Group B Streptococci serotype distribution in pregnant women in Ghana: assessment of potential coverage through future vaccines
Abstract
enObjective
Group B streptococcal (GBS) colonization of pregnant women can lead to subsequent infection of the new-born and potentially fatal invasive disease. Data on GBS colonization prevalence and serotype distribution from Africa are scarce, although GBS-related infections are estimated to contribute substantially to infant mortality. In recent years, GBS vaccine candidates provided promising results in phase I and II clinical trials. We aimed to assess the prevalence and serotype distribution of GBS in Ghana since this knowledge is a prerequisite for future evaluation of vaccine trials.
Methods
This double-centre study was conducted in one rural and one urban hospital in central Ghana, West Africa. Women in late pregnancy (≥35 weeks of gestation) attending the antenatal care clinic (ANC) provided recto-vaginal swabs for GBS testing. GBS isolates were analysed for serotype and antibiotic susceptibility. GBS-positive women were treated with intrapartum antibiotic prophylaxis (IAP) according to current guidelines of the Center for Disease Control and Prevention (CDC).
Results
In total, 519 women were recruited at both study sites, recto-vaginal swabs were taken from 509. The overall prevalence of GBS was 19.1% (18.1% in rural Pramso and 23.1% in urban Kumasi, restrospectively). Capsular polysaccharide serotype (CPS) Ia accounted for the most frequent serotype beyond all isolates (28.1%), followed by serotype V (27.1%) and III (21.9%). No resistance to Penicillin was found, resistances to second line antibiotics clindamycin and erythromycin were 3.1% and 1%, respectively.
Discussion
Group B Streptococcus serotype distribution in Ghana is similar to that worldwide, but variations in prevalence of certain serotypes between the urban and rural study site were high. Antibiotic resistance of GBS strains was surprisingly low in this study.
Abstract
frObjectif
Evaluer la distribution de la prévalence et du sérotype du Streptococcus du groupe B (SGB) au Ghana car la colonisation des femmes enceintes peut entraîner une infection subséquente du nouveau-né et potentiellement la maladie invasive mortelle. Cette connaissance est aussi un prérequis pour l’évaluation de futurs essais vaccinaux.
Méthodes
Etude bi-centrique réalisée dans un hôpital rural et un hôpital urbain dans le centre du Ghana, en Afrique de l'ouest. Les femmes en fin de grossesse (≥35 semaines de gestation) se présentant à la clinique des soins prénatals (ANC) ont fourni des prélèvements recto-vaginaux par écouvillon pour la recherche du SGB. Les isolats SGB ont été analysés pour le sérotype et la sensibilité aux antibiotiques. Les femmes positives pour le SGB ont été traitées avec une antibio-prophylaxie intra-partum (IAP), conformément aux directives actuelles du Center for Disease Control and Prevention (CDC).
Résultats
519 femmes ont été recrutées dans les deux sites de l’étude, les prélèvements recto-vaginaux ont été effectués chez 509 d'entre elles. La prévalence globale du SGB était de 19,1% (18,1% à Pramso, zone rurale et 23,1% à Kumasi, zone urbaine). Le sérotype polysaccharide capsulaire (CPS) I.a était plus fréquent (28,1%), suivi du sérotype V (27,1%) et III (21,9%). Aucune résistance à la pénicilline n'a été détectée; mais il y avait une résistance aux antibiotiques de deuxième ligne, la clindamycine (3,1%) et l’érythromycine (1%).
Discussion
La distribution des sérotypes de SGB au Ghana est similaire à celle dans le monde entier, mais les variations de la prévalence de certains sérotypes entre l’étude urbaine et l’étude rurale étaient élevées. La résistance aux antibiotiques des souches de SGB était étonnamment faible dans cette étude.
Abstract
esObjetivo
Evaluar la prevalencia y distribución de serotipos del Estreptococo del Grupo B (EGB) en Ghana, ya que la colonización de mujeres embarazadas puede conllevar a la infección del recién nacido y a una enfermedad invasiva potencialmente letal, y este conocimiento es un prerequisito para la evaluación futura de ensayos de vacunas.
