Clinicopathological correlates, oncological impact, and validation of Oncotype DX™ in a European Tertiary Referral Centre
Abstract
Oncotype DX™ (ODX) score estimates prognosis and predicts breast cancer recurrence. It also individualizes patient adjuvant chemotherapy prescription in breast cancer. This assay relies on genetic and molecular markers; the clinicopathological phenotype of which are tested routinely. The aim of this study was determine whether clinicopathological and immunohistochemical information predicts ODX recurrence score (RS). Secondly, to assess the impact on adjuvant chemotherapy (AC) and oncological outcome of ODX testing in patients in a European tertiary referral center. Estrogen receptor positive (ER+), human epidermal growth factor receptor-2 negative (HER2-), lymph node negative (LN-), and female breast cancer patients with ODX testing performed between 2007 and 2015 were categorized into low- (<11), intermediate- (11–25), and high-risk (>25) groups. Clinicopathological and immunohistochemical correlates of RS were determined. Predictors of RS were assessed using binary logistic regression. Oncological outcome was assessed using Kaplan-Meier and Cox regression analyses. ODX was performed in 400 consecutive ER+LN- patients. Median follow-up was 74.1 months (3.0–144.4). Low grade (odds ratio [OR]:2.39; 95% confidence interval [CI]:1.04–5.51, p = 0.041) independently predicted low ODX, while high grade (OR:2.04; 95% CI: 1.19–3.49, p = 0.009) and reduced progesterone receptor (PgR) expression (OR: 2.57, 95% CI: 1.42–4.65, p = 0.002) independently predicted high ODX. Omission of AC in intermediate- (p = 0.159) and high-risk (p = 0.702) groups did not negatively impact survival. In conclusion, tumor grade independently predicts low and high RS, while PgR negativity predicts high RS. ODX reduced AC prescription without compromising oncological outcome.
1 INTRODUCTION
Breast cancer is the most prevalent cancer among women and a leading cause of cancer-related death.1 The management of early estrogen receptor positive (ER+) breast cancer has evolved with our improved understanding of the molecular mechanisms driving the disease. A tailored approach is now utilized with refined surgical techniques and bespoke hormonal, radiotherapy (XRT), and chemotherapeutic strategies optimized for each individual patient.2, 3 The goal of this strategy is to deliver efficacious, cost-effective treatment that enhances survival while minimizing treatment-related toxicities. Management of early ER+, human epidermal growth factor receptor-2 negative (HER2-) primarily involves locoregional management and systemic endocrine treatment, with chemotherapy limited to certain high-risk cohorts.4-6
Fisher established that early ER+disease is more effectively treated by breast-conserving surgery (BCS) in combination with radiation therapy, adjuvant chemotherapy (AC), and/or endocrine hormonal therapy (EHT), than by radical mastectomy.7-10 This mantra included adjuvant chemotherapy (AC) for a majority of ER+patients, despite marginal benefit for most recipients.4-6 The development and clinical validation of genomic assays such as Oncotype DX™ (ODX) (Genomic Health Inc., Redwood City, California) have since attempted to substratify those most likely to derive benefit from systemic chemotherapy, in an effort to spare those patients known to derive excellent outcomes with locoregional (surgery +/- XRT) and EHT management from the deleterious effect of AC.4-6 Consequently, these assays have been incorporated into modern management of patients with early-stage, ER+/HER2 disease.11, 12 In particular results of the Trial Assigning IndividuaLized Options for Treatment (Rx) or TAILORx have led to judicious use of AC in the low- and intermediate-risk recurrence score (RS) groups.13
Despite these developments, disadvantages of ODX include the cost and a local turnover time taking up to 6 weeks (Genomic Health Inc® headquarters is in California).14 As a component of the RS can be derived from accessible clinicopathological factors such as hormonal receptor status and tumor grade, it may be feasible to substratify patients into low- or high-risk groups without routine application of RS. This would have a significant cost-saving impact on current management of this cohort of early-stage, ER+patients. The primary aim of this study was to determine clinicopathological predictors of RS. Secondly, to assess the impact on chemotherapy prescription and oncological outcome of RS testing in a European tertiary referral center.
