Experimental comparison of pathogenic potential of two sibling species Anisakis simplex s.s. and Anisakis pegreffii in Wistar rat
Abstract
enObjectives
There are little data available on the pathology caused by the sibling species Anisakis simplex s.s. and Anisakis pegreffii. The differences shown in their ability to penetrate the muscle of fish may also be manifested in humans. The purpose of this study is to confirm possible differences in pathogenicity between A. simplex s.s. and A. pegreffii using an experimental model which simulates infection in humans.
Methods
Female Wistar rats were infected with 190 Anisakis type I L3 larvae from the Iberian coastline. After the animal was sacrificed, these L3 larvae were then recovered and identified via PCR-RFLP of the ITS1-5.8S-ITS2. A logistic regression analysis was performed searching for association between experimental pathogenic potential and species.
Results
The distribution of A. simplex s.s. and A. pegreffii between Atlantic and Mediterranean waters of the Iberian Peninsula showed statistically significant differences (P < 0.001) which were not observed in the hybrid genotypes (P > 0.3). 21.6% showed pathogenic potential, interpreted as the capacity of the larvae to cause lesions, stick to the gastrointestinal wall or penetrate it. The species variable showed association with the pathogenic role of the larva (P = 0.008). Taking A. simplex s.s. as our reference, the OR for A. pegreffii is 0.351 (P = 0.028).
Conclusions
Despite this difference, A. pegreffii is also capable of causing anisakiasis, being responsible for 14.3% of the penetrations of the gastric mucosa found in rats, which justifies both species being considered aetiologic agents of this parasitic disorder.
Abstract
frObjectifs
Il y a peu de données disponibles sur la pathologie causée par les espèces sœurs A. simplex s.s. et A. pegreffii. Les différences indiquées dans leur capacité à pénétrer dans le muscle de poisson peuvent aussi se manifester chez l'homme. Le but de cette étude est de confirmer d’éventuelles différences de pathogénicité entre A. simplex s.s. et A. pegreffii à l'aide d'un modèle expérimental qui simule l'infection chez l'homme.
Méthodes
Des rats Wistar femelles ont été infectés avec 190 larves d’Anisakis de type I L3 de la côte ibérique. Après sacrifice de l'animal, ces larves L3 ont été récupérées et identifiées par PCR-RFLP pour l’ITS1-5.8S-ITS2. Une analyse de régression logistique a été effectuée pour la recherche d'association entre le potentiel pathogène expérimental et l'espèce.
Résultats
La distribution de A. simplex s.s. et A. pegreffii entre les eaux atlantiques et méditerranéennes de la péninsule ibérique a montré des différences statistiquement significatives (P < 0,001) qui n’étaient pas été observées dans les génotypes hybrides (P > 0,3). 21,6% ont montré un potentiel pathogène, interprétée comme la capacité des larves à provoquer des lésions, d'adhérer à la paroi gastro-intestinale ou la pénétrer. La variable d'espèces a montré une association avec le rôle pathogène de la larve (P = 0,008). En prenant A. simplex s.s. comme référence, le rapport de côtes (OR) pour A. pegreffii était de 0,351 (P = 0,028).
Conclusions
En dépit de cette différence, A. pegreffii est aussi capable de causer de l'anisakiase, étant responsable de 14,3% des pénétrations dans la muqueuse gastrique chez les rats, ce qui justifie que les deux espèces soient considérées comme des agents étiologiques de cette maladie parasitaire.
Abstract
esObjetivos
Existen pocos datos disponibles sobre la patología causada por las especies crípticas A. simplex s.s. y A. pegreffii. Las diferencias observadas en su habilidad para penetrar el músculo de los peces también podría manifestarse en los humanos. El propósito de este estudio es confirmar posibles diferencias entre la patogenicidad de A. simplex s.s. y de A. pegreffii, utilizando un modelo experimental que simula la infección en humanos.
Métodos
Se infectaron hembras de ratas Wistar con 190 larvas L3 de Anisakis del tipo I de la costa Ibérica. Después se sacrificaron los animales y las larvas L3 se recuperaron e identificaron por PCR-RFLP de la región ITS1-5.8S-ITS2. Se realizó un análisis de regresión logística para determinar la asociación entre el potencial patogénico experimental y las especies.
