Injectable Methylcellulose/Hyaluronic Acid/Collagen Hydrogel Loaded With Adipose-Derived Stem Cells Alleviates Skin Photoaging via Inhibition of HIF-1α: An in Vitro and in Vivo Study
Funding: This research was funded by the Wenzhou Municipal Science and Technology Bureau (Y2023461).
ABSTRACT
Background
Adipose-derived stem cells (ADSCs) exhibit good anti-photoaging activity, yet inadequate homing numbers and low cell survival rates remain urgent challenges to overcome, and the underlying mechanisms are still unclear.
Methods
Methylcellulose (MC)/hyaluronic acid (HA)/Collagen I hydrogel (Gel) loaded with ADSCs (MHAC/ADSCs Gel) was prepared using the thermal dispersion method. Scanning electron microscopy was used to observe its microstructure, while swelling and degradation rates were determined by the pycnometer method. Subsequently, a photoaging HaCat cell model was established to evaluate the anti-photoaging potential of MHAC/ADSCs using the CCK-8 assay and Transwell experiment. Furthermore, qRT-PCR, WB, TargetScan bioinformatics analysis, and dual-luciferase reporter assays were conducted to investigate the underlying mechanisms of MHAC/ADSCs. Finally, a photoaging mouse model was established to assess the anti-photoaging activity of MHAC/ADSCs using H&E staining, Masson staining, immunohistochemistry, qRT-PCR, and WB.
Results
MHAC/ADSCs exhibited a 3D porous structure, remaining in a sol state at 20°C and forming a gel at 37°C. Additionally, it demonstrated excellent swelling properties and degradability. The results also revealed that MHAC/ADSCs showed significant anti-photoaging activity, promoting the migration of HaCat cells, increasing the expression of miR-18a and Collagen I, and inhibiting the expression of HIF-1α. In animal experiments, MHAC/ADSCs reversed epidermal thickening and collagen loss in mice and reduced the expression level of CD31.
Conclusion
MHAC/ADSCs exhibit anti-photoaging activity by inhibiting the expression of HIF-1α through the upregulation of miR-18a, providing a promising approach for clinical treatment of photoaging.
1 Introduction
Skin photoaging is a degenerative skin disease [1] that increases the risk of developing malignant tumors. It results from the combined effects of natural aging and ultraviolet radiation [2], with Ultraviolet B (UVB) having a particularly strong aging effect on the skin [3]. When the skin epidermis is penetrated by UVB, a series of adverse effects are triggered [4], including epidermal thickening [5], degenerative changes in the elastic tissue of the dermis, and increased collagen degradation by matrix metalloproteinases (MMPs) [6]. These changes further lead to the degeneration of the extracellular matrix (ECM), accelerating the process of skin aging [7]. In the treatment of photoaging, adipose-derived stem cells (ADSCs) have shown promising results [8]. Their anti-aging effect is mainly exerted by influencing the expression of various related factors through their paracrine function [9, 10], but the specific mechanism of action is still unclear.
Hypoxia-inducible factor-1α (HIF-1α) is a transcription factor responsible for maintaining tissue homeostasis and regeneration [11]. It is sensitive to various stressors. It activates many growth factors and plays a crucial role in angiogenesis and skin photoaging [12]. The formation of skin wrinkles and cutaneous angiogenesis in mice was led by acute UVB exposure [13]. High levels of HIF-1α can be detected in UVB-treated dermal grafts [14], indicating that HIF-1α mediates significant angiogenesis. Additionally, miR-18a is a microRNA that regulates gene expression levels by binding to the 3' untranslated region (3'UTR) of target mRNAs [15], inhibiting their translation or promoting their degradation. Therefore, a hypothesis arises, anti-photoaging activity may be exerted by ADSCs through the promotion of miR-18a expression and the inhibition of HIF-1α levels. Despite the promising prospects of cell delivery therapy in medicine [16], insufficient homing numbers [17] and low cell survival rates [18] remain challenges that urgently need to be overcome.
