Supramolecular Assembly of Dendrobium officinale Polysaccharides-Hyaluronic Acid and Its Moisturizing Properties
Funding: The authors received no specific funding for this work.
Abstract
Background
Supramolecular technology has been widely applied in cosmetics due to its ability to enhance the stability of active substances and reduce their irritation potential. The synergistic interaction between polysaccharides can produce new products with characteristics and rheological properties that make them suitable for various applications.
Objective
The supramolecularly assembly of Dendrobium officinale polysaccharide (DOP) and hyaluronic acid (HA) was produced, and its moisturizing effect was verified to provide a basis for the application of supramolecular technology in cosmetic functional raw materials.
Methods
The supramolecular structure of polysaccharide assembly samples was verified through scanning electron microscopic observation. Moisture absorption and the ability of samples to resist cell drying damage before and after supramolecular assembly were evaluated by moisture absorption experiments and a cell drying damage model, respectively. Furthermore, this study validated the moisturizing effect of the supramolecular compositions during their application in cosmetics through tests of skin moisture content in extremely dry environments (during the winter season of Beijing, China), skin roughness and scale index, and trans epidermal water loss.
Results
The supramolecular composition appeared as a large sheet-like structure under a scanning electron microscope. Further, the supramolecular composition had significantly improved moisture absorption performance and could effectively resist cell drying damage. In an extremely dry environment, the composition showed excellent moisturizing performance and effectively improved skin scale and roughness.
Conclusions
The supramolecular assembly of DOP and HA has a synergistic moisturizing effect; thus, it can be expected to have a good moisturizing effect when applied to cosmetics.
1 Introduction
With the continuous development of science and technology and the cross-integration of various disciplines, a variety of technologies are increasingly being applied in cosmetics. This includes supramolecular technology, which has been widely applied in cosmetics in recent years. Supramolecular technology is defined as the process of combining two or more molecules through intermolecular forces, such as hydrogen bonds and van der Waals forces, to form supramolecular compounds. Supramolecules are applied in cosmetics to enhance the stability of active substances and reduce their irritating effects [1, 2]. For example, studies have reported that a supramolecular gel provides the photostability to vitamin A palmitate and has no effect on the physical and chemical properties of active substances [3]. Researchers have formulated supramolecular complexes by utilizing the interaction between arabinogalactose [4], L-phenylalanine [5], and salicylic acid molecules to improve the stability and activity of salicylic acid, while simultaneously controlling the release of salicylic acid and reducing its irritation. Therefore, supramolecular technology has potential applications in cosmetics. The interaction between polysaccharides in supramolecular association is another important research direction that researchers should focus on. The synergistic interaction between polysaccharides can generate new characteristics for products, allowing them to have rheological properties that are suitable for various applications. Mixtures of polysaccharides may have different characteristics depending on the proportion and nature of each polysaccharide [6, 7].
Among various types of polysaccharides, hyaluronic acid (HA) is a polyanionic polysaccharide that is rich in hydroxyl groups and has the common structural basis required to form supramolecular complexes such as hydrogen bonds and van der Waals forces. Its solution has a high viscosity and is usually arranged in a three-dimensional structure through intramolecular hydrogen bonds [8] that also promote its physical and chemical interactions with other molecules [9-11]. Silvani et al. [12] combined HA with arabinogalactan to form a novel artificial eye drop that can synergistically reduce xanthine oxidoreductase activity and inhibit the formation of UA (uric acid) and ROS (reactive oxygen species), which in turn help to treat dry eye, reduce irritation, and other related pathological conditions. This formulation may also contribute to resolving the dry eye syndrome. Martin et al. [13] investigated a possible synergistic effect between HA and two different types of galactomannans through rheological and nuclear magnetic resonance analyses. In addition to producing characteristic hydrogels, this polymer combination can also partially replace HA in many applications, such as nutritional food, tissue engineering and cosmetics, to help reduce costs.
