Polynucleotides Enhance Skin Barrier Function and Reduce Inflammation in a 2,4-Dinitrochlorobenzene-Induced Mouse Model of Atopic Dermatitis
ABSTRACT
Background
Atopic dermatitis (AD) is a chronic inflammatory dermatological disorder characterized by skin barrier dysfunction, dry skin, pruritus, and aberrant immune responses to external stimuli. Although polynucleotides (PNs) have anti-inflammatory properties, their effect on AD remains unexplored.
Materials and Methods
This study investigated the effects of PNs on a 2,4-dinitrochlorobenzene (DNCB)-induced AD mouse model. The effects were evaluated by the dermatitis severity score (DSS), the spleen index, the serum immunoglobulin E (IgE) concentration, trans-epidermal water loss (TEWL), histological findings, and the expression levels of cytokine mRNA and filaggrin protein in skin tissue.
Results
Topical application of PNs significantly reduced the DSS, the spleen index, the serum IgE concentration, and TEWL compared with the control. Additionally, histopathological analysis showed that PNs reduced epidermal and dermal thickness, the mast cell count, collagen deposition, and eosinophil infiltration in the dermis. Moreover, PNs significantly downregulated the expression of key inflammatory cytokines, including interleukin (IL)-4, IL-5, IL-13, IL-25, IL-33, and thymic stromal lymphopoietin (TSLP), in affected skin tissue. Immunohistochemical (IHC) staining and Western blot revealed that PNs inhibited DNCB-induced suppression of filaggrin. A combination of hyaluronic acid (HA) and PNs showed enhanced efficacy compared with PNs alone, particularly for reducing the serum IgE concentration and TEWL and increasing filaggrin expression.
Conclusion
These results suggest that PNs are potential candidates to treat AD because they possess anti-inflammatory properties and improve skin barrier function.
1 Introduction
Atopic dermatitis (AD) is a highly prevalent, chronic inflammatory skin disorder that affects patients over extended periods. It is a representative refractory disease characterized by skin barrier dysfunction and aberrant immune responses [1-4]. Immune dysfunction in AD can be represented by increased expression of interleukin (IL)-6 and IL-1β and changes in immunoglobulin E (IgE)-mediated hypersensitivity [5]. This renders the skin more sensitive and vulnerable to irritants, allergens, and other environmental stimuli. AD is treated by avoiding factors that exacerbate the condition, enhancing the skin barrier through adequate skincare, and treating the underlying skin inflammation.
Polynucleotides (PNs) are natural and highly purified DNA biopolymers extracted from trout gonads [6]. These molecules exhibit a strong affinity for water, forming a gel that provides prolonged hydration and replenishes viscosity. They are raw materials for medical devices such as dermal fillers and intra-articular injections. Polydeoxyribonucleotides (PDRNs), DNA-based PN, stimulate skin regeneration and repair by facilitating cellular proliferation, differentiation, and migration, which are essential to maintain skin barrier integrity [7]. In patients with oral mucositis, PDRNs exert positive effects by selectively activating the adenosine A2A receptor, a key regulator of inflammation, cell growth, and angiogenesis [8]. Additionally, PDRNs potentially mitigate neuronal cell death by downregulating the JAK/STAT pathway and inflammatory responses in an in vitro ischemia/reperfusion injury model [9].
Hyaluronic acid (HA) is a high-molecular-weight compound comprising repeating β-1,4-D-glucuronic acid and β-1,3-N-acetylglucosamine units and has distinct physicochemical properties. HA possesses excellent viscoelasticity, a hydroscopic nature, remarkable moisture retention capacity, high biocompatibility, and hygroscopic properties [10-12]. It influences the wound healing process by controlling skin inflammation and remodeling the skin [13]. HA alleviates inflammatory responses in AD mouse models, suggesting it may be useful for the treatment of AD [14].
Clinical trials targeting patients with knee osteoarthritis have shown that injections of PN and HA exhibit good tolerability and efficacy in alleviating joint pain, with the combination relieving pain and controlling wound contraction more effectively than either substance alone [15]. Moreover, PNs combined with HA are superior to HA alone for promoting human fibroblast proliferation and extracellular matrix production [15-17].
