Liver transplantation from active COVID-19 donors: Is it ethically justifiable?
Alessandra Agnese Grossi
Center for Clinical Ethics, Department of Biotechnologies and Life Sciences, University of Insubria, Varese, Italy
Department of Human Sciences, Innovation and Territory, University of Insubria, Varese, Italy
Search for more papers by this authorFederico Nicoli
Center for Clinical Ethics, Department of Biotechnologies and Life Sciences, University of Insubria, Varese, Italy
Clinical Ethics Service, Domus Salutis Clinic, Teresa Camplani Foundation, Brescia, Italy
Search for more papers by this authorMassimo Cardillo
Italian National Transplantation Center (CNT), Italian National Institute of Health, Rome, Italy
Search for more papers by this authorSalvatore Gruttadauria
Department for the Treatment and Study of Abdominal Diseases and Abdominal Transplantation, IRCCS-ISMETT, UPMC (University of Pittsburgh Medical Center), Palermo, Italy
Department of Surgery and Medical and Surgical Specialties, University of Catania, Catania, Italy
Search for more papers by this authorGiuseppe Tisone
Department of Surgical Sciences, University of Rome - Tor Vergata, Rome, Italy
Search for more papers by this authorGiuseppe Maria Ettorre
Department of General and HBP Surgery, Liver Transplantation Service, San Camillo Forlanini Hospital, Rome, Italy
Search for more papers by this authorLuciano De Carlis
General Surgery and Abdominal Transplantation Unit, University of Milano-Bicocca and Niguarda-Cà Granda Hospital, Milan, Italy
Search for more papers by this authorRenato Romagnoli
General Surgery 2U and Liver Transplantation Center, AOU Città della Salute e della Scienza di Torino, University of Turin, Turin, Italy
Search for more papers by this authorCarlo Petrini
Bioethics Unit, Italian National Institute of Health (ISS), Rome, Italy
Search for more papers by this authorCorresponding Author
Paolo Antonio Grossi
Italian National Transplantation Center (CNT), Italian National Institute of Health, Rome, Italy
Department of Medicine and Surgery, Infectious and Tropical Diseases Unit, University of Insubria, ASST Sette Laghi, Varese, Italy
Correspondence
Paolo Antonio Grossi, Department of Medicine and Surgery, Infectious and Tropical Diseases Unit, University of Insubria, ASST Sette Laghi, Varese, Italy.
Email: [email protected]
Search for more papers by this authorMario Picozzi
Center for Clinical Ethics, Department of Biotechnologies and Life Sciences, University of Insubria, Varese, Italy
Search for more papers by this authorAlessandra Agnese Grossi
Center for Clinical Ethics, Department of Biotechnologies and Life Sciences, University of Insubria, Varese, Italy
Department of Human Sciences, Innovation and Territory, University of Insubria, Varese, Italy
Search for more papers by this authorFederico Nicoli
Center for Clinical Ethics, Department of Biotechnologies and Life Sciences, University of Insubria, Varese, Italy
Clinical Ethics Service, Domus Salutis Clinic, Teresa Camplani Foundation, Brescia, Italy
Search for more papers by this authorMassimo Cardillo
Italian National Transplantation Center (CNT), Italian National Institute of Health, Rome, Italy
Search for more papers by this authorSalvatore Gruttadauria
Department for the Treatment and Study of Abdominal Diseases and Abdominal Transplantation, IRCCS-ISMETT, UPMC (University of Pittsburgh Medical Center), Palermo, Italy
Department of Surgery and Medical and Surgical Specialties, University of Catania, Catania, Italy
Search for more papers by this authorGiuseppe Tisone
Department of Surgical Sciences, University of Rome - Tor Vergata, Rome, Italy
Search for more papers by this authorGiuseppe Maria Ettorre
Department of General and HBP Surgery, Liver Transplantation Service, San Camillo Forlanini Hospital, Rome, Italy
Search for more papers by this authorLuciano De Carlis
General Surgery and Abdominal Transplantation Unit, University of Milano-Bicocca and Niguarda-Cà Granda Hospital, Milan, Italy
Search for more papers by this authorRenato Romagnoli
General Surgery 2U and Liver Transplantation Center, AOU Città della Salute e della Scienza di Torino, University of Turin, Turin, Italy
Search for more papers by this authorCarlo Petrini
Bioethics Unit, Italian National Institute of Health (ISS), Rome, Italy
Search for more papers by this authorCorresponding Author
Paolo Antonio Grossi
Italian National Transplantation Center (CNT), Italian National Institute of Health, Rome, Italy
Department of Medicine and Surgery, Infectious and Tropical Diseases Unit, University of Insubria, ASST Sette Laghi, Varese, Italy
Correspondence
Paolo Antonio Grossi, Department of Medicine and Surgery, Infectious and Tropical Diseases Unit, University of Insubria, ASST Sette Laghi, Varese, Italy.