In addition, HA has a good moisturizing effect and has been widely used as a humectant, usually added in the form of sodium salt (sodium hyaluronate) in skin care products. It is a viscous polysaccharide whose molecules are highly stretched and intertwined in an aqueous solution to form a continuous network structure. Water molecules can combine with HA molecules through hydrogen bonds within this network structure, thereby allowing HA to have “molecular sponge-like properties” that can absorb and retain several thousand times its weight in water [13]. With the increasing demand for green and safe cosmetics, people are focusing on finding safer and more effective moisturizing ingredients from natural plants; thus, plant polysaccharides, such as Tremella fuciformis Berk polysaccharide and Dendrobium officinale Kimura et Migo (D. officinale) polysaccharide, are increasingly applied in cosmetics as moisturizing agents [14, 15]. D. officinale is known as one of the “Nine Immortals” of Chinese herbal medicine. Studies have shown that D. officinale is rich in polysaccharides that play an important role in enhancing immunity, acting against cancer, and delaying aging [16]. Studies have reported that D. officinale polysaccharides are effective as skin moisturizers by increasing the expression of filaggrin (FLG), caspase-14, tight junction proteins (occludin and claudin1), and aquaporin 3 (AQP3) gene, which promotes skin hydration [17, 18].
At present, many studies have demonstrated the effect of supramolecular assemblies of polysaccharides in enhancing the stability of functional components and enhancing drug release performance [19, 20]; however, only a few studies have reported the moisturizing effects of supramolecular assemblies of Dendrobium polysaccharides. Therefore, based on the current application of supramolecular technology in cosmetics, polysaccharides from the Chinese characteristic plant D. officinale and moisturizing polysaccharide HA, which is commonly used in cosmetics, were selected to form a supramolecular polysaccharide assembly. We then compared the moisturizing properties of the polysaccharides before and after the supramolecular assembly to provide a basis for the application of supramolecular technology in cosmetic functional raw materials.
2 Materials and Methods
2.1 Materials and Instruments
Materials used in this study include: D. officinale (Kunming Institute of Botany, Chinese Academy of Sciences); sodium hyaluronate (Bloomage Freda Biopharm CO., LTD.); keratinocyte (National Infrastructure of Cell Line Resource); fetal bovine serum (Gibco); PBS (Gibco); MTT (Sigma, Darmstadt); and DMSO (Sigma, Darmstadt).
Instruments used in this study included: a CO2 incubator (Thermo, 150I); an inverted microscope (Olympus, CKX41); an electric suction aid (Eppendorf); a microplate reader (Bio Tek, VT); a gel permeation chromatograph (Agilent 1260 InfinityII MDS); a scanning electron microscope (SEM JSM 6700F); the skin scale index and roughness measurement device (VisioScan, Courage and Khazaka); a skin moisture content tester (Corneometer CM 825, Courage and Khazaka); a skin imaging device (Multi-Functional Scope, BOMTECH) and a TEWL measurement device (Aquaflux AF 200, EOTECH SAS).
2.2 Sample Preparation
2.2.1 Preparation of D. officinale Polysaccharide
Dried stems of D. officinale were ground into a fine powder, mixed with water at a mass ratio of 1:60, stirred at room temperature for 20–30 min, and then extracted at 55°C–60°C for 1.5–2 h. D. officinale polysaccharide with molecular weights of 100k-500 kDa was collected and then concentrated at 70°C–75°C and 0-0.01 MPa until it contained a solid content of 10%–15%.
The concentrated solution was mixed with 80% ethanol at a ratio of 1:4 (w/w), then kept at 4°C for 12 h. The precipitate was collected by filtration. The precipitate was dispersed in water at a mass ratio of 1:2 (w/w, precipitate to water) and then freeze-dried.