2 METHODS
2.1 Study design and patient selection
Local ethical approval was granted. A single-center, retrospective cohort study was undertaken involving consecutive breast cancer patients diagnosed and treated in a tertiary referral center between 2007 and 2015. Included patients were identified from a prospectively maintained database and had a diagnosis of ER+, HER2-, lymph node negative (LN-) breast cancer that underwent resection and ODX. Patients had either T1-T3 disease. Patients over the age of 75 years were excluded. Clinicopathological data, RS, adjuvant treatment, and clinical outcomes were collected from medical records. ODX genomic testing was carried out at the Genomic Health laboratory.
2.2 Methodology of staging
All patients underwent triple assessment: Clinical examination was conducted by a consultant breast surgeon, and radiological assessment was performed via mammography and/or ultrasound. Imaging was then analyzed by a consultant breast radiologist. Diagnosis was confirmed via histological analysis of tumor specimens using a standardized reporting template. All patients underwent surgical resection of their cancer. Following resection, specimens were analyzed by a consultant breast pathologist in an accredited pathology laboratory. Staging was performed in accordance with the American Joint Committee on Cancer (AJCC), version 8 Guidelines.15
2.3 Histopathological assessment and immunohistochemistry
ER and progesterone receptor (PgR) status was analyzed using the Allred scoring system.15 HER2 status was assessed using immunohistochemistry, and those scoring 2+ were submitted for fluorescence in situ hybridization (FISH). Tumor specimens were graded using the Nottingham grading system.16 Tumor lymphatic invasion was evaluated using D2-40 staining 17 and vascular invasion using CD34.18 Tumor perineural invasion was evaluated using S-100 staining.19 Ki-67 indices were determined using MIB1 antibody testing.20
2.4 Multidisciplinary approach to care
Each case was staged and discussed prior to definitive treatment at the breast multidisciplinary meeting at the tertiary referral center. Decisions regarding treatment relied upon clinicopathological, radiological, patient performance status, family history, genetic testing results as well as the patient's own wishes with regard to treatment. Adjuvant chemotherapy prescription was guided by ODX.
2.5 Follow-up
Patient follow-up was recorded through a prospectively maintained database. The median and mean follow-up were calculated using the reverse Kaplan-Meier method.21 Disease recurrence and overall survival data were collected from medical records. Cause of death was confirmed from data obtained from National Registries. Invasive disease-free survival was defined as “freedom from invasive disease recurrence, a second primary cancer or death.”
2.6 Statistical analysis
Clinicopathological correlates of ODX were determined using descriptive statistics. Univariable logistic regression analysis assessed the association between clinicopathological variables and RS expressed in crude odds ratios (OR) with 95% confidence intervals (CIs). Variables with p < 0.200 in univariable analysis were included in the multivariable logistic regression analysis. Binary logistic regression analysis using the forward conditional selection method was used to identify variables that contributed independently to ODX. Kaplan-Meier, Log-rank (Mantel-Cox), and Cox regression analyses were used to associate survival with clinicopathologic characteristics expressed as hazard ratios (HR) with 95% CIs. All tests of significance were 2-tailed, with p < 0.050 indicating statistical significance. Data were analyzed using Statistical Package for Social Sciences™ (SPSS™) version 25.
3 RESULTS
3.1 Patient demographics and application of ODX
There were 400 consecutive patients included in the study. The mean age at diagnosis was 56.2 ± 9.4 years (range 27–75). One hundred and eight patients (27.0%) were premenopausal, 22 (5.5%) were peri-menopausal, and the majority were postmenopausal at the time of diagnosis (n = 270, 67.5%). Clinicopathological data and frequency of findings per RS group are outlined in Supplementary Appendices S1 and S2. The median follow-up was 74.1 months (range 3.0–144.4).21
All 400 patients underwent ODX testing: 46 patients were in the low-risk group (RS0-10) (11.5%), 294 in the intermediate-risk (RS11-25) (73.5%) and 60 patients in the high-risk (RS>25) (15.0%). Seventy-nine patients underwent ODX testing through the TAILORx trial (19.8%).