No study has evaluated the application of PNs or a PN+HA complex as a potential therapy for AD. Therefore, this study was designed to investigate the effects of PNs and the synergistic effects of PNs and HA on skin barrier function and cutaneous inflammation in AD.
2 Materials and Methods
2.1 Animals and Reagents
All animal experiments in this study were approved by the Institutional Animal Care and Use Committee (IACUC No. 2022-02-327) of Asan Institute for Life Sciences (Asan Medical Center, Korea). Six-week-old male BALB/c mice were obtained from Orient Bio Inc. (Seongnam, Korea) and maintained as previously described [18]. PNs (14 mg/mL), HA (6 mg/mL), and PN+HA were supplied by BRPHARM (Wonju, Korea). 2,4-Dinitrochlorobenzene (DNCB) and dexamethasone (DEX) were sourced from Sigma-Aldrich (St. Louis, MO, USA).
2.2 Experimental Design
The therapeutic effects of PNs on AD were evaluated using a DNCB-induced AD model and 200 µL of 0.01% DEX as the positive control. The concentration of DEX was determined based on a study by Park et al. [19]. After 1 week of acclimation, the dorsal region of the mice was carefully shaved, and the mice were randomly assigned to six groups, with six mice per group: normal (unstimulated), control (DNCB-stimulated and saline-treated), PN (DNCB-stimulated and 200 µL of 14 mg/mL PN-treated), HA (DNCB-stimulated and 200 µL of 6 mg/mL HA-treated), PN+HA (DNCB-stimulated and PN+HA-treated), and DEX (DNCB-stimulated and 0.01% DEX-treated group). One day after shaving, mice (except those in the normal group), were sensitized by painting their shaved back skin with 100 µL of 1% DNCB prepared in acetone/olive oil (3:1, v/v) every day for 5 days. Subsequently, after a latency period of 3 days, the mice were challenged by painting 100 µL of 0.5% DNCB on the same area every 3 days for 3 weeks. Mice in the normal group were treated with a 1:1 mixture of acetone and olive oil. The experimental model and DNCB concentration were established based on previous studies [20-22], with slight modifications to optimize the protocol for this study. To examine the potential beneficial effects of PN+HA on AD, the back of each mouse (except those in the normal group) was topically treated with saline, PN, HA, PN+HA, or DEX every day after the latency period (Figure 1A). Mice in the normal group remained untreated and monitored until the end of the experiment. On Day 30, the mice in all groups were sacrificed.
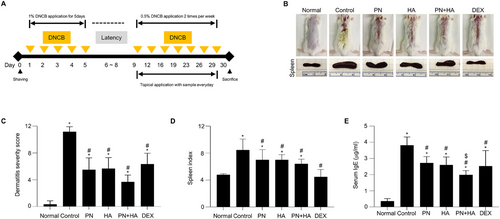
Experimental schedule and effect of PNs on AD symptoms in a DNCB-induced AD model.
(A) Experimental schedule. (B) Dorsal skin lesions and spleen from each group on Day 30. (C) Dermatitis severity score is quantified based on edema, erythema/hemorrhage, oozing/crust, and dryness. (D) Spleen index (spleen weight [mg]/body weight [g]). (E) Serum IgE concentration was determined via an enzyme-linked immunosorbent assay. Data are presented as the mean ± SD (n = 6). * p < 0.05 versus normal group. # p < 0.05 versus control group. $ p < 0.05 PN group versus PN+HA group. Normal: unstimulated group; control: DNCB-stimulated and saline-treated group; PN: DNCB-stimulated and PN-treated group; HA: DNCB-stimulated and HA-treated group; PN+HA: DNCB-stimulated and PN+HA-treated group; DEX: DNCB-stimulated and 0.01% dexamethasone-treated group.
2.3 Evaluation of the Severity of Dermatitis
After sacrificing the mice on Day 30, the dermatitis severity score (DSS) was determined based on four clinical parameters: edema, erythema/hemorrhage, oozing/crust, and dryness. Each symptom was graded from 0 to 3 (0 = none, 1 = mild, 2 = moderate, and 3 = severe), and the total DSS was obtained by summing these scores, yielding a maximum score of 12. To ensure rigor and minimize bias, the evaluations were conducted in a blinded manner by independent assessors who were unaware of the treatment groups.