2.2.2 Supramolecular Assembly of D. officinale Polysaccharide and HA
D. officinale polysaccharide prepared in 2.2.1 was mixed with HA at a mass ratio of 5:2 before being dissolved in water at a ratio of 1:40 (w/w). After being stirred at 50°C–60°C for 20 min, the sample was ultrasonicated for 20–30 min, homogenized for 10–20 min, and then freeze-dried to obtain Dendrobium polysaccharide-HA supermolecular polysaccharide (DOP-HA). The presence of supramolecularly assembled DOP-HA was confirmed by rheological tests and infrared spectrum tests.
2.2.3 Preparation of Mixed Samples of D. officinale Polysaccharide and HA
D. officinale polysaccharide prepared in 2.2.1 was mixed with HA at a mass ratio of 5:2, and the mixture was dissolved in water at a ratio of 1:40 (w/w). After being stirred at 50°C–60°C for 20 min, the mixture was freeze-dried to obtain mixed samples of D. officinale polysaccharide and HA (DOP+HA).
2.2.4 Treatment of D. officinale Polysaccharide and HA
To eliminate the secondary influence of freeze-drying on the intermolecular forces and hydrogen bonds within the supramolecular structure of samples, D. officinale polysaccharides prepared in 2.2.1 and HA were dissolved in water at a ratio of 1:40 (material: liquid, w/w). The mixture was stirred at 50°C–60°C for 20 min and then freeze-dried to obtain D. officinale polysaccharides (DOP) and HA samples.
2.3 Polysaccharide Test
2.3.1 Determination of Polysaccharide Content
The content of polysaccharides in DOP after separation and purification was determined by the phenol-sulfuric acid method.
2.3.2 Determination of Molecular Weight of DOP
DOP sample was dissolved in 0.1 M NaNO3 aqueous solution to a final concentration of 2 mg/mL, and the solution was then filtered through a 0.45 µm microporous filter. The molecular weight distribution of DOP was determined using gel permeation chromatography (Agilent 1260 InfinityII MDS). The operation conditions were as follows: chromatographic column, PL aquagel-OH mixed-H (8 µm, 7.5×300 mm, molecular weight range = 200—10 000 000); detector, differential RI detector (Optilab T-rEX, Wyatt Technology Co., Santa Barbara, CA, USA); flow rate. 1.0 mL/min; column temperature, 45°C; injection volume, 50 µL; mobile phase, 0.1 M NaNO3 (0.01%NaN3), and elution, isocratic elution.
2.4 Scanning Electron Microscopic Observation
HA, DOP, DOP+HA, and DOP-HA samples prepared in 2.2.2-2.2.4 were pressed and secured on the sample stage of a scanning electron microscope (model: JSM 6700F) using a conductive adhesive. The surface morphology of the samples was then observed.
2.5 Moisture Absorption Properties
The improvement of the water-absorption ability of the supramolecular polysaccharide was evaluated through moisture absorption experiments [21].
2.6 Assessment of Cell Protection Rate
2.7 Determination of Skin Moisture Content in Extremely Dry Environments
| Content (%) | ||
|---|---|---|
| Materials | 0.5% DOP-HA | Formula matrix |
| Water | To 100 | To 100 |
| Ammonium acryloyldimethyltaurate/VP copolymer | 1.00 | 1.00 |
| DOP-HA | 0.50 | / |
| 1,3-Butylene glycol | 1.00 | 1.00 |
| 1,2-Hexanediol | 0.50 | 0.50 |
| Hydroxyacetophenone | 0.50 | 0.50 |
| Viscosity | 43 550 mPa.s (25°C, 12 rpm/min) | 36 100 mPa.s (25°C, 12 rpm/min) |
- Note: DOP-HA is the supramolecular assembly of D. officinale polysaccharide and HA prepared in 2.2.2.