Email: [email protected]
Search for more papers by this authorMario Picozzi
Center for Clinical Ethics, Department of Biotechnologies and Life Sciences, University of Insubria, Varese, Italy
Search for more papers by this authorAbstract
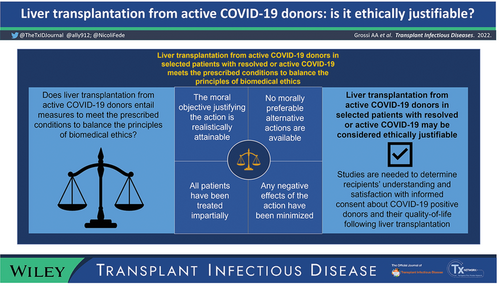
The debate on the opportunity to use organs from donors testing positive for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in recipients with naïve resolved or active COVID-19 is ongoing. We aim to present the ethical analyses underlying the decision to perform liver transplantation (LT) in selected patients with resolved or active COVID-19 in Italy. We used Jonsen, Siegler, and Winslade's Four-Boxes casuistic method, addressing the four topics considered as constitutive of the essential structure of single clinical cases for their ethical analysis (medical indications, patient preferences, quality of life, and contextual features) to enable decision-making on a case-by-case basis. Based on these topics, we elucidate the meaning and balance among the principles of biomedical ethics. Clinical ethics judgment based on the relation between the risk of acquiring SARS-CoV-2 along with its potentially negative effects and the expected benefits of transplant lead to consider LT as clinically appropriate. Shared decision-making allows the integration of clinical options with the patient's subjective preferences and considerations, enabling a valid informed consent specifically tailored to the patients’ individual circumstances. The inclusion of carefully selected SARS-CoV-2 positive donors represents an opportunity to offer lifesaving LT to patients who might otherwise have limited opportunities to receive one. COVID-19 positive donor livers are fairly allocated among equals, and respect for fundamental rights of the individual and the broader community in a context of healthcare rationing is guaranteed.The ethical analysis of the decision to perform LT in selected patients shows that the decision is ethically justifiable.
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose as described by Transplant Infectious Disease.
Supporting Information
| Filename | Description |
|---|---|
| tid13846-sup-0001-appendix.docx17 KB | Supplementary Appendix |
| tid13846-sup-0002-GraphicalAbstract.tif1.6 MB | Supplementary Appendix |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1Loupy A, Aubert O, Reese PP, Bastien O, Bayer F, Jacquelinet C. Organ procurement and transplantation during the COVID-19 pandemic. Lancet. 2020; 395(10237): e95-e96. https://doi.org/10.1016/S0140-6736(20)31040-0
- 2 Centro Nazionale Trapianti. Report 2020 - Italian National Transplantation Network. 2021. http://www.trapianti.salute.gov.it/imgs/C_17_cntPubblicazioni_415_allegato.pdf
- 3Angelico R, Trapani S, Manzia TM, Lombardini L, Tisone G, Cardillo M. The COVID-19 outbreak in Italy: initial implications for organ transplantation programs. Am J Transplant. 2020; 20(7): 1780-1784. https://doi.org/10.1111/ajt.15904
- 4Aubert O, Yoo D, Zielinski D, et al. COVID-19 pandemic and worldwide organ transplantation: a population-based study. Lancet Public Heal. 2021; 6(10): e709-e719. https://doi.org/10.1016/S2468-2667(21)00200-0/ATTACHMENT/FD1D3229-1966-47DA-A46C-430EC232534A/MMC1.PDF
10.1016/S2468?2667(21)00200?0/ATTACHMENT/FD1D3229?1966?47DA?A46C?430EC232534A/MMC1.PDF PubMed Web of Science® Google Scholar
- 5Shah MB, Lynch RJ, El-Haddad H, Doby B, Brockmeier D, Goldberg DS. Utilization of deceased donors during a pandemic: argument against using SARS-CoV-2-positive donors. Am J Transplant. 2020; 20(7): 1795-1799. https://doi.org/10.1111/AJT.15969
- 6Koval CE, Poggio ED, Lin Y-C, Kerr H, Eltemamy M, Wee A. Early success transplanting kidneys from donors with new SARS-CoV-2 RNA positivity: a report of 10 cases. Am J Transplant. 2021; 21: 3743-3749. https://doi.org/10.1111/AJT.16765
- 7Romagnoli R, Gruttadauria S, Tisone G, et al. Liver transplantation from active COVID-19 donors: a lifesaving opportunity worth grasping? Am J Transplant. 2021; 21(12): 3919-3925. https://doi.org/10.1111/AJT.16823
- 8 Centro Nazionale Trapianti. Idoneità alla donazione di donatori SARS-CoV-2 positivi. 2020. [date last accessed January 10, 2022]. http://www.trapianti.salute.gov.it/imgs/C_17_cntAvvisi_281_0_file.pdf
- 9 Centro Nazionale Trapianti. Ulteriori specifiche sull'utilizzo di organi da donatore deceduto SARS-CoV-2 positivo, aggiornamento nota del 21/08/2020 (Prot. 1413/CNT 2020). 2020. [date last accessed January 10, 2022]. http://www.trapianti.salute.gov.it/imgs/C_17_cntAvvisi_299_0_file.pdf
- 10Kates OS, Fisher CE, Rakita RM, Reyes JD, Limaye AP. Use of SARS-CoV-2-infected deceased organ donors: should we always “just say no?” Am J Transplant. 2020; 20(7): 1787-1794. https://doi.org/10.1111/ajt.16000
- 11 Italian National Transplantation Center. Covid: in 15 mesi realizzati 71 trapianti grazie a donatori positivi. 2022. [date last accessed April 10, 2022]. https://www.trapianti.salute.gov.it/trapianti/dettaglioComunicatiNotizieCnt.jsp?lingua=italiano&area=cnt&menu=media&sottomenu=news&id=748
- 12Jonsen AR, Siegler M, Winslade WJ. Clinical Ethics: A Practical Approach to Ethical Decisions in Clinical Medicine. McGraw Hill; 2015.