Métodos
Estudio doble céntrico realizado en un hospital rural y un hospital urbano en Ghana central, África Occidental. Las mujeres atendidas en una maternidad durante las últimas semanas de embarazo (≥35 semanas de gestación) proveyeron dos frotis recto-vaginales para realizar la prueba EGB. Los aislados de EGB obtenidos fueron analizados para determinar el serotipo y la susceptibilidad a antibióticos. Las mujeres positivas para EGB recibieron profilaxis antibiótica intraparto (PAI) siguiendo las guías actuales del Centro para el Control y la Prevención de Enfermedades (CDC).
Resultados
Se reclutaron 519 mujeres en ambos centros de estudios, y se tomaron frotis recto-vaginales de 509 de ellas. La prevalencia general de EGB era del 19.1% (18.1% en la zona rural de Pramso y del 23.1% en la zona urbana de Kumasi). El serotipo Ia con polisacárido capsular (PSC) era el más frecuente (28.1%), seguido por el serotipo V (27.1%) y el III (21.9%). No se detectó resistencia a la penicilina; pero había resistencia a antibióticos de segunda línea como la clindamicina (3.1%) y la eritromicina (1%).
Discusión
La distribución de serotipos de EGB en Ghana era similar a la que existe globalmente, pero las variaciones en prevalencia de algunos serotipos entre zonas urbanas y rurales era alta. La resistencia a antibióticos de cepas de EGB era sorprendentemente baja en este estudio.
Introduction
The reduction of childhood mortality is one of the millennium development goals (MDG 4) of the World Health Organization (WHO). Although substantial improvements have been made, more than 40% of deaths in children under 5 years of age worldwide occur during the neonatal period 1. Streptococcus agalactiae, also referred to as Lancefield Group B Streptococcus (GBS), has been identified as a major contributor to neonatal morbidity and mortality since the 1970s 2-5. Recent investigations from the African continent confirmed GBS as an important pathogen causing neonatal sepsis 6, 7.
Newborns usually acquire the encapsulated gram-positive cocci vertically from their mothers before or during delivery. Worldwide, between 10–37% of healthy pregnant women are colonised recto-vaginally by GBS 8-10. Of these, 50% transfer the bacteria to their neonates, of whom approximately 1% develops invasive disease 11.
Early onset disease (EOD) occurs with sepsis within the first 6 days of life and tends to progress rapidly, particularly since some neonates are already septicaemic at birth 12. Late onset disease (LOD) affects newborns between the 7th and 90th day of life with a peak 1 month after birth and commonly presents with bacteraemia and meningitis.
An extensive meta-analysis by Edmond et al. 13 included 74 studies from countries all over the world and revealed a mean incidence of GBS-related invasive disease in infants 0–98 days of age of 0.53 per 1000 live births (95% confidence interval (CI): 0.44–0.62) and a mean case fatality rate of 9.6% (95% CI: 7.5–11.8). Dagnew et al. 1 found even higher incidences of up to 3.06 per 1000 live births in resource-poor settings in South Africa. Notably, variations between countries and continents were high overall. Yet, data from Africa are scarce because only 5% of the available studies were conducted on the continent 13.
Group B Streptococcus contains an antigenically diverse polysaccharide antigen (capsular polysaccharides (CPS)), by which it can be further divided into ten unique subtypes (Ia, Ib, II – X). As one of the major virulence factors, CPS enables bacterial evasion from phagocytic clearance 14. Globally, the five serotypes Ia, Ib, II, III and V are most frequently associated with invasive disease and account for 85% of cases 13. Serotype III alone caused 37% of EOD and 53% of LOD worldwide 13. However, predominating serotypes vary geographically, as for instance serotypes Ia and III cause the majority of infections in the United States, while serotypes IV and VIII were the most common strains in pregnant women in Japan 15, 16.