2.8 Skin Scale Index and Roughness Test
Fifteen healthy volunteers (aged 50–60) with dry skin were selected for the experiment. The volunteers voluntarily participated in the test and signed an informed consent form. The volunteers sat quietly in an indoor environment (20.1°C–21.8°C with humidity [RH] = 44%–50%) for 30 min before conducting the test. Gels containing 0.5% HA, 0.5% DOP, 0.5% DOP+HA, 0.5% DOP-HA, and gel formula matrix were applied to different parts of lateral leg of volunteers. Samples were prepared according to the composition in Table 1. The skin scale index and roughness were measured before (baseline) and after 1, 2, and 4 h of application. The test was repeated five times, and the values were averaged. The skin data were collected using the VisioScan VC98 image analyzer (CK, Germany) before (baseline) and after 1, 2, and 4 h of application. Test environment was 20.1°C–21.8°C with humidity (RH) = 44%–50%.
2.9 Measurement of Transepidermal Water Loss (TEWL)
Fifteen healthy volunteers (aged 50–60) with dry skin and obvious scales on the outside of their lateral leg were selected for the experiment. All volunteers voluntarily participated in the test and signed an informed consent form. Testers applied 0.5% DOP-HA sample and sample matrices on both sides of their lateral leg daily. The sample composition is shown in Table 2. After 0, 2, and 4 weeks, the TEWL was measured using the AquaFlux. The volunteers sat quietly in an indoor environment (20.1°C–21.8°C with humidity [RH] = 44%–50%) for 30 min before conducting the test. The test environment was 20.1°C–21.8°C with humidity (RH) = 44%–50%.
| Materials | 0.5% DOP-HA | Sample formula (%) |
|---|---|---|
| Coco-glucoside | 1.50 | 1.50 |
| Glyceryl stearate | 0.80 | 0.80 |
| Cetearyl alcohol | 0.30 | 0.30 |
| Hydrogenated polyisobutene | 2.00 | 2.00 |
| Caprylic/capric triglyceride | 3.00 | 3.00 |
| Dimethicone | 3.00 | 3.00 |
| Water | 79.69 | 80.19 |
| Disodium EDTA | 0.05 | 0.05 |
| Carbomer | 0.10 | 0.10 |
| Glycerine | 5.00 | 5.00 |
| Xanthan gum | 0.10 | 0.10 |
| Dimethiconol | 3.00 | 3.00 |
| Triethanolamine | 0.06 | 0.06 |
| DOP-HA | 0.50 | / |
| Fragrance | 0.10 | 0.10 |
| 1,2-hexanediol | 0.80 | 0.80 |
| Viscosity | 1736 mPa.s (25°C, 30 rpm/min) | 1520 mPa.s (25°C, 30 rpm/min) |
- Note: DOP-HA is the supramolecular assembly of D. officinale polysaccharide and HA prepared in 2.2.2.
2.10 Statistical Analysis
Results are presented as mean ± standard deviation. Statistical analysis was carried out using SPSS 17.0 software. Statistical differences were determined by t-test or one-way analysis of variance (ANOVA), and the difference with p < 0.05 was considered statistically significant.
3 Results
3.1 Polysaccharide Content and Molecular Weight Distribution of DOP
The polysaccharide content of DOP was 90.12%, indicating that it had high purity. The polydispersity index (Mw/Mn value) is an index that characterizes the fractional width of polymer molecular weight: the larger its value, the wider the molecular chain distribution. The polydispersity index was 1.364 and it indicated that DOP had good homogeneity. As shown in Tables 3, the DOP with molecular weights of 100–500 kDa accounted for 93.92%, which is consistent with the membrane separation results.