- 13Beauchamp TL, Childress JF. Principles of Biomedical Ethics. 7th ed. Oxford University Press; 2012.
- 14Cho HJ, Koo JW, Roh SK, et al. COVID-19 transmission and blood transfusion: a case report. J Infect Public Health. 2020; 13(11): 1678-1679. https://doi.org/10.1016/j.jiph.2020.05.001
- 15Hong HL, Kim SH, Choi DL, Kwon HH. A case of coronavirus disease 2019–infected liver transplant donor. Am J Transplant. 2020; 20(10): 2938-2941. https://doi.org/10.1111/ajt.15997
- 16Leclerc M, Fourati S, Menouche D, Challine D, Maury S. Allogeneic haematopoietic stem cell transplantation from SARS-CoV-2 positive donors. Lancet Haematol. 2021; 8(3): e167-e169. https://doi.org/10.1016/S2352-3026(21)00025-9
- 17Kumar D, Humar A, Keshavjee S, Cypel M. A call to routinely test lower respiratory tract samples for SARS-CoV-2 in lung donors. Am J Transplant. 2021; 21(7): 2623-2624. https://doi.org/10.1111/ajt.16576
- 18 Organ Procurement and Transplantation Network. Summary of current evidence and information– donor SARS-CoV-2 testing & organ recovery from donors with a history of COVID-19. 2021. Accessed January 16, 2022. https://optn.transplant.hrsa.gov/media/kkhnlwah/sars-cov-2-summary-of-evidence.pdf
- 19Kaul DR, Valesano AL, Petrie JG, et al. Donor to recipient transmission of SARS-CoV-2 by lung transplantation despite negative donor upper respiratory tract testing. Am J Transplant. 2021; 21(8): 2885-2889. https://doi.org/10.1111/ajt.16532
- 20Lumley SF, O'Donnell D, Stoesser NE, et al. Antibodies to SARS-CoV-2 are associated with protection against reinfection. medRxiv. 2020. https://doi.org/10.1101/2020.11.18.20234369
10.1101/2020.11.18.20234369 Google Scholar
- 21Durand CM, Segev D, Sugarman J. Realizing HOPE: the ethics of organ transplantation from HIV infected donors. Ann Intern Med. 2016; 165(2): 138. https://doi.org/10.7326/M16-0560
- 22Nangia G, Borges K, Reddy KR. Use of HCV-infected organs in solid organ transplantation: an ethical challenge but plausible option. J Viral Hepat. 2019; 26(12): 1362-1371. https://doi.org/10.1111/JVH.13130
- 23Picozzi M, Pegoraro R. Taking care of the vulnerable: the criterion of proportionality. Am J Bioeth. 2017; 17(8): 44-45. https://doi.org/10.1080/15265161.2017.1340997
- 24Grossi AA, Nicoli F, De Feo TM, et al. The 3-T model of informed consent for non-standard risk donors: a proposal for transplant clinical practice. Transplant Direct. 2021; 7(11):e782. https://doi.org/10.1097/TXD.0000000000001238
- 25Whitney SN, McGuire AL, McCullough LB. A typology of shared decision making, informed consent, and simple consent. Ann Intern Med. 2004; 140(1): 54-59. https://doi.org/10.7326/0003-4819-140-1-200401060-00012
- 26Åberg F. Quality of life after liver transplantation. Best Pract Res Clin Gastroenterol. 2020; 46-47:101684. https://doi.org/10.1016/J.BPG.2020.101684
- 27Fleetwood VA, Lusciks J, Poirier J, Hertl M, Chan EY. Utilization of public health service increased risk donors yields equivalent outcomes in liver transplantation. J Transplant. 2016; 2016: 1-7. https://doi.org/10.1155/2016/9658904
10.1155/2016/9658904 Google Scholar
- 28Stock PG, Wall A, Gardner J, et al. Ethical issues in the COVID era: doing the right thing depends on location, resources, and disease burden. Transplantation. 2020; 104(7): 1316-1320. https://doi.org/10.1097/TP.0000000000003291
- 29Jaffe A, Schilsky ML, Deshpande R, Batra R. Liver transplantation in the time of COVID19: barriers and ethical considerations for management and next steps. Hepatol Commun. 2020; 4(9): 1242-1256. https://doi.org/10.1002/HEP4.1568
- 30Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020; 382(21): 2049-2055. https://doi.org/10.1056/nejmsb2005114