The first risk- and screening-based prophylactic approaches against invasive GBS-related neonatal diseases were implemented in the early 1990s 17, 18 and have led to a significant decline of incidences of invasive disease 19-21. Screening of pregnant women between 35 and 37 weeks gestation for GBS carriage and subsequent administration of intravenous intrapartum antibiotic prophylaxis (IAP) to positive women turned out to be the most effective intervention to protect newborns against GBS-EOD 22-24. However, IAP appears to be less effective against LOD. Moreover, despite IAP being routinely performed, EOD may still occur as women may be colonised after being screened 20, 23, 25. Above all, developing countries that have the highest incidences of neonatal invasive disease are widely lacking the infrastructure to implement routine screening programmes.
In the last two decades, extensive efforts have been undertaken to develop GBS vaccines for women of childbearing age 26, 27. Effective immunisation against the most prevalent serotypes could protect neonates from GBS infection and subsequent GBS-related disease. To date, several polysaccharide and conjugated vaccine candidates have been assessed or are still assessed in clinical trials 28-30.
The aim of this study was to provide data on the prevalence and serotype distribution of GBS in women in late pregnancy living in rural and urban areas of Ghana, West Africa, to serve as a basis for future vaccine trials in the region.
Methods
Study site
The study was conducted between May and September 2013 in Kumasi and Pramso in the Ashanti region of central Ghana. With an estimated population of 2 million people, Kumasi is the second biggest city in Ghana. The rural village of Pramso is located 22 km south-east of Kumasi.
Participants were recruited in late pregnancy within their regular visit scheme to the antenatal care clinic (ANC) operated by the Campus Hospital of the Kwame Nkrumah University of Science and Technology (KNUST) in Kumasi (urban site) and St. Michael's Hospital Pramso (rural site).
Study population
All women aging ≥18 years, who had been pregnant for at least 35 weeks and who were willing to participate and able to understand the procedures, purpose and potential risks of participation in the study were eligible for inclusion. Pregnant women who were already in labour were excluded. Consent had to be granted by signature or thumbprint.
In case a woman was illiterate, a trained field worker explained the purpose of the study and translated from English to the local language Twi, if necessary. The process was witnessed by a local nurse who was not involved in the study.
After consenting, the women were interviewed by a member of the study team and a case report form (CRF) with pre-defined questions concerning the current pregnancy, socio-economic background and living situation was completed.
A trained member of the study team collected a vaginal swab from the lower part of the vagina and a rectal swab according to CDC guidelines 13. The swabs were collected using sterile cotton carriers and placed in Amies medium (BBL Venturi Liquid Amies Medium Transport swabs, Becton Dickinson, USA).
Samples were transported to the laboratory of the Kumasi Center for Collaborative Research in Tropical Medicine, which is located on the campus of the KNUST in Kumasi. There, they were stored in a locked refrigerator at 2–8 °C.
Laboratory procedures
Following the CDC guidelines, selective enrichment of pooled sample pairs (rectal and vaginal swab of each individual) was performed on the day of collection using 5-ml Lim broth (Becton Dickinson, USA), a selective enrichment media based on Todd-Hewitt broth supplement containing colistin (10 μg/ml), nalidixic acid (15 μg/ml) and 1% yeast extract 24. The inoculated Lim broth tubes were incubated at 35–37 °C for 18–24 h in normal atmosphere.
After incubation, 10 μl of the enriched Lim broth was spread on Columbia blood agar plates (Oxoid, UK) prepared according to the manufacturer's instructions. The inoculated plates were incubated for 18–24 h at 35–37 °C under conditions of elevated carbon dioxide levels using commercially available candle jars (Anaerojar, Oxoid, UK). Next, culture plates were inspected for growth of β-haemolytic colonies suspicious for GBS. If no β-haemolytic growth was observed after the first incubation period, plates were re-incubated for another 18–24 h.