To overcome these challenges, cell encapsulation technology is regarded as a promising solution [19]. In cell encapsulation, biomaterial scaffolds serve as a surrogate ECM for cells [20]. This not only addresses the issue of mechanical dispersion of cells from the injection site but also significantly enhances cell survival rates and improves cell retention, enabling cells to exert therapeutic effects over an extended period. Research, such as that conducted by Zhu's team [21], has demonstrated that embedding ADSCs into chitosan microspheres can boost cell implantation rates. When compared to material microspheres, hydrogels (Gel) are more particularly advantageous for cell encapsulation due to their stable three-dimensional structure and polymer networks formed through physical or chemical interactions [22], creating an ideal environment for cell proliferation and growth. In particular, thermosensitive Gel enables precise delivery of cells to the injured area [23], effectively addressing cell retention challenges and further elevating cell survival rates, ensuring stable and long-lasting cellular function within the target tissue. In addition, Gel with an uneven surface can increase the contact area between drugs and the hydrogel matrix, which promotes drug adsorption and encapsulation, ultimately improving drug delivery efficiency [24]. Furthermore, porous hydrogels are conducive to maintaining higher cell viability [25].
Methylcellulose (MC), a water-soluble cellulose derivative capable of forming physically crosslinked gels at specific temperatures [26], is considered an ideal material for gel formation. Despite exhibiting low protein adsorption and cellular adhesion properties, they can be effectively overcome by functionalizing MC with ECM components [27] such as hyaluronic acid (HA) and collagen. Based on this, MC/HA/Collagen I Gel loaded with ADSCs (MHAC/ADSCs Gel) was prepared, and the feasibility of its treatment for skin photoaging was evaluated, while the mechanisms were also explored. This study aimed to enhance the survival rate and homing quantity of ADSCs, providing a potential therapeutic strategy for the clinical treatment of skin photoaging.
2 Materials and Methods
2.1 Preparation and Characterization of MHAC/ADSCs
MHAC hydrogel was prepared through a thermal dispersion method by generating a 7.0% w/v MC solution. After heating 4 mL of Hank's balanced salt solution (HBSS, H6648, Sigma-Aldrich) to 70°C, 0.70 g of MC (M7027, Sigma-Aldrich) was added and stirred using a magnetic stirrer until the MC powder was completely wetted. Continuously, 6 mL of HBSS (prechilled at 4°C for 24 h) was added to the system. As the temperature decreased, the MC polymer became more soluble in water, achieving complete hydration (MC solution). Additionally, HA (924474, Sigma-Aldrich) was dissolved in HBSS and stirred overnight to prepare a 0.45% w/v HA solution. Once prepared, the two solutions were mixed in equal volumes, and an amount of Collagen I (234138, Sigma-Aldrich) equivalent to that of HA was added to prepare the MHAC solution.
1 mL of the MHAC prepolymer solution was aspirated, and 1 × 106 ADSCs (SNP-H350, Sunncell), along with their dedicated culture medium (SNLM-597, Sunncell), were added to it to prepare the MHAC/ADSCs Gel. After the material was prepared, its microstructure was characterized using a scanning electron microscope (SEM, SU8600, Hitachi).
2.2 Determination of Degradation Rate
2.3 Measurement of Swelling Performance
2.4 Cell Culture and Treatment
HaCaT cells were purchased from the American Type Culture Collection (ATCC) and cultured in DMEM medium (D0822, Sigma-Aldrich) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin at 37°C in a 5% CO2 incubator.
To determine the effective dose of MHAC/ADSCs for subsequent experiments, the cells were divided into five groups and treated with 0, 50, 100, 150, and 200 µL/mL of MHAC/ADSCs Gel, respectively. After incubating for 24 h, cell viability was assessed using the CCK-8 assay.
To determine the appropriate UVB exposure duration for subsequent experiments, after co-incubation with 150 µL/mL of MHAC/ADSCs Gel, the cells were divided into two groups: the MHAC/ADSCs Gel group and the MHAC/ADSCs Gel + UVB group. The MHAC/ADSCs Gel + UVB group was exposed to UVB radiation, and cell viability was measured at 0, 3, 6, 12, and 24 h.
2.5 CCK-8 Assay
HaCaT cells were seeded into 96-well plates (5 × 103). After treatment with MHAC/ADSCs, 10 µL of CCK-8 solution (C0037, Beyotime) was added to each well. The cells were then incubated in a 37°C incubator for 1 h. Finally, the absorbance values at 450 nm were measured using a microplate reader (SpectraMax M5, Molecular Devices).