| Final molecular weight (Da) | Initial molecular weight (Da) | Cumulative (%) |
|---|---|---|
| 996 125 | 500 000 | 3.68 |
| 500 000 | 300 000 | 25.63 |
| 300 000 | 200 000 | 30.80 |
| 200 000 | 100 000 | 32.23 |
| 100 000 | 50 000 | 5.26 |
| 50 000 | 30 106 | 2.39 |
3.2 Scanning Electron Microscopic Observation
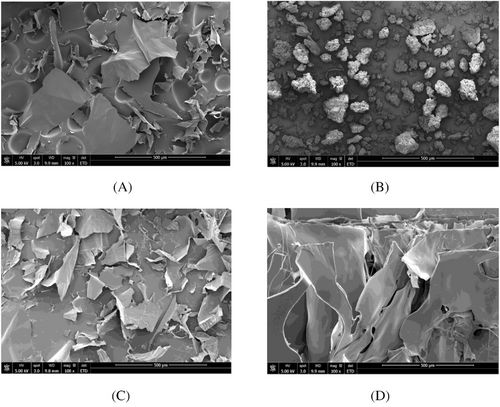
SEM images of different samples are shown in Figure 1. Among all samples, DOP had a small lamellar structure, while HA had a granular structure (Figure 1A and B). A mixture of DOP and HA solutions could be observed to have a lamellar structure similar to Dendrobium (Figure 1C), while supramolecularly assembled DOP and HA formed a large multilayered lamellar structure (Figure 1D) with a compact and smooth surface. The material was homogeneous, and micro-scale phase separation was not observed. This confirms good compatibility of DOP and HA in the supramolecules. Their appearance as a large sheet structure also indicates that they form a large network structure through hydrogen bonding and entanglement of polymer chains.
3.3 Verification of Enhancement Due to Supramolecular Assembly
3.3.1 Moisture Absorption Experiment
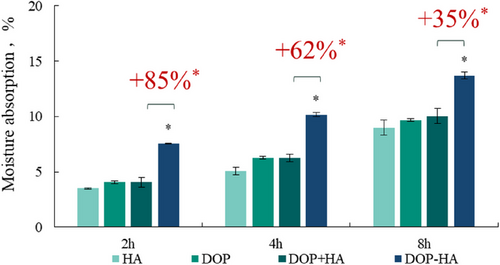
Moisture absorption of samples before and after supramolecular assembly was evaluated by moisture absorption experiments. The results are shown in Table 4 and Figure 2. Within 8 h, the moisture absorption of the DOP-HA sample was higher than that of the other samples. Compared with the DOP+HA group (p < 0.05), the moisture absorption of the DOP-HA sample at 2, 4, and 8 h increased by 85%, 62%, and 35%, respectively. This shows that the supramolecular assembly can significantly improve the water absorption performance of polysaccharides, thereby enhancing their moisturizing effect.
| Sample weight (g) | ||||
|---|---|---|---|---|
| Sample | 0 h | 2 h | 4 h | 8 h |
| HA | 0.5099 | 0.5278 | 0.5358 | 0.5558 |
| DOP | 0.5123 | 0.5330 | 0.5444 | 0.5618 |
| DOP+HA | 0.4787 | 0.4982 | 0.5087 | 0.5268 |
| DOP-HA | 0.4988 | 0.5365 | 0.5495 | 0.5671 |
3.3.2 Cell Protection Rate
Using the keratinocyte drying injury model, the ability of cells to resist drying and prevent dehydration death was detected by MTT assay to reflect the effectiveness of the sample in resisting drying injury.
After incubating the cells in the medium containing test samples for 24 h, the medium was removed and the cells were dried for 20 min at a medium wind speed on a small ultra clean bench. Then, the medium (excluding the test samples) was added and cultured for another 24 h. The cell protection rate was determined by the MTT assay.