Resultados
La distribución de A. simplex s.s. y A. pegreffii en las aguas del Atlántico y el Mediterráneo de la Península Ibérica mostraron diferencias estadísticamente significativas (P < 0.001) que no se observaron en los genotipos híbridos (P > 0.3). Un 21.6% tenía potencial patogénico, interpretado como la capacidad de las larvas de causar lesiones, pegarse a la pared gastrointestinal o penetrarla. La variable mostró una asociación con el papel patogénico de la larva (P = 0.008). Tomando a A. simplex s.s. como referencia, el OR para A. pegreffii es de 0.351 (P = 0.028).
Conclusiones
A pesar de esta diferencia A. pegreffii también es capaz de causar anisakiasis, siendo responsable del 14.3% de las penetraciones de la mucosa intestinal observada en ratas, lo cual justifica considerar a ambas especies como agentes etiológicos de este desorden parasitario.
Introduction
There is no doubt that one of the main risk factors for anisakiasis is the consumption of fish, especially the way in which it is consumed, that is, raw or undercooked; another factor is the susceptibility of man as an accidental host of this parasite. The third larval stage (L3) of species of the Anisakis genus occurs as parasites in a large number of fish and cephalopods which form part of the human diet (Pereira-Bueno 1992; Koie 1993; Valero et al. 2006). The accidental ingestion of the parasite causes gastrointestinal symptoms ranging from minor to severe and/or allergic reactions whose symptoms vary from urticaria to anaphylactic shock (Daschner et al. 2000; Del Rey Moreno et al. 2006; Solas et al. 2009). The scarcity or even absence of distinguishing morphological features in the third larval stage complicates or prevents species differentiation. This means that we must resort to molecular techniques, mainly to PCR-RFLP of the ribosomal ITS1-5.8S-ITS-2 fragment (D'Amelio et al. 2000; Martín-Sánchez et al. 2005; Abattouy et al. 2011). Almost all of the thousands of cases of parasitism in humans reported in 27 countries are caused by the morphotype I of Anisakis (Repiso Ortega et al. 2003; Umehara et al. 2007). Although few larvae from human cases have been identified by molecular methods, the most important study carried out in Japan by Umehara et al. (2007) and more recently Arizono et al. (2012) identified Anisakis simplex s.s. as the principal aetiologic agent of anisakiasis in this country, whilst in Italy, Anisakis pegreffii is attributed this same status with a significantly lower number of cases (D'Amelio et al. 1999; Mattiucci & Nascetti 2008; Fumarola et al. 2009). On this subject, some authors have found that A. pegreffii larvae have less penetrative power, not only in the muscle of certain fish but also in solid agar, and even worse survival in artificial gastric juice than A. simplex s.s., which may indicate a lower pathogenic potential in the case of A. pegreffii (Suzuki et al. 2010; Quiazon et al. 2011; Arizono et al. 2012).
The purpose of this study is to corroborate the existence of differences in pathogenicity between A. simplex s.s. and A. pegreffii through the infection of laboratory animals, specifically using the Wistar rat.
Materials and methods
Parasites, fish and geographical area
The parasites were obtained from blue whiting (Micromesistius poutassou) caught at different points along the Atlantic and Mediterranean coastlines of the Iberian Peninsula (Figure 1). We selected 190 Anisakis type I L3 larvae measuring at least 20 mm, all of which were highly mobile. The criteria used for their morphological identification were those indicated by Berland (1961) and Koie (1993).
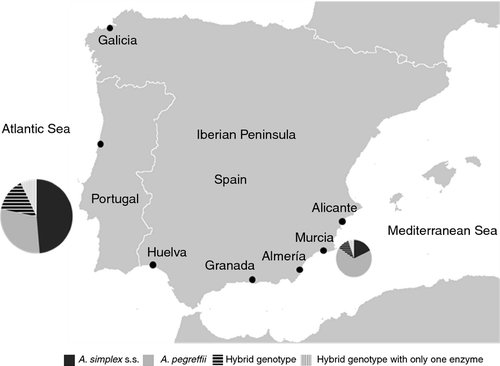
Among the larvae extracted from blue whiting for use in the experimental infection, 79.5% (151/190) came from fish caught off the Atlantic Ocean coastlines surrounding the Iberian Peninsula, of which 70.2% (106/151) were from Galicia, 15.2% (23/151) from Portugal and 14.6% (22/151) from Huelva. The remaining 20.5% (39/190) were from the Spanish Mediterranean coastline: 23.1% (9/39) from Alicante, 7.7% (3/39) from Murcia, 17.9% (7/39) from Almería and 51.3% (20/39) from Motril (Granada).