2.6 Transwell Experiment
HaCaT cells were seeded into the upper chambers of Transwell inserts (1 × 105), and 0.7 mL of DMEM complete medium containing 10% FBS was added to the lower chambers of the 24-well plates. Each group had three replicate wells, and they were incubated in a 37°C incubator for 24 h. Subsequently, 1 mL of 4% paraformaldehyde solution (P0099, Beyotime) was added to each well to fix the cells at room temperature for 10 min. The fixative was aspirated, and the cells were washed before adding 0.5% crystal violet solution (C0121, Beyotime) for staining for 30 min. Finally, the cells were observed under a microscope (DM4b, Leica).
2.7 qRT-PCR
Total RNA was extracted using TRIzol reagent (R0016, Beyotime). Subsequently, cDNA synthesis was performed using the Hifair II First Strand cDNA Synthesis Kit (11119ES60, Yeasen) with approximately 1 µg of RNA from each sample as the starting material. The synthesized cDNA samples were diluted 10-fold and used as templates for subsequent analyses. Real-time PCR was conducted on a Roche Light Cycler 96 (Roche) using SYBR Green PCR Master Mix (04707516001, Roche). Actin was used as the internal reference gene, and relative gene expression levels were calculated using the 2^(−ΔΔCt) method. The primer sequences for qRT-PCR are shown in Table 1.
| Forward | Reverse | |
|---|---|---|
| miR-18a | UAAGGUGCAUCUAGUGCAGAUAG | AUCUGCACUAGAUGCACCUUAUU |
| HIF-1α | CAGCCGCTGGAGACACAATC | TTTCAGCGGTGGGTAATGGA |
| Collagen I | GCTCCTCTTAGGGGCCACT | ATTGGGGACCCTTAGGCCAT |
| Actin | CCCATCTATGAGGGTTACGC | TTTAATGTCACGCACGATTTC |
2.8 WB Assay
Total protein was extracted from mice skin tissue, followed by quantitative analysis using a BCA kit (P0012, Beyotime). Subsequently, equal amounts of protein were separated by SDS-PAGE and transferred onto PVDF membranes (IPVH00010, MerckMillipore) for immunoblotting analysis. To minimize nonspecific binding, the PVDF membranes were blocked and then incubated with primary antibodies overnight at 4°C. The membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies (1:2000, A0208, Beyotime) for 1 h at room temperature. Finally, protein bands were visualized using an ECL detection system (GelView 6000Plus), and their intensities were quantitatively analyzed by densitometry. The primary antibodies used included anti-Collagen I (1:1000, ab138492, Abcam), anti-HIF-1α (1:1000, ab317044, Abcam), and anti-GAPDH (1:1000, ab8245, Abcam).
2.9 Transfection Assay
The miR-8a mimic, miR-8a inhibitor, siRNA targeting HIF-1α (CCTGAAGAATTGGAAGAAATCAGAA), and scrambled control were synthesized by Shanghai GenePharma Co., Ltd. According to the manufacturer's instructions, transfection experiments were performed using Lipofectamine 3000 reagent (L3000001, Invitrogen).
2.10 Dual Luciferase Reporter Gene Assay
The wild-type (HIF-1α-WT) and mutant (HIF-1α-MUT) versions of HIF-1α were cloned into the firefly luciferase reporter vector PGL3. Subsequently, primary HaCat cells were transfected with HIF-1α-WT+miR-NC, HIF-1α-WT+miR-18a mimics, HIF-1α-MUT+miR-NC, and HIF-1α-MUT+miR-18a mimics, respectively. After 48 h of incubation post-transfection, luciferase activity was measured using a dual luciferase reporter gene assay kit (RG088S, Beyotime).
2.11 Establishment and Administration of Photoaging Mice
C57BL/6 mice (6–8 weeks, 18–22 g) were purchased from Sipeifu (Beijing) Biotechnology Co., Ltd. They were housed in a sterile environment with a 12-h light/12-h dark cycle. Free access to food and water is allowed. All animal experimental procedures adhered to the ARRIVE 2.0 guidelines and were approved by the Experimental Animal Ethics Committee of Wenzhou Medical University (wydw2024-0555).