3.2 Oncological impact on adjuvant treatment
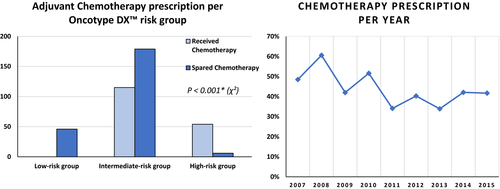
All patients received EHT. In the low-risk group (LRG), no patients received AC. In the IRG, 115 patients (39.1%) received AC as did 54 patients (90.0%) in high-risk group (HRG) (Figure 1.A). Omission of AC in patients in the HRG was based upon patient's personal preference (5/6, 83.3%) and poor performance status (1/6 patients, 16.7%). Figure 1.B demonstrates the reduction in AC prescription in patients who underwent ODX testing during this study.
3.3 Associations and predictors of RS from clinicopathological characteristics
Increased tumor grade (p < 0.001), PgR score of 8 (p = 0.040), and reduced PgR expression (p = 0.002) and were significantly associated with RS. Clinicopathological characteristics not associated with RS are outlined in Supplementary Appendix S2.
Using univariable analysis, age greater than 65 at diagnosis (OR: 0.223, 95% CI: 0.053–0.943, p = 0.041) and grade 1 tumors (OR: 2.632, 95% CI: 1.200–5.773, p = 0.016) were significantly associated with RS <11. Grade 1 disease was the sole independent predictor using multivariable analysis (OR: 2.391, 95% CI: 1.038–5.511, p = 0.041) (Table 1).
| Parameter | OR | 95% CI | p-value | OR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Univariable | Multivariable | |||||
| Age >65 | 0.223 | 0.053 – 0.943 | 0.041* | |||
| Postmenopausal | 0.640 | 0.345 – 1.184 | 0.155 | |||
| T1 | 1.183 | 0.647 – 2.163 | 0.585 | |||
| LVI | 0.906 | 0.442 – 1.858 | 0.788 | |||
| Grade 1 | 2.632 | 1.200 – 5.773 | 0.016* | 2.391 | 1.038 – 5.511 | 0.041* |
| PgR- | 0.389 | 0.116 – 1.297 | 0.124 | |||
| Ki67% <6 | 1.227 | 0.639 – 2.551 | 0.488 | |||
- Abbreviations: CI, Confidence interval; LVI, Lymphovascular invasion; OR, Odds ratio; PgR, Progesterone receptor; T1, Histopathological tumor stage 1.
- * Denotes statistical significance.
Grade 3 tumors (OR: 2.180, 95% CI: 1.307–3.633, p = 0.003) and PgR negativity (PgR-) (OR: 2.494, 95% CI: 1.402–4.435, p = 0.002) were significantly associated with RS >25. Multivariable analysis demonstrated that grade 3 tumors (OR: 2.024, 95% CI: 1.138–3.464, p = 0.010) and PgR- (OR: 2.552, 95% CI: 1.408–4.623, p = 0.002) independently predicted RS>25 (Table 2).
| Parameter | OR | 95% CI | p-value | OR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Univariable | Multivariable | |||||
| Age >65 | 1.302 | 0.749 – 2.263 | 0.350 | |||
| Postmenopausal | 1.397 | 0.905 – 2.156 | 0.131 | |||
| T3 | 1.357 | 0.903 – 2.040 | 0.141 | |||
| LVI | 1.038 | 0.649 – 1.660 | 0.876 | |||
| Grade 3 | 2.190 | 1.314 – 3.651 | 0.003* | 2.037 | 1.190 – 3.485 | 0.009* |
| PgR- | 2.505 | 1.409 – 4.455 | 0.002* | 2.568 | 1.418 – 4.651 | 0.002* |
| Ki67% >14 | 1.207 | 0.603 – 2.416 | 0.595 | |||
- Abbreviations: CI, Confidence interval; LVI, Lymphovascular invasion; OR, Odds ratio; PgR, Progesterone receptor; T3, histopathological tumor stage 3.