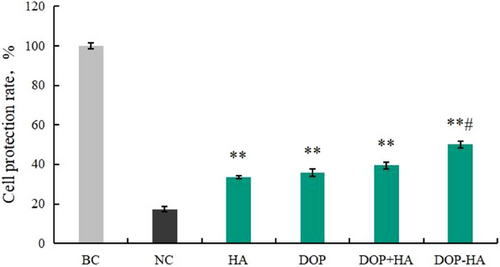
The ability of samples to resist cell drying damage before and after supramolecular assembly was tested using a cell drying damage model. The results are shown in Figure 3. The results showed that the protection rates of 0.0625% HA, 0.0625% DOP, and 0.0625% DOP+HA samples were 33.56%, 35.68%, and 39.51%, respectively. The cell protection rate of 0.0625% DOP-HA was 50.14%, which was significantly higher compared to that of the NC group (damaged by drying; p < 0.001) and was significantly different (p < 0.05) from that of the DOP+HA group (p < 0.05). As could be seen, the supramolecular assembly could improve the cell protection ability, preventing cell water loss and maintaining cell structural integrity, in turn protecting cells from drying injury.
3.4 Moisturizing Effect of DOP-HA in Protecting Skin from Extremely Dry Environment
The moisturizing effects of supramolecular composition in an extremely cold and dry environment were evaluated by tests run in alternating indoor and outdoor environments during the winter season (in Beijing, China) for 5 h. The results are shown in Figure 4. As shown in Figure 4A, compared with the baseline skin moisture content, the skin moisture content after using 0.5% DOP-HA gel for 0.5, 2.5, 3, and 5 h significantly increased (p < 0.05) with relative changes of 43.7%, 40.8%, 39.5%, and 36.9%, respectively. Moreover, the use of the DOP-HA group resulted in a significantly better outcome (p < 0.05) compared to the use of the gel matrix group for the same time period.
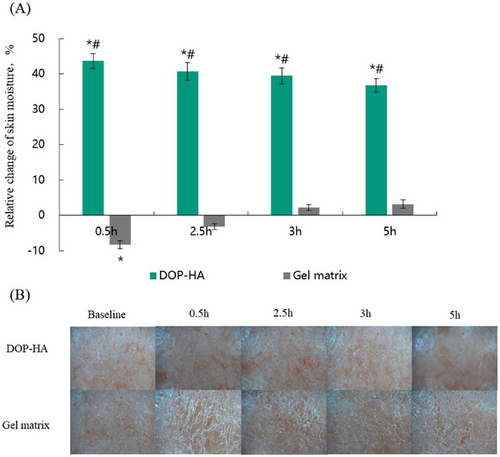
A skin analyzer (Multi-Functional Scope, BOMTECH) was used to collect images of the facial skin in the test area, and the results are shown in Figure 4B. Within 5 h after using 0.5% DOP-HA gel, the skin remained smooth and hydrated. However, after using the formula matrix for 0.5 h, scales appeared on the skin, which were significantly different from those observed in the sample group. Therefore, 0.5% DOP-HA had the moisturizing effect that could significantly increase and maintain the moisture content of skin in an extremely dry environment.
3.5 Changes in Skin Roughness and Scale Index
Skin roughness can be represented by smoothness (SEsm): the lower the SEsm value, the smoother the skin. That is, a lower SEsm value is an indication of high skin smoothness. As shown in Table 5 and Figure 5A, after using 0.5% DOP-HA gel for 1, 2 and 4 h, the SEsm values decreased by 19.2%, 20.6% and 18.1% respectively, which were significantly lower than those obtained after using the gel matrix group (p < 0.05). Additionally, these values were statistically significantly different from the values obtained after using DOP+HA solution-mixed group for 2 and 4 h. Moreover, the values obtained after using DOP-HA for 2 and 4 h were also statistically significantly different compared to those obtained after using DOP+HA group (p < 0.05).