All mice were randomly assigned to a Control group (n = 6) or a UVB intervention group (n = 18). Mice in the UVB intervention group were exposed to UVB lamp irradiation once daily for 4 weeks. Specifically, during the first and second weeks, the mice received a daily irradiation dose equivalent to one time the minimal erythema dose (MED), which was 120 mJ/cm2. In the third and fourth weeks, the irradiation dose was increased to 180 mJ/cm2 daily to induce a mouse model of skin photoaging. Meanwhile, mice in the Control group were housed under normal conditions without UVB irradiation.
Following successful induction of the skin photoaging model, mice in the UVB intervention group were further randomized into three subgroups: the UVB group, the UVB+MHAC Gel group, and the UVB+MHAC/ADSCs Gel group, with six mice in each subgroup. Mice in the UVB+MHAC Gel group received 200 µL of MHAC via subcutaneous injection, mice in the UVB+MHAC/ADSCs Gel group received 200 µL of MHAC/ADSCs via subcutaneous injection, and mice in the Control group and the UVB group received 200 µL of PBS via subcutaneous injection. This procedure was performed once daily for 2 weeks. At the end of the experimental period, all mice were euthanized by cervical dislocation after isoflurane anesthesia. Skin tissue (0.5 cm × 0.5 cm) was harvested from the backs of the mice for subsequent pathological staining.
2.12 H&E Staining
Skin tissue samples of 0.5 cm × 0.5 cm were obtained from the injection area on the backs of mice and prepared as sections. After being processed with xylene (X821391, Macklin) and ethanol (91125, Macklin), the sections were immersed in hematoxylin solution (ST2067, Beyotime) for 5 min and then rinsed with running water. Subsequently, they were differentiated in 1% hydrochloric acid ethanol (C0161S, Beyotime) and stained in eosin solution (C0109, Beyotime) for 5 min. Finally, the sections were dehydrated, cleared, mounted, and observed under a microscope to record the tissue morphology.
2.13 Masson Staining
To detect collagen in skin tissue, Masson's trichrome staining kit (C0189S, Beyotime) was used to stain sections of mouse dorsal skin tissue. Specifically, the sections were immersed in hematoxylin solution for 5 min and differentiated in hydrochloric acid ethanol for 3 s. Subsequently, they were stained in Ponceau acid fuchsin solution for 5 min, rinsed with running water, and then treated with aqueous phosphotungstic acid for 5 min. Next, they were counterstained with aniline blue solution for 5 min. Finally, after dehydration, clearing, and mounting, the sections were observed under a microscope.
2.14 Immunohistochemistry
The tissue sections were washed with PBS and immersed in 3% H2O2 (5843989, Macklin) for 15 min. After being blocked with blocking solution for 1 h, they were incubated with anti-CD31 antibody (1:50, ab28364, Abcam) overnight at 4°C. The sections were then rinsed with PBS and co-incubated with horseradish peroxidase (HRP)-labeled goat anti-rabbit secondary antibody (1:50, A0208, Beyotime) at 37°C for 30 min. Subsequently, they were stained with diaminobenzidine (DAB, ST3205, Beyotime) for 15 min, dehydrated, cleared, and mounted. Microscopic observations were conducted to compare the differences between the sections of various groups.
2.15 Statistical Analysis
All experiments were repeated at least three times. Statistical analysis and graphing of the data were performed using GraphPad Prism software. Data are presented as the mean ± standard deviation (SD) for each group. The differences between the two groups were compared using the t-test, while comparisons among multiple groups were performed using one-way ANOVA. A p value < 0.05 was considered to indicate a statistically significant difference.
