Antibody, cell-mediated response and infection susceptibility in allogeneic hematopoietic stem cell recipients after COVID-19 mRNA vaccination
Abstract
Background
Patients undergoing allogeneic stem-cell transplantation (allo-SCT) have reduced responses to vaccines due to immunosuppressive status linked to GvHD prophylaxis and treatment. In our study, we compared humoral responses to anti-SARS-CoV-2 mRNA vaccine, and infection onset, according to patients and transplant features; we also evaluated cellular response in patients without seroconversion.
Methods
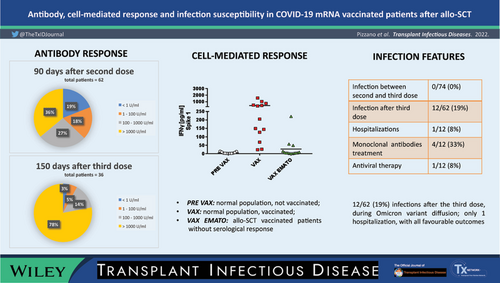
We tested antibodies titer after second and third vaccine doses. Antibodies were detected through an immune-enzymatic assay. In a patients’ subgroup without seroconversion, we tested cell-mediated responses evaluating interferon-gamma release by T-lymphocytes exposed to virus spike protein.
Results
Seroconversion rate increased from 66% at 30 days to 81% at 90 days after the second dose; it was 97% at 150 days after the third dose. We found a significant association between seroconversion after the second dose and two variables: shorter interval between allo-SCT and vaccination; ongoing immunosuppression. Twelve of 19 patients (63%) without antibodies after the second dose did not show cellular responses. Nineteen percent of patients developed SARS-CoV-2 infection after the third dose, with favorable outcome in all cases. Patients within 12 months after allo-SCT showed a significantly higher infection risk.
Conclusions
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.