Experimental infection
The study was conducted on 51 female Wistar rats weighing between 100 and 120 g. Three to four larvae (depending on the number of larvae available) were introduced into the lumen of the stomach of each rat using a gastric probe. After necropsy (Zúñiga et al. 2011) performed 4 h post-infection, data were gathered on the larvae in the rats. To detect the presence of lesions, a thorough visual examination was performed under stereoscopic microscope of the content and wall of the digestive tract, organs (liver, pancreas, kidneys and ovaries) and adjacent body cavity fat.
The larvae recovered from the rodent were then washed in 0.9% NaCl solution and placed individually into Eppendorf tubes at −20°C to await genetic analysis.
Identification of the larvae
PCR-RFLP was used to identify the species, extracting genomic DNA by using the RealPure kit (Ref RBMEG01). PCR amplification of rDNA fragment ITS1-5.8S-ITS2 used primers NC5 (forward), 5′ GTA GGT GAA CCT GCG GAA GGA TCA TT 3′ and NC2 (reverse), 5′ TTA GTT TCT TTT CCT CCG CT 3′ reported by Zhu et al. (1998), followed by digestion with 10 U/μl restriction enzymes HinfI (Bioron international), TaqI (Bioron international). The digestion product was subjected to 3% agarose gel electrophoresis for species identification according to the band pattern (D'Amelio et al. 2000; Martín-Sánchez et al. 2005; Abattouy et al. 2011).
Statistical study
A logistic regression analysis was performed using the categorical variable pathogenic potential of L3 larvae (no, yes) – defined as its capacity to cause lesions, attach itself onto the gastric or intestinal wall, or penetrate through them to reach the abdominal cavity – as the dependent variable (N = 190). The independent variables consisted of the species or genotype (A. simplex s.s., A. pegreffii and hybrid genotypes) and the geographical origin of the larva, classified as Mediterranean/Atlantic or as more specific geographical locations: Galicia, Portugal, Huelva, Alicante, Murcia, Almeria and Granada. The statistical study was carried out using SPSS 15.0.; P-values of ≤0.05 were considered significant.
Ethical clearance
All experiments were carried out in accordance with European Parliament and of the Council of 22 September 2010 (2010/63/UE and RD 1201/2005). The ethical clearance for conducting the experiments was obtained from the Sociedad Española para las Ciencias del Animal de Laboratorio (Zúñiga et al. 2011).
Results
Experimental infection
A thorough examination of the sacrificed animals made it possible to locate 100% of the larvae used in the experimental infection, all of which were alive (Table 1). 16.3% of the L3 larvae (31/190) had caused lesions in the digestive tract. These lesions were mainly (87.1%, 27/31) located in the greater curvature of the wall of the body of stomach and pyloric antrum, whilst the rest (12.9% 4/31) were found in the small intestine. The lesions varied in size from <1 to 24 mm2. When the larva attaches onto the wall of the gastrointestinal tract, the lesion consists of a small haemorrhagic ulcer accompanied by oedema of various dimensions, and on occasions, the parasite leaves tunnels with oedema. Some of the larvae were seen just as they passed through the stomach or intestine wall of the rodent and moved towards the abdominal cavity, all with a hood surrounding the lips. The larvae that passed through the stomach wall left authentic holes in it. In one of the rats, three L3s were found attached to the abdominal muscle. In the abdominal cavity of the animal were found 12.1% of the larvae (23/190); 34.8% of the larvae (8/23) found in the abdominal cavity did not leave any lesions which were perceivable with the method used.