Catalase testing was performed for colonies suspicious for GBS using 6% hydrogen peroxide. If the test was negative, latex agglutination test (Streptococcal grouping kit, OXOID, UK) was used to determine the respective Lancefield group of the isolate according to manufacturer's instructions. Validation of the quality of latex reagents was performed on a daily basis.
Antibiotic susceptibility testing was carried out for all isolated GBS strains using the Kirby–Bauer disc diffusion method in accordance with the current guidelines of the Clinical and Laboratory Standards Institute (CLSI) 31. Mueller–Hinton blood agar was prepared according to manufacturer's instructions (OXOID, UK). GBS colonies were picked from blood agar plates and suspended in 0.9% sterile saline to a turbidity of 0.5 McFarland. Afterwards, the suspension was brought on 4-mm Mueller–Hinton agar plates using sterile cotton swabs. Antibiotic discs (OXOID, UK) were dispended, and agar plates were incubated for 18–24 h in normal atmosphere, followed by measurement of inhibition zone diameters.
For serotyping, samples were transported in liquid nitrogen to Bernhard Nocht Institute of Tropical Medicine (BNITM) in Hamburg, Germany, at the end of the recruitment period using cryovials (Microbank, PRO LAB Diagnostics, Canada). Before, during and after the transport GBS colonies in the cryovials were stored at −80 °C. Serotyping of GBS isolates was performed for strains Ia, Ib, II, III and V using the IMMULEX STREP-B-LATEX agglutination test (Statens Serum Institute, Denmark) according to the manufacturer's instructions 32.
Treatment of GBS-positive women
Positive results were forwarded to the treating clinician at the study site, who added the information to the patient's folder. If the pregnant woman delivered her baby at the respective hospital, she was identified as study participant and was treated with 5 million units penicillin G IV initially after onset of labour followed by another 2.5 million units every 4 h until delivery 24. For women with known allergy against penicillin second-line antibiotics were chosen according to the CDC guidelines and local availability 24.
Data management and statistical analyses
All individual data were pseudonymised using subject code labels. Only, the local principal investigator was able to trace back personal information of the individuals in order to report information of GBS-positive women to the respective hospital. Data were entered daily (Excel 2007, Microsoft, USA) by trained fieldworkers and cross-checked for inconsistencies by another study team member. Statistical analyses were carried out using STATA SE 12 (StataCorp, USA). Demographic, clinical and microbiological parameters were compared between study sites applying Mann–Whitney U-test and chi-square test for nonparametric and categorical variables, as appropriate.
Ethical considerations
Ethical approval of the study protocol and all study related documents was obtained from the Committee on Human Research, Publications and Ethics (CHRPE), School of Medical Sciences, KNUST, Kumasi, Ghana.
Results
Study population
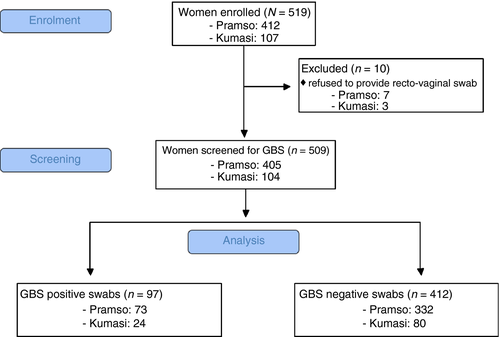
A total of 519 women were enrolled in the study, 107 at the KNUST hospital Kumasi and 412 at St. Michael's hospital Pramso. Ten women withdrew consent to provide a vaginal and rectal swab and were excluded from subsequent analyses (Figure 1). The mean age of participants was 28 (range 18–43) years. Socio-demographic data are reported in Table 1.