| SEsm | ||||
|---|---|---|---|---|
| Sample | 0 h | 1 h | 2 h | 4 h |
| HA | 340.2 | 303.3 | 301.9 | 317.4 |
| DOP | 323.0 | 292.5 | 281.7 | 297.7 |
| HA+DOP | 327.9 | 275.0 | 275.5 | 293.7 |
| HA-DOP | 328.3 | 265.3 | 260.6 | 268.9 |
| Gel matrix | 351.5 | 334.5 | 357.3 | 371.4 |
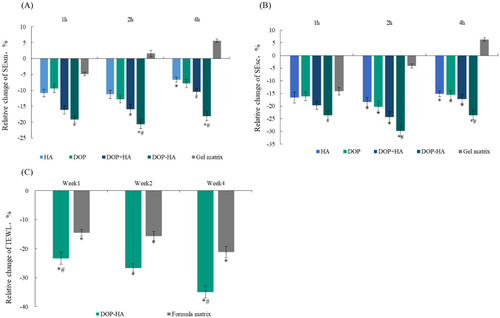
Scaliness (SEsc) was employed to characterize the degree of skin scaling: the smaller the value, the less peeling of the skin stratum corneum, which is the indication of an improvement in scaling. The SEsc test results are shown in Table 6 and Figure 5B. After using 0.5% DOP-HA gel for 1, 2, and 4 h, the SEsc decreased by 23.6%, 29.8%, and 23.6%, respectively, compared to those before using. Moreover, after 2 and 4 h, the use of the DOP-HA group resulted in a significantly better outcome (p < 0.05) compared to the use of the DOP+HA group. These results indicate that the combination of supramolecules has beneficial effects on skin roughness and scales and can effectively smooth the skin.
| SEsc | ||||
|---|---|---|---|---|
| Sample | 0 h | 1 h | 2 h | 4 h |
| HA | 1.83 | 1.52 | 1.49 | 1.55 |
| DOP | 1.74 | 1.46 | 1.39 | 1.47 |
| HA+DOP | 1.61 | 1.29 | 1.22 | 1.33 |
| HA-DOP | 1.74 | 1.33 | 1.22 | 1.33 |
| Gel matrix | 1.90 | 1.63 | 1.82 | 2.02 |
3.6 Changes in TEWL
TEWL is an important indicator for evaluating the skin barrier function: the lower the value, the better the skin barrier and water retention capacity. TEWL is one of the most important indicators for evaluating moisturizing efficacy [22, 23]. As shown in Table 7 and Figure 5C, TEWL decreased significantly (p < 0.05) after the use of 0.5% DOP-HA for 1, 2, and 4 weeks with the reduction rates of 23.3%, 26.6%, and 34.9%, respectively. After using the formula matrix for 1, 2, and 4 weeks, the reduction rates of TEWL were 14.5%, 15.6%, and 21.2%, respectively. It appears that the sample containing 0.5% DOP-HA can significantly reduce skin water loss, and the reduction rate of TEWL after 4 weeks of using this sample was significantly higher than that after using formula matrix for the same time period (p < 0.05). These results indicate that the supramolecular compositions can effectively improve the skin barrier function, thereby allowing for more effective moisturization.
| TEWL | ||||
|---|---|---|---|---|
| Sample | Week 0 | Week 1 | Week 2 | Week 4 |
| HA-DOP | 11.11 | 8.52 | 8.15 | 7.23 |
| Formula matrix | 10.87 | 9.29 | 9.17 | 8.57 |
4 Discussion
A supramolecular compound is a complex and organized aggregate that is bound together by intermolecular interactions. The application of supramolecular technology in cosmetics can be used to optimize the solubility, stability, transdermal effect and efficacy of raw materials [1, 2]. Modification of natural functional raw materials by supramolecular assembly can greatly improve their properties and overcome their limitations in terms of formulatability.
Salicylic acid is an attractive bionic acid that is widely used in cosmetics and drugs for the treatment of skin diseases. However, salicylic acid is a relatively strong irritant and cannot be used on sensitive skin, and its poor water solubility limits the amounts that can be included in formulas, resulting in poor transdermal efficiency and bioavailability. Wang et al. [24] developed matrinium salicylate, a supramolecular ionic salt that is less cytotoxic and irritates the skin less than salicylic acid, while exhibiting enhanced water-solubility, transdermal-delivery, and bioactivity properties.