| Species or genotypes | Pathogenic larvae (%) | Non-pathogenic larvae (%) | ||||
|---|---|---|---|---|---|---|
| Cavity | Stomach | Intestine | Stomach | Intestine | Total | |
| Anisakis simplex s.s. | 11 (5.8) | 5 (2.6) | 3 (1.6) | 21 (11) | 40 (21) | 80 (42.1) |
| Anisakis pegreffii | 5 (2.6) | 2 (1.1) | – | 35 (18.4) | 29 (15.3) | 71 (37.4) |
| Hybrid genotype with the two restriction enzymes | 4 (2.1) | 5 (2.6) | 2 (1) | 9 (4.8) | 6 (3.2) | 26 (13.7) |
| Hybrid genotype with only one enzyme | 3 (1.6) | 1 (0.5) | – | 4 (2.1) | 5 (2.6) | 13 (6.8) |
| Total | 23 (12.1) | 13 (6.9) | 5 (2.6) | 69 (36.3) | 80 (42.1) | 190 |
Identification of species
42.1% of the L3 larvae (80/190) belonged to A. simplex s.s.; 37.4% (71/190) were identified as A. pegreffii. The remaining 13.7% (26/190) showed a hybrid PCR-RFLP band pattern with the two restriction enzymes, HinfI and TaqI, which is the sum of the patterns generated for the species mentioned above, and the remaining 6.8% (13/190) showed this hybrid genotype with only one enzyme, whilst with the other, the pattern generated was indistinguishable from A. pegreffii. The distribution of these species between Atlantic and Mediterranean waters of the Iberian Peninsula showed statistically significant differences (P = 0.001); namely, in the proportions of A. simplex s.s. and of A. pegreffii (P < 0.001), but not in those of the hybrid genotypes (P > 0.3). In this study, we observed that is six times more likely (CI95% 2.4–15.0) to find A. pegreffii L3 larvae in blue whiting from the Mediterranean than in those from the Atlantic.
Association between experimental pathogenic potential and genotype
Within the framework of the method used, the pathogenic potential of the larva was defined as its capacity to cause lesions, attach itself onto the gastric or intestinal wall, or penetrate through them to reach the abdominal cavity. 21.6% (41/190) of the larvae used displayed this pathogenic role (Table 1).
A logistic regression analysis of the data was conducted in order to detect the potential association between experimental pathogenic potential and characteristics of the larva such as the Anisakis species and the geographical origin. The variable of geographical origin (Mediterranean/Atlantic) was not associated with experimental pathogenic potential (P = 0.785), nor was it when the geographical origins of the larvae were specified more precisely (P = 0.761). Only the species variable showed association with the pathogenic role of the larva (P = 0.008). Taking A. simplex s.s. as our reference, the OR for A. pegreffii is 0.351 (P = 0.028), whilst there are no statistically significant differences with the hybrid genotypes.
Discussion and conclusions
Anisakis simplex s.s. and A. pegreffii are parapatric species and the coasts of the Iberian Peninsula are areas in which the two species are found in sympatric conditions, sharing both permanent and intermediate/paratenic hosts (Mattiucci & Nascetti 2008). Differentiation between the two species using ribosomal DNA markers is based exclusively on the existence of two fixed differences in the ITS-1 sequence, which means different restriction patterns are produced with HinfI and TaqI enzymes (Ceballos-Mendiola et al. 2010). The restriction enzyme Cfo was not used in this study because it generates the same pattern for the two sibling species. The detection of the mix of genotypes of both species (hybrid genotypes) has been the cause of some controversy in terms of its interpretation: whilst some authors believe these mixed genotypes reflect hybridisation, others adduce ‘incomplete homogenisation in a multiple-copy repeated DNA region’ (Martín-Sánchez et al. 2005; Hermida et al. 2012). For the purposes of this study, we chose to consider them to be different genotypes, which is in line with either of the above interpretations.