| Characteristics | Kumasi | Pramso | Total | P |
|---|---|---|---|---|
| n = 104 | n = 405 | n = 509 | ||
| Median age in years (range) | 28 (18–41) | 28 (18–43) | 28 (18–43) | 0.824a |
| Highest level of education | ||||
| None or primary education (%) | 12 (11.5) | 75 (18.5) | 87 (17.1) | <0.001b |
| Junior high school (%) | 31 (29.8) | 231 (57.0) | 262 (51.5) | |
| Senior high school (%) | 28 (26.9) | 63 (15.6) | 91 (17.9) | |
| Tertiary education (%) | 33 (31.7) | 36 (8.9) | 69 (13.5) | |
| Literate (%) | 96 (92.3) | 344 (84.9) | 440 (86.4) | 0.054b |
| English speaking (%) | 84 (80.1) | 248 (61.2) | 332 (65.2) | <0.001b |
| Marital status (%) | ||||
| Never married (%) | 28 (26.9) | 183 (45.2) | 211 (41.5) | 0.003b |
| Married (%) | 76 (73.1) | 221 (54.6) | 297 (58.4) | |
| Divorced (%) | 0 (0.0) | 1 (0.2) | 1 (0.2) | |
| n = 103 | n = 399 | n = 502 | ||
|---|---|---|---|---|
| Mean household size (range) | 3.4 (1–10) | 4.5 (1–16) | 4.2 (1–16) | <0.001a |
- n-values may vary because of missing values.
- a P-value obtained with Mann–Whitney U-test.
- b P-value obtained with chi-square test.
Pregnancy-related information
The majority (Table 2) of women had been pregnant before. As shown in Table 2, only 14.8% stated to be in their first pregnancy. The highest percentage of women (36.5% in Kumasi and 42.0% in Pramso) reported to be in their 3rd or 4th pregnancy.
| Characteristics | Kumasi | Pramso | Total | P |
|---|---|---|---|---|
| n = 104 | n = 405 | n = 509 | ||
| Gravidity | ||||
| Primigravida (%) | 25 (24.0) | 50 (12.4) | 75 (14.8) | 0.019a |
| Secundigravida (%) | 23 (22.1) | 88 (21.7) | 111 (21.8) | |
| Gravida 3–4 (%) | 38 (36.5) | 170 (42.0) | 208 (40.9) | |
| Gravida 5+ (%) | 18 (17.3) | 97 (24.0) | 115 (22.6) | |
| Complicationsb | ||||
| All (%) | 24 (23.1) | 73 (18.1) | 97 (19.1) | 0.247 |
| Vaginal discharge (%) | 15 (14.4) | 46 (11.4) | 61 (12.0) | 0.655 |
| Vaginal bleeding (%) | 8 (7.7) | 25 (6.2) | 33 (6.5) | 0.396 |
| Other | 3 (2.9) | 8 (2.0) | 11 (2.2) | – |
| Pre-existing morbidityc (%) | 6 (5.8) | 29 (7.2) | 35 (6.9) | 0.828a |
| Mean gestational week (range) | 37 (35–40) | 36.7 (35–41) | 36.7 (35–41) | 0.063d |
- a P-value obtained with chi-square test.
- b complications (one or multiple) during the current pregnancy, prompting the woman to seek medical help.
- c women who mentioned to have any known diseases or allergies.
- d P-value obtained with Mann–Whitney U-test.
Women were also asked if they experienced pregnancy-related illness or complications during the current pregnancy. In Kumasi, 23.1% of the participants stated to have sought medical help for complications, 18.1% in Pramso. The most frequent complication was vaginal discharge (14.4% in Kumasi vs. 11.4% in Pramso) followed by vaginal bleeding (7.7% in Kumasi vs. 6.2% in Pramso), respectively. Overall 6.9% of women mentioned any pre-existing medical conditions at both study sites.
Group B Streptococcus prevalence and serotype distribution
Of 509 women who provided a recto-vaginal swab, 97 (19.1%) tested positive for GBS by cultivation and latex agglutination testing: 24 (23.1%) in Kumasi and 73 (18.0%) in Pramso. Pure cultures could be recovered from 96 of 97 GBS-positive samples after transport to BNITM in Germany.
| Serotype | Kumasi n = 24 | Pramso n = 72 | Total N = 96 | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Ia | 2 | 8.3 | 25 | 34.7 | 27 | 28.1 |
| Ib | 5 | 20.8 | 3 | 4.2 | 8 | 8.3 |
| II | 3 | 12.5 | 6 | 8.3 | 9 | 9.4 |
| III | 7 | 29.2 | 14 | 19.4 | 21 | 21.9 |
| V | 5 | 20.8 | 21 | 29.2 | 26 | 27.1 |
| n.t. | 2 | 8.4 | 3 | 4.2 | 5 | 5.2 |
- a P-value = 0.027 obtained with chi-square test.