3 RESULTS
3.1 Preparation and Characterization of MHAC/ADSCs
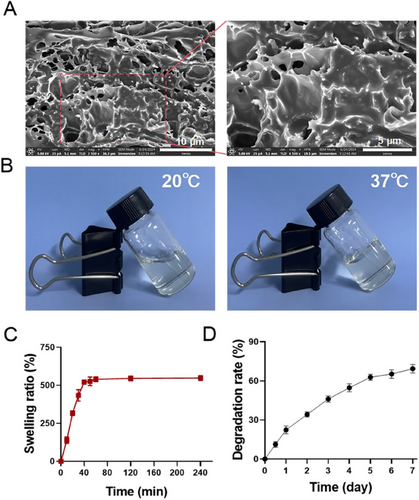
First, MHAC/ADSCs were prepared by the thermal dispersion method, and their micromorphology was observed by SEM. The result indicated that MHAC/ADSCs exhibited a 3D porous structure with pores of varying sizes (Figure 1A). Subsequently, changes in its state at 20°C and 37°C were recorded. MHAC/ADSCs were in a sol state at 20°C and formed a gel at 37°C (Figure 1B). Next, the swelling ratio of MHAC/ADSCs was determined, and the results showed that MHAC/ADSCs demonstrated good swelling properties, reaching swelling equilibrium around 60 min (Figure 1C). Additionally, the degradation rate of MHAC/ADSCs was also measured. The results indicated that MHAC/ADSCs possessed good degradability, with a degradation rate reaching 70% on the seventh day (Figure 1D).
3.2 MHAC/ADSCs Promoted HaCaT Cell Migration
To evaluate the viability of ADSCs within MHAC/ADSCs Gel, the CCK-8 method was employed. The results showed that ADSCs in the Gel still maintained strong viability after continuous culture for 3 and 7 days; however, their viability slightly decreased by Day 15 (Figure 2A). Next, the effect of different concentrations of MHAC/ADSCs on the viability of HaCaT cells after UVB irradiation was determined. The results indicated a positive correlation between the increase in HaCaT cell viability and the concentration of MHAC/ADSCs. Notably, when the concentration of MHAC/ADSCs was 150 µL/mL, cell viability significantly increased (Figure 2B). Therefore, a treatment concentration of 150 µL/mL was selected for subsequent experiments. Additionally, the effect of different UVB irradiation durations on HaCaT cell viability was also assessed, revealing a negative correlation between HaCaT cell viability and UVB irradiation duration. Specifically, when the irradiation duration was 12 h (Figure 2C), a significant difference in viability was observed compared to unirradiated cells. Consequently, a UVB irradiation duration of 12 h was chosen for subsequent experiments.
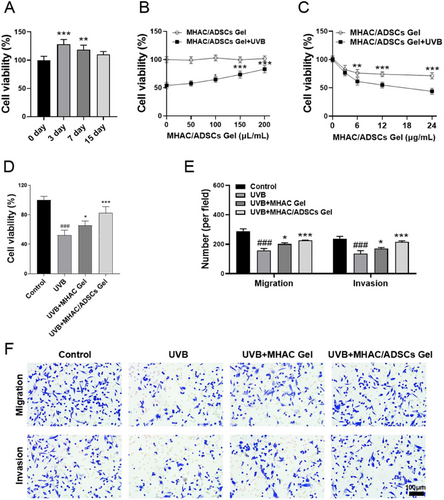
After determining the optimal dose concentration of MHAC/ADSCs and UVB irradiation duration, the protective effect of MHAC/ADSCs on HaCaT cells after UVB irradiation was evaluated using the CCK-8 method. The results demonstrated that cell viability significantly decreased after 12 h of UVB irradiation, but recovered somewhat, albeit limitedly, after MHAC intervention. In contrast, intervention with MHAC/ADSCs significantly enhanced the viability of HaCaT cells (Figure 2D). To further explore the potential of MHAC/ADSCs in vitro against photoaging, a Transwell experiment was conducted. The result showed that compared to the Control group, the cell migration ability of the UVB group was significantly reduced; however, intervention with MHAC/ADSCs significantly reversed this decrease (Figure 2E–F), suggesting that MHAC/ADSCs have significant anti-photoaging potential in vitro.
3.3 MHAC/ADSCs Inhibited the Expression of HIF-1α by Increasing the Level of miR-18a
After UVB irradiation, the expression of HIF-1α significantly increases. Inhibiting HIF-1α may contribute to reversing skin photoaging. It has been reported that miR-18a can inhibit the expression of HIF-1α [28]. Therefore, we hypothesize that ADSCs may inhibit the expression of HIF-1α by regulating the level of miR-18a, thereby exerting an activity to reverse photoaging. To validate this hypothesis, the expression levels of miR-18a mRNA and HIF-1α mRNA in HaCaT cells were determined by qRT-PCR. The results indicated that compared to the Control group, the level of miR-18a mRNA in the UVB group was significantly decreased (Figure 3A), while the level of HIF-1α mRNA was significantly increased (Figure 3B). When treated with MHAC/ADSCs, this trend was significantly reversed, with an increase in the level of miR-18a mRNA and a decrease in the level of HIF-1α mRNA. Subsequently, TargetScan bioinformatics analysis was utilized to predict the complementary sites between the 3'-UTR of HIF-1α and miR-18a, further supporting our hypothesis. Additionally, the results of luciferase reporter gene experiments showed that cotransfection of miR-18a mimics and HIF-1α-WT reduced luciferase activity, while cotransfection of HIF-1α-MUT and miR-18a mimics restored luciferase activity (Figure 3C), suggesting that HIF-1α is a direct target of miR-18a.