In Japan, most cases of human anisakiasis for which molecular species identification has been carried out are caused by infection by A. simplex s.s., whilst in Italy, the species responsible is A. pegreffii (D'Amelio et al. 1999; Mattiucci et al. 2007, 2011; Umehara et al. 2007; Fumarola et al. 2009; Arizono et al. 2012). In Spain, despite an increase in the number of cases published in recent years, the species involved has not been identified (Repiso Ortega et al. 2003). In reality, little is known about the pathology caused by each of these sibling species in their different hosts. Experimental studies on fish conducted by Suzuki et al. (2010) and Quiazon et al. (2011) highlighted differences between the two sibling species indicating a greater tendency for A. simplex s.s. to migrate towards the muscle than A. pegreffii. These differences in ability to penetrate the muscle of fish may also be manifested in their capacity to penetrate into the tissue of accidental hosts such as man, thus influencing their pathogenic potential. Recently, Arizono et al. 2012 have demonstrated that A. simplex s.s. larvae survived the acidic artificial gastric juice for approximately 1 day more than A. pegreffii larvae. With this in mind, this study attempts to shed some light on the many questions still surrounding these parasites regarding their pathology in humans, using the Wistar rat as an experimental model. The rat has a larger digestive system than the mouse and fewer space and care requirements than other larger animals; moreover, the size of the parasite requires the use of a cannula 2 mm wide, which makes the rat an ideal animal for this type of study (Zúñiga et al. 2011; Romero et al. 2012). Furthermore, administering the Anisakis larvae orally is an effective and simple technique which allows us to simulate human infection, as the Anisakis L3 larvae display distribution and behaviour patterns in the rat digestive system which are similar to those seen in human cases (Navarro-Moll et al. 2011; Zuloaga et al. 2013). Given that the number of larvae found in fish muscle can be high (Pereira-Bueno 1992; Jurado-Palomo et al. 2010), we considered it appropriate to use several larvae per laboratory animal.
The majority of human anisakiasis cases occur in the stomach and ileum due to the barrier effect against larvae created, respectively, by both the pylorus and the Bauhin valve, causing greater numbers of larvae to concentrate in these areas (Ishikura et al. 1992). In our experimental model, 87.1% (27/31) of the larvae which penetrate or attach onto the gastrointestinal wall do so in the stomach, the preferred area being the wall of the body of stomach or sometimes the pyloric antrum (Paulsen 2011). This preference may be due, on one hand, to a progressive peristaltic movement in the fundic region which would push the larvae towards the region of the body of stomach, and on another, to there being less secretion in the stomach body area, which would facilitate their remaining and becoming attached. In the remaining 12.9%, the area affected is the intestine. Both species of Anisakis have been incriminated as aetiologic agents of gastric and intestinal anisakiasis in humans (D'Amelio et al. 1999; Suzuki et al. 2010; Mattiucci et al. 2011). Among the different cases documented in Spain, gastric complaints represent 40–70% of parasitisation (Castán et al. 2002; Repiso Ortega et al. 2003). In these cases, superficial erosions and ulcers similar to those observed in this study were reported. In our case, we were unable to perform a histological examination of biopsies; thus, we cannot confirm the existence of a non-specific inflammatory infiltrate with or without an increase in eosinophils and foreign body granulomas as occurs in humans. We found some larvae in the abdominal cavity with an absence of lesions in the digestive tract (three A. simplex s.s., three A. pegreffii and two hybrid genotypes); self-limiting clinical signs which disappear within hours of treatment of symptoms have also been reported in humans (Repiso Ortega et al. 2003).
The results obtained show that pathogenic potential is associated with the species or genotype to which the larva belongs. Thus, we found that the risk of A. pegreffii penetrating is 65% lower than that of A. simplex s.s. (CI 95%: 11–86%). Conversely, the hybrid genotypes with the two restriction enzymes show double the penetration risk of A. simplex s.s. (CI 95% 1–6) with a P-value close to statistical significance (P = 0.072). The larvae identified as hybrid genotypes with only one enzyme did not display statistically significant differences in pathogenic potential when compared to A. simplex s.s. (P = 0.588). In summary, we have demonstrated through our experimental model in rats that A. simplex s.s. is a more pathogenic species than A. pegreffii, a factor which, coupled with its greater penetration capacity into fish muscle and acid tolerance (Suzuki et al. 2010; Quiazon et al. 2011; Arizono et al. 2012) could justify its purported status of aetiologic agent in the majority of human cases, even in areas where A. pegreffii is the predominant species (Umehara et al. 2007; Arizono et al. 2012). In spite of this difference, A. pegreffii is also capable of causing anisakiasis (responsible for 14.8% of the penetrations of the gastric mucosa in rats), thus justifying it being considered the aetiologic agent of anisakiasis in Italy, given that in Italian waters A. simplex s.s. is not found, and A. pegreffii is widely represented in all species of fish (Mattiucci et al. 2007). Obviously, there are several other aspects which could affect the pathogenetic role of these zoonotic species to humans such as human susceptibility (Audicana & Kennedy 2008).
Acknowledgements
We are grateful to the Junta de Andalucía (Regional Autonomous Government of Andalusia) for the project grant P07-CVI-03249, and Ictioparasitology group cvi-03249.




