3′ (forward) and 5′-TGTTGTGTTTACTTTGAAGGGT-3′ (reverse); and for At_Aba4, 5′-AGTGGTGAAGATTTGAATCAGA-3′ (forward) and 5′-ACAACTTAACCCATTACAGCTA-3′ (reverse). To construct the pGEM-Nxd1 plasmid, we amplified fragments by PCR from M82 cDNA using the following primers: 5′-TTGGTATTTTGGAGTTCATGGA-3′ (forward) and 5′-ACCAACAATCTTATGAAGGCG-3′ (reverse).
All fragments were inserted into plasmids using the pGEM®-T Easy Vector System (Promega, http://www.promega.com/).
To construct the pGEM-At_Nxd1 + At_ABA4 plasmid, we inserted into the plasmid pGEM-At_ABA4 a PCR-amplified fragment from Col-0 Arabidopsis cDNA using the following primers: 5′-TTCATACCGCGGTCCATGGACGTTGAAGAAAAG-3′ (forward) and 5′-CTTGCCCCATGGTGTTGTGTTTACTTTGAAGGGT-3′ (reverse). Both plasmid and insert DNA were cut with SacII and NcoI, and ligated using T4 ligase (New England Biolabs).
Escherichia coli cells of strain XL1 Blue were transfected by electroporation with the above plasmids separately or in combinations and selected on chloramphenicol or chloramphenicol + ampicillin Luria–Bertani medium plates. Streaks were made from four colonies and grown on LB plates at the appropriate antibiotic selection overnight at 37°C followed by two more days at room temperature (23 ± 1 °C). Bacterial cells were collected and carotenoids extracted in acetone as previously described (Isaacson et al., 2004).
Measurements of photosynthetic efficiency
The efficiency of PSII was estimated in leaves of dark-adapted plants by measuring chlorophyll fluorescence with a computer-controlled pulse amplitude modulated (PAM) fluoro-meter IMAG-MAX/L (Walz, http://www.walz.com/) as previously described (Barry et al., 2012). Non-photochemical quenching of chlorophyll fluorescence was calculated as NPQ = (Fm – Fm′)/Fm ‘, as described in (Eppel et al., 2013).
Field measurements
Plants grown in the field of the Akko Regional Experimental Station from April to August were harvested. The weight of fruit and vegetative above-ground organs was measured for each plant. Ten plants of each genotype were analyzed over three seasons.
Acknowledgements
We thank Dr Nir Keren and Etan Salomon for their help in photosynthesis measurements. This research was supported by grants 1685/09 from the Israel Science Foundation and EU–FP7 METAPRO 244348.