Overall, CPS-serotype Ia accounted for the most frequent (28.1%) serotype, followed by serotype V (27.1%) and III (21.9%). In urban Kumasi, serotype III was the most frequent one (29.2%), followed by Ib (20.8%) and V (20.8%). In rural Pramso, serotype Ia (34.7%) accounted for the most frequent isolate, followed by V (29.2%) and III (19.4%). Differences of distribution of serotypes across both study sites were significant (chi-square: P = 0.027) (Table 3).
Antibiotic susceptibility
Testing for antibiotic susceptibility was performed in Ghana for all 97 GBS isolates. All samples were sensitive to penicillin, ampicillin and ampicillin/sulbactam. One sample each was intermediately resistant to erythromycin and fully resistant to erythromycin. One sample was intermediately resistant, and 3 were fully resistant to clindamycin. Twelve samples were resistant to chloramphenicol (Table 4).
| Antibiotic agent, potency | Sensitive | Intermediately resistent | Resistent | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Penicillin, 10 units | 97 | 100 | 0 | 0.0 | 0 | 0.0 |
| Ampicillin, 10 μg | 97 | 100 | 0 | 0.0 | 0 | 0.0 |
| Ampicillin, 10 μg/sulbactam, 10 μg | 97 | 100 | 0 | 0.0 | 0 | 0.0 |
| Erythromycin, 15 μg | 95 | 97.9 | 1 | 1.0 | 1 | 1.0 |
| Clindamycin, 2 μg | 93 | 95.9 | 1 | 1.0 | 3 | 3.1 |
| Chloramphenicol, 30 μg | 85 | 87.6 | 0 | 0.0 | 12 | 12.4 |
- a Antibiotic resistance was tested using Kirby–Bauer disc diffusion. Minimal inhibitory concentrations (MIC) were determined in accordance with current Clinical and Laboratory Standards Institute (CLSI) guidelines.
Discussion
This study is the first to provide data on the prevalence of recto-vaginal GBS colonisation and serotype distribution in pregnant women in Ghana. Overall, every fifth woman carried GBS.
Group B Streptococcus carriage was more frequent in Kumasi (urban) than Pramso (rural). CPS serotypes Ia, V and III were the predominant serotypes, while serotype distribution differed between the study sites.
It has been shown earlier that Group B Streptococcus prevalence in high- and middle-income countries is similar to that in developing countries 10. Studies from the African continent revealed maternal colonisation rates of around 20% 33-35, while prevalences in developed countries are estimated between 20 and 37% 8, 9, 36, 37.
We described GBS prevalence and serotype distribution at two study sites. According to the 2010 census of the 24.6 million inhabitants of Ghana, 51% lived in urban areas 38. To assess whether our study sites are representative for urban and rural regions in Ghana, we compared living standards of our population with data from Ghana Living Standards Survey Round 6 (GLSS 6) from 2014. The survey showed that people from urban areas lived in smaller households, were more frequently literate, had more frequent access to water toilets and more likely lived in concrete houses than their rural counterparts 39. Taking into account these five aspects, characteristics of our urban and rural population match those found in the survey. Thus, we are confident that we indeed describe a representative population for rural and urban areas in Ghana.