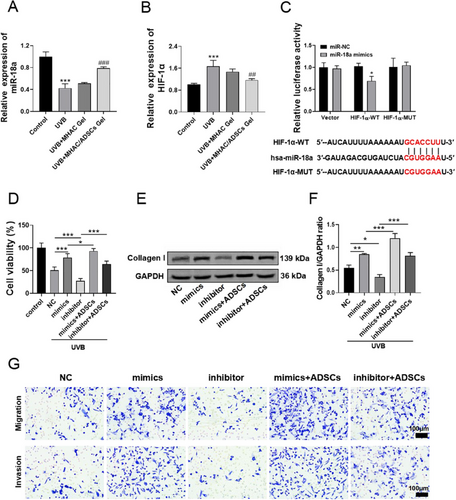
Subsequently, miR-18a was overexpressed or inhibited in HaCat cells, and the cells were exposed to UVB for 12 h. CCK-8 was used to measure changes in cell viability. The results showed that inhibiting the expression of miR-18a further decreased cell viability compared to the UVB group. Conversely, overexpressing miR-18a significantly increased cell viability, and co-incubation with ADSCs further enhanced cell viability (Figure 3D). To further assess the impact of overexpressing or inhibiting miR-18a in HaCat cells on skin photoaging, the expression of Collagen I protein within the cells was determined by WB. The results indicated that the expression of collagen I in the cells was increased by overexpressing miR-18a, and this expression was further significantly elevated by co-incubation with ADSCs. In contrast, the expression of Collagen I was decreased when miR-18a expression was inhibited, but this effect was reversed when co-incubated with ADSCs (Figure 3E–F). Additionally, Transwell experiments also demonstrated that overexpressing miR-18a promoted cell migration, and co-incubation with ADSCs further increased the number of migrating cells. Conversely, inhibiting the expression of miR-18a reduced the number of migrating cells (Figure 3G).
3.4 MHAC/ADSCs Alleviated Skin Photoaging in Mice
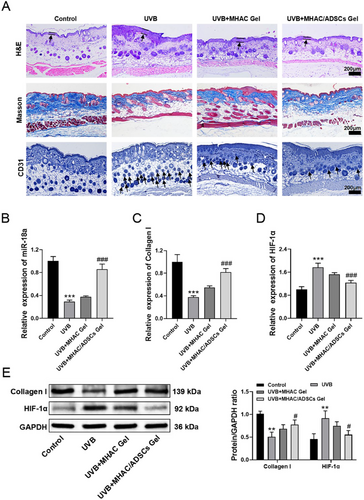
To further evaluate the anti-photoaging activity of MHAC/ADSCs in vivo, a mouse model of skin photoaging was established. Subsequently, the therapeutic effects of MHAC/ADSCs were assessed through H&E staining, Masson's trichrome staining, and immunohistochemical analysis. The results showed that after UVB exposure, the dorsal epidermis of the mice significantly thickened, Masson's staining appeared lighter, and CD31 expression was upregulated. Following the treatment of MHAC/ADSCs, the epidermal thickness decreased, and CD31 expression was reduced (Figure 4A). Subsequently, the expression of miR-18a, HIF-1α, and Collagen I in mouse skin was measured by qRT-PCR. The results showed that after the intervention of UVB, the expression of miR-18a mRNA and Collagen I mRNA significantly decreased, while HIF-1α mRNA expression increased. However, after the treatment of MHAC/ADSCs, the expression of miR-18a mRNA and Collagen I mRNA significantly increased, and HIF-1α mRNA expression significantly decreased (Figure 4B-D). Similarly, WB analysis also reached the same conclusion (Figure 4E), further confirming that MHAC/ADSCs may exert their effects by influencing the expression of HIF-1α and Collagen I through miR-18a.