- * Denotes statistical significance.
3.4 Oncological outcomes
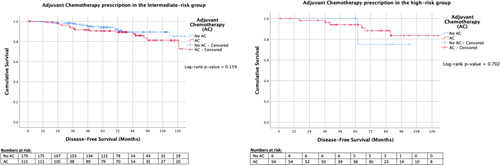
Patients in our series demonstrated a mean invasive disease-free survival of 87.0% (348 of 400 patients) at 74.0 months (median, range 3.0–143.9), and an overall survival of 96.5% (386 of 400 patients) at 74.1 months (median, range 3.0–144.4). There was no difference in DFS (HR: 0.964, 95% CI: 0.531–1.751, p = 0.904) between the ODX categories using Cox regression and Kaplan-Meier analyses (p = 0.991) (Figure 2). Furthermore, there was no difference in OS (HR: 0.582, 95% CI: 0.205–1.653, p = 0.310) between the ODX categories using Cox regression and Kaplan-Meier analyses (p = 0.819) (Figure 2). Survival outcomes were similar for intermediate- and high-risk ODX groups irrespective of AC prescription (IRG: p = 0.159, HRG: p = 0.702, respectively) (Figure 3).

3.5 Clinicopathological factors associated with DFS and OS
There was no independent predictor of improved DFS (Supplementary Appendix S3A). Age at diagnosis >65 years (HR: 2.316, 95% CI: 0.843–6.360, p = 0.103), tumor stage 3 (HR: 2.213, 95% CI: 0.767–6.382, p = 0.142) and adjuvant XRT (HR: 0.370, 95% CI: 0.141–0.973, p = 0.044) were included in the multivariable model for OS based on univariable analysis. However, only age >65 (HR: 2.800, 95% CI: 1.001–7.842, p = 0.049) and receipt of adjuvant XRT (HR: 0.339, 95% CI: 0.163–0.912, p = 0.032) was independently associated with OS (Supplementary Appendix S3B).
3.6 Patterns of disease recurrence and death
Within the LRG, there were no patients with distant recurrence or breast cancer-related deaths (0.0%, 0/46). In the IRG, 5.1% of patients had distant recurrence or breast cancer-related deaths (15/294), with 3.7% having breast cancer-related deaths (11/294) at a median follow-up of 74.1 months. The HRG had 5.0% distant recurrences or deaths (3/60), with just 3.3% breast cancer progression-related deaths (2/60). Locoregional recurrence was also low within all of the cohorts with just 2.2%, 1.3%, and 1.7% ipsilateral breast cancers/local recurrence and 6.6%, 4.1%, and 3.3% of contralateral breast cancers across the groups. The patterns of disease recurrence and death per ODX category are detailed in Supplementary Appendix S4.