Overall, the most common serotypes isolated in this study equal those from other parts of the world, for example Europe and the United States 4, 13. Only, two other studies provide GBS serotype distribution of colonisation isolates from Africa: in both Tanzania and Zimbabwe, serotype III was the most frequent serotype, followed by serotypes V and Ia, respectively 37, 40. Serotype III is believed to be responsible for the majority of LOD 11, 13. In our study, it was found in more than one-fifth of all isolates. However, variations in serotype distribution between the study sites were high, especially for serotype Ia, which was the most frequent in Pramso, whereas its prevalence was only 8.3% in Kumasi. A similar difference occurred for serotype Ib, which was much more common in Kumasi but rarely isolated in Pramso. Indeed, spatial variations of serotype prevalences have been described earlier, but our results might be also influenced by the inequality of sample sizes of the study sites 10.
Intrapartum antibiotic prophylaxis with penicillin is recommended by CDC for women colonised by GBS in late pregnancy. Second-line treatment comprises ampicillin, clindamycin, erythromycin and vancomycin. In particular for women who report an allergy to penicillin, clindamycin and erythromycin are the antibiotics of choice 32. In recent years, increasing rates of resistances against these antibiotics have been reported from various areas in the world 41, 42. A study from Switzerland revealed a clindamycin and erythromycin resistance of 28% and 30%, respectively 43. Researchers in China even reported 55.7% resistance to clindamycin and 66.2% to erythromycin 44. Data from Africa are scarce. In a study from South Africa, all isolates from pregnant women were sensitive to penicillin and 5.5% were resistant to macrolides 45. In another study from Tanzania, rectal and vaginal GBS isolates were tested. Here, 9.4%, 18.7% and 15.7% of isolates were resistant to penicillin, erythromycin and clindamycin, respectively. In contrast to these reports, in our study, only 4.1% of the isolates were resistant against 2nd line antibiotics like clindamycin and erythromycin.
For a long time, the development of GBS vaccines focused on composition using the antigenic characteristics of the serotype-specific CPS 46. Trials with polysaccharide and tetanus toxoid-conjugated vaccines both revealed acceptable safety and good immunogenicity profiles 47-49. In contrast to polysaccharide vaccines, conjugated vaccines proved not only to have the potential for induction of B-cell memory, but also to be capable of reducing the levels of vaginal and rectal colonisation with GBS 49. Through maternal transfer of the CPS-specific antibodies, a mother could possibly protect newborns of multiple pregnancies against invasive disease. The mother herself could also benefit from personal protection against clinical GBS disease. In developing countries, vaccination of women of childbearing age could lead to a broad protection of pregnant women independently from their ability to visit the ANC regularly and their place of delivery. Further, the enormous effort of implementation of GBS screening schemes and provision of IAP at the site of delivery could be circumvented, which would save costs.
This study has several limitations. First, women in this study may be not representative for the overall female population of childbearing age in Ghana. They belong to a group of individuals that has access to the health system and is open to medical advice. It is questionable whether our results are representative of women for whom this is not the case: although 96% of pregnant women in Ghana visit an ANC for at least one time, only 67% of births are attended by skilled health personnel 50.
Another limitation of our results is the unequal amount of collected samples at both study sites, because approximately 80% of samples were collected in rural Pramso. Thus comparisons and conclusions may be exaggerated. Also, we collected samples at only two sites in the Ashanti region in Ghana within a study period of 5 months. It is therefore not possible to exclude any differences in prevalence or serotype distribution in other parts of the country or variations in GBS colonisation over the year. However, the latter has never been described in literature.
In conclusion, our data provide important information for potentially upcoming vaccine trials in sub-Saharan Africa. A potential GBS vaccine – which is not yet available – containing antigenic profiles of the five serotypes Ia, Ib, II, III and V would, indeed, cover approx. 95% of the serotypes carried by women in our study because only 5.2% of serotypes were not typeable or attributed to other GBS serotypes.
Acknowledgements
The authors would like to thank all women who participated in this study. We also would like to thank the entire staff of the ANCC at St. Michael's Hospital Pramso, in particular Sister Doris and her team and Eric Fomevor, as well as the team around Sister Dora at the KNUST Hospital, Kumasi, for their enduring and valuable work.