4 Discussion
In this study, we revealed that MHAC/ADSCs exhibit potent anti-skin photoaging potential, which is associated with their ability to upregulate miR-18a and downregulate HIF-1α expression. ADSCs are one of the most commonly used stem cell sources in clinical research, attracting significant attention due to their high availability and accessibility. They have been proven to prevent UVB-induced aging in human keratinocytes (KCs) and skin fibroblasts [29]. However, the underlying mechanisms remain unclear, prompting extensive research into their modes of action. Zha team suggested that ADSCs may mitigate UVB-induced damage by secreting exosomes and transferring circ-Ash1l [30]; Zhang team proposed that the exovesicles of ADSCs alleviate UVB-induced photoaging by inhibiting the TIMP1/Notch1 pathway [31]. As important mediators of intercellular communication, ADSC-derived exosomes contain various bioactive molecules, including proteins, lipids, and microRNAs, which play crucial roles in the process of skin aging [32]. It has been proven that cell proliferation, migration, and matrix remodeling can be effectively promoted by ADSC-derived exosomes [33]. miR-18a, a microRNA contained in ADSC-derived exosomes, has been reported to be associated with epithelial-mesenchymal transition, cell proliferation, and migration [34]. Therefore, we speculated that after transplantation to the target site, ADSCs promote cell migration and alleviate UVB-induced photoaging by secreting exosomes containing miR-18a. Our study showed that UVB exposure caused a decrease in miR-18a expression in HaCat cells; intervention with MHAC alone failed to increase miR-18a levels, but after MHAC/ADSCs intervention, miR-18a expression significantly increased, effectively inhibiting skin photoaging in mice, suggesting that miR-18a played a key role in the ADSCs-mediated anti-photoaging process.
On the other hand, Sonja's team pointed out that the phenomenon of photoaging can be improved by regulating HIF-1α. Specifically, when HIF-1α is specifically knocked out in mice, the mice show increased sensitivity to UVB, with an increase in the number of apoptotic cells and delayed barrier repair [35]. However, HIF-1α is regulated by miR-18a [36]. In this study, this conclusion was validated, namely that miR-18a can directly bind to and negatively regulate the expression of HIF-1α. To support this conclusion, the complementary binding sites between the 3'-UTR region of HIF-1α and miR-18a were predicted using the TargetScan bioinformatics tool, and it was further confirmed that HIF-1α was a direct target of miR-18a through a dual luciferase reporter assay. Based on this, we believe that the implantation of ADSCs exerts anti-photoaging activity by increasing the expression of miR-18a, inhibiting the level of HIF-1α, and promoting cell migration.
Clinical studies have shown that skin photoaging can be significantly improved by local injection of ADSCs [37]. However, it has the disadvantages of low cell homing numbers and short duration of activity. To overcome these disadvantages, MHAC Gel was designed as a carrier material for ADSCs. MHAC Gel is a thermosensitive hydrogel that can effectively overcome the issue of low stem cell homing numbers. Furthermore, we found that when ADSCs were loaded onto MHAC Gel, their activity remained strong for 7 days, with a slight decline in vitality observed only on the 15th day of culture. Overall, our study validated the feasibility of MHAC/ADSCs in treating UVB-induced skin photoaging. However, in comparison with autologous gel, MHAC/ADSCs could cause immune rejection reactions and might lead to infections and inflammation, especially in patients sensitive to implanted materials [38]. Additionally, the mechanism by which miR-18a regulates Collagen I was not elucidated in this study. Wakana's research indicated that miR-18a can regulate IL-23 [39], affecting the expression of Collagen I in dermal fibroblasts. This conclusion still requires more experiments to verify in UVB-induced photoaging models.
5 Conclusion
In summary, our research has shown that MHAC/ADSCs alleviate UVB-induced skin photoaging by promoting the expression of miR-18a and negatively regulating the level of HIF-1α. This discovery provides a novel therapeutic approach for the clinical treatment of photoaging.
Acknowledgments
This work was supported by Wenzhou Municipal Science and Technology Bureau (Y2023461).
Conflicts of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