4 DISCUSSION
In recent years, the treatment paradigm of ER+, HER2-, and LN- breast cancer has shifted toward a more judicious systemic chemotherapy prescription. The landmark TAILORx trial demonstrated that chemotherapy only improves prognosis when RS >25 and that AC prescription in early ER+, HER2- breast cancer should be restricted to this group with very few exceptions.13 The present study demonstrates the correlation between readily available clinicopathological information and RS, and the predictability of low- and high-risk ODX scores in certain subsets of early ER+disease. This study also highlights the successful reduction in AC prescription in ER+, HER2-, and LN- patients who undergo ODX testing and the overall excellent prognosis of early ER+breast cancer treated with modern multimodality management. These findings validate the role of the genomic assay in clinical practice, while also supporting the literature demonstrating that statistical models based on clinicopathological information may act as a cost-effective surrogate to ODX where it is not affordable, available, or necessary 22-26; or give additional prognostic and predictive information in combination with ODX where clinical uncertainty exists.27
In our series, PgR- was an independent predictor of increased RS. This is unsurprising as RS is derived from an equation which is dependent upon ER, PgR, ERBB2 (HER2) scores and the proliferation of reverse-transcription polymerase chain reaction products and subsequently values for PgR are negatively deducted from the algorithm producing RS.6 Recent evidence suggests low grade, PgR- tumors are unlikely to generate low-risk RS,28, 29 and thus, these biomarkers may be useful in the substratification of these cancers for conventional cytotoxic chemotherapy prescription in the adjuvant setting. A number of observational studies and a recent meta-analysis have also shown that PgR- status is associated with worse outcomes in ER+breast cancer.30 Moreover, while ER+/HER- patients traditionally respond poorly to neoadjuvant chemotherapy (NAC), the PgR- subgroup are more likely to achieve breast and axillary complete pathological response rates (pCR) 31 as well as being more amenable to BCS. The prognostic significance of quantitative PgR is also demonstrated in established clinicopathological models such as the IHC424and the Rochester Modified Magee Equations for predicting RS.25, 26 In this study, 94.6% (53/56) of PgR- tumors were in the intermediate or high RS category and only 5.4% (3/56) were deemed low risk. These findings suggest PgR assessment should remain a necessary component of routine breast histopathological reporting.30
In keeping with previous reports, Nottingham grade independently predicted low- and high-risk RS using multivariable analysis.32, 33 Tumor grade is a composite score based on the degree of nuclear atypia, mitotic index, and tubular formation, all of which are features known to indirectly contribute to individual ER, HER2 and proliferation genes assessed in the ODX assay.32, 34 Almost all low grade, LN-breast cancers are ER+/PR+/HER2- 35and consequently are eligible for ODX testing according to international guidelines.11, 12 “Classical” invasive lobular cancer (ILC)(ER+/PR+, low grade), in particular is molecularly identified as Luminal A intrinsic subtype of tumor and except for the pleomorphic variant, ILC has low to intermediate RS.36, 37 Multiple studies have demonstrated ILC to be largely chemoresistant.36-38 In this study, 0.0% (0/41) of low grade and just 7.4% of double hormone positive ILC tumors (5/68) had a high RS, with all the latter cases having a nuclear grade 2 or 3 signifying the pleomorphic variant of ILC.39 These findings suggest that performing ODX testing on low grade, ER+and/or classical ILC tumors may represent an unnecessary expense as it is not useful in guiding clinical management decisions in this cohort of patients.
Initial aspirations for ODX were to provide a cost-effective, personalized approach to treating early, ER+breast cancer by reducing the cost and toxicity of overtreatment with AC.40, 41 However, studies have begun to question the cost-effectiveness of this strategy given the primacy of the locoregional and EHT management of ER+disease, and the difficulty in separating early disease into clinically relevant subgroups to which systemic chemotherapy can improve outcomes.42, 43 The knowledge that AC can be safely omitted for most patients in low- and intermediate-risk groups has resulted in consideration of an even more judicious approach to patient selection. 42, 43 There is an increasing realization of the predictive nature of clinicopathological information in determining low-risk RS groups.22-26 Our study demonstrates that grade 1 cancers independently predict RS <11, suggesting all 41 patients with grade 1 tumors would have been correctly allocated to EHT alone. Furthermore, a large number of patients are stratified into these potentially predictable nonactionable groups following genomic testing,43, 44 a result replicated within our study (342/402, 85.1% having ODX scores less than 25). With recent focus on global surgery initiatives such an approach may be particularly advantageous in underfunded and inadequately resourced health care systems. 45 A number of groups have similarly demonstrated that utilizing clinicopathological information in order to generate composite scoring systems to predict RS may be a plausible, more cost-efficient screening option to help avoid overtreatment of ER+, HER2-, and LN- breast cancer with AC in future.22, 23, 25-27