The vast majority of natural polysaccharides contain hydrophilic groups such as hydroxyl and carboxyl groups, which have high water solubility and dispersion stability. At the same time, their molecular chains are composed of various types of functional groups that can be modified by chemical and biochemical methods to obtain functionalized polysaccharide supramolecular derivatives with wider practical applications. Supramolecular assembly based on polysaccharides has a wide range of benefits. For example, the supramolecular assembly of polysaccharides can be used as a protective carrier to improve the stability and biological activity and as a sustained and controlled release carrier to achieve controlled drug release under selective conditions. In addition, polysaccharides can be used as targeted carriers or stabilizing agents to synthesize nanoparticle assemblies to enhance biological activity [25-28].
In this study, the supramolecular assembly of DOP and HA was generated and the surface morphology of the supramolecular structure was observed by SEM. The supramolecularly assembled DOP and HA formed a large multilayered lamellar structure (Figure 1D) with a compact and smooth surface. In addition, the structure of supramolecular assemblies can also be observed through transmission electron microscopy (TEM) [29]. Fourier Transform Infrared Spectrometer (FT-IR) is commonly used to confirm specific functional groups and chemical bonds in supramolecules, such as C = O, C-N, in order to infer their chemical structure and composition [30]. There are also studies using viscometers to measure the viscosity of supramolecular samples, determining the fluidity of solutions and intermolecular interactions [31]. In this study, we compared the effect of adding the supramolecular compositions to the formulation on the viscosity of the system. The results showed that the viscosity increased after the addition of DOP-HA to the formulation compared with the formulation matrix, indicating a synergistic interaction between polysaccharides (Tables 1 and 2). According to the previous studies, the enhancement of the three-dimensional polymeric network increases the viscosity of the system [13, 28]. Therefore, the viscosity enhancement observed in the supramolecular polysaccharide assembly samples, influenced by the interaction of DOP-HA, can be attributed to the increased entanglement and intermolecular interactions between DOP and HA chains. Meanwhile, hydrogen bonding is also enhanced, forming a more stable network structure and supramolecular aggregates that can bind water molecules, ultimately leading to reduced fluidity and increased viscosity.
Furthermore, this study validated the moisturizing effect of the supramolecular compositions during their application in cosmetics. The results showed that the supramolecular compositions had superior moisturizing ability, thus could protect the skin from extremely dry environments and extremely dry areas. This moisturizing ability is closely related to the network structure formed after the supramolecular assembly of polysaccharides. During supramolecular assembly, DOP and HA formed an interpenetrating network structure through strong intermolecular hydrogen bonds, exposing more active functional groups. This generates the supramolecular assembly of DOP and HA with significantly improved water absorption capacity. At the same time, the formed water-locking network structure can fix a large number of water molecules, thereby preventing water loss; as a result, the “water retention” of the sample was improved despite the extremely dry environment.
5 Conclusions
In this study, the supramolecular assembly of DOP and HA was generated, and its moisturizing effects were verified.The results showed that the supramolecular assembly could effectively improve the water absorption ability and resistance to cell drying damage of DOP and HA. Efficacy studies showed that the supramolecular composition formed by the two polysaccharides could significantly increase and maintain skin moisture content in extremely dry environments, while effectively improving skin scales and roughness and reducing water loss. Overall, the supramolecular assembly of DOP and HA has a synergistic moisturizing effect; thus, it may be applied as a raw material to improve the moisturizing effect of cosmetics.
Ethics Statement
The human body test was carried out in EviSkin Testing Technology (Beijing) Co., Ltd., Beijing, China. The research protocol (A004-2022120701R5) was examined and approved by the Ethical Commission of EviSkin Testing Technology (Beijing) Co., Ltd. Before the study, benefits and potential risks were explained to the subjects, and the informed written consent of the participants was obtained, and each of the participant signed the informed consent.
Consent
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare that they have no conflicting interests.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