Despite these encouraging results, our analysis highlights the presence of “outliers” within each risk category. This suggests that caution should be taken, in particular before any surrogate for RS testing is introduced to routine practice. The clinical utility of ODX is evidenced by the fact that despite the reduction in chemotherapy prescription there were comparable survival outcomes between the low- and high-risk cohorts. This may indicate that ODX is correctly stratifying patients to receive tailored adjuvant chemoendocrine prescription, ameliorating the worse prognosis of the high RS groups. However, subgroup analysis of treatment with AC stratified according to RS, failed to demonstrate a benefit from AC regardless of ODX category, and freedom from disease recurrence and OS was excellent for all groups, albeit we acknowledge there are limited follow-up and few events from which to draw meaningful conclusions. Interestingly, there was inverse relationship between the incidence of new primary and contralateral breast cancers, and increasing ODX category (Supplementary Appendix S4). An explanation for this finding is chemotherapy-induced ovarian ablation, as one-third of patients in this study were either pre- or peri-menopausal at the time of diagnosis.46, 47 Ovarian suppression and endocrine therapy might provide a useful alternative (or adjunct in high RS groups) to chemoendocrine therapy in this cohort.48
Included patients were treated prior to the publication of results from the TAILORx trial in 2018.13 Consequently, while the majority of patients in our study will have been treated on the basis of results of the NSABP-14 and NSABP-20 trials, a proportion of participants in this study were randomized as part of the TAILORx trial (n = 79), which may have impacted treatment decision-making.5, 6, 13 However, we have attempted to account for this by recategorizing RS risk groups as per TAILORx. Moreover, the typical patient enrolled in TAILORx was 55 years old, had a 1.5 cm grade-2 PgR+tumor with an RS of 17 making it difficult to extrapolate this data for younger women, with high-grade tumors and PgR-. Consequently, age, grade and PgR status may also help inform clinical decision-making when used in combination with ODX, especially in intermediate-risk groups.27 The lack of any independent predictor of DFS is likely explained by Type II error due to the small study population and few event rates one would expect with early ER+, HER2-, and LN- disease. It may appear surprising that use of XRT predicted OS but not DFS, it being an integral component of locoregional therapy; however, randomized controlled trials and meta-analyses have demonstrated adjuvant XRT, regardless of surgical approach (mastectomy or BCS), can reduce the risk of distant recurrences, and death.49-54 This may suggest an “abscopal” or immunogenic effect beyond the immediate zone of locoregional irradiation,55 although selection bias to spare older, more comorbid patients the additional burden of XRT is an important confounder.
To conclude, this study demonstrates the shift in administration of AC through the implementation of ODX testing in a European tertiary referral center, with the personalization of therapy based on each patients’ likelihood of recurrence and the overall excellent prognosis of early ER+breast cancer treated with modern multimodality management. The correlation between readily available clinicopathological information and RS, specifically Nottingham grade and PgR status, was also demonstrated. These results suggest the potential for the development of a clinicopathological and immunohistochemical tool that could act as a surrogate for predictable RS in specific situations or provide supplementary prognostic information to aid clinicians’ decision-making when used in combination with RS, especially in intermediate-risk groups. This could be of importance with the likely expansion of indications for ODX testing in clinical practice with results of the Treatment (Rx) for node POsitive, eNDocrinE Responsive breast cancer (or RxPONDER) trial to incorporate those with 1–3 +LNs, as well as validation of RS in guiding NAC following analysis from core biopsies for ER+ disease.56, 57
ACKNOWLEDGMENT
Open access funding provided by IReL.
ETHICAL APPROVAL
Local hospital ethical approval was obtained for this study. None of the authors have any conflicts of interest to disclose. Data are available upon reasonable request from the corresponding author via email.