2.4 Measurement of Trans-Epidermal Water Loss (TEWL)
On Day 30, skin barrier function was evaluated by measuring the TEWL using the GPSkin device (GPower, Seoul, Korea) on the dorsal skin of each mouse as previously described [18].
2.5 Enzyme-Linked Immunosorbent Assay (ELISA)
Whole blood was obtained via orbital collection from mice and subsequently subjected to centrifugation at 5000 × g for 10 min to isolate serum. The IgE level in serum was measured using a Mouse IgE ELISA Kit (Mybiosource, San Diego, CA, USA) following the manufacturer's protocol.
2.6 Histological and Immunohistochemical (IHC) Staining
On Day 30, spleens were collected from the sacrificed mice and weighed using an electronic analytical balance. The dorsal skin tissues were excised and fixed as previously described [18]. Tissue sections were stained with hematoxylin and eosin (H&E) to evaluate epidermal and dermal thickness, as well as the eosinophil infiltration count. Toluidine blue (TB) staining was performed to quantify mast cell infiltration, and Masson's trichrome (MT) staining was performed to identify connective tissue elements, collagen, and muscle fibers. Skin sections were immunostained with an anti-filaggrin antibody (1: 500, orb10662; Biorbyt, Durham, NC, USA) according to the manufacturer's guidelines. The slide images were scanned and analyzed as previously described [18].
2.7 Real-Time RT-PCR Analysis
Total RNA was isolated from dorsal skin tissues using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer's protocol. Complementary DNA was synthesized from these total RNA preparations using random primers and SuperScript II reverse transcriptase (Thermo Fisher Scientific, Waltham, MA, USA). Quantitative real-time RT-PCR was then conducted on a Roche LightCycler 96 system (Roche, Mannheim, Germany) with SYBR Green I Master Mix (BIOFACT, Daejeon, Korea). The primer sequences of IL-4, IL-5, IL-13, IL-25, and IL-33 were designed based on previous study [23]. In addition, primers for thymic stromal lymphopoietin (TSLP) (forward 5′-GCAAATCGAGGACTGTGAGAGC-3′, reverse 5′-TGAGGGCTTCTCTTGTTCTCCG-3′), COL1A1 (forward 5′-CGATGGATTCCCGTTCGAGT-3′, reverse 5′-TTCGATGACTGTCTTGCCCC-3′), and glyceraldehyde 3-phosphate dehydrogenase GAPDH (forward 5′-CATCACTGCCACCCAGAAGACTG-3′, reverse 5′-ATGCCAGTGAGCTTCCCGTTCAG-3′) were designed using the Primer3 software.
2.8 Western Blot Analysis
Skin tissues were homogenized in RIPA buffer (Biosesang, Seongnam, Korea) supplemented with a protease inhibitor cocktail (Roche). Protein concentrations were measured using a Bradford assay kit (Thermo Fisher Scientific). Equal amounts of protein were separated via SDS-PAGE, transferred onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA), and probed with a filaggrin antibody (1:500, orb10662, Biorbyt). Protein bands were detected through an enhanced chemiluminescence detection system (Thermo Fisher Scientific) and their relative intensities were analyzed using ImageJ software.
2.9 Statistical Analysis
Statistical analyses were performed using SPSS21 (IBM Corporation, Armonk, NY, USA). Group comparisons were conducted using Student's t-test and one-way analysis of variance (ANOVA) to assess statistical significance. A p value below 0.05 was regarded as statistically significant.
3 Results
3.1 Effect of PNs on AD Symptoms in a DNCB-Induced AD Model
After 30 days of induction according to the schedule, morphological changes were observed (Figure 1B), and the spleen index (spleen weight [mg]/body weight [g]) and serum IgE concentration were measured. The DSS, encompassing erythema, edema, and eczematous skin lesions, was significantly lower in the PN, HA, PN+HA, and DEX groups than in the control group (PN: 5.5 ± 0.7, HA: 5.7 ± 0.7, PN+HA: 3.7 ± 0.4, and DEX: 6.3 ± 0.7 vs. control: 11.2 ± 0.3, p < 0.05; Figure 1C). The spleen index was markedly elevated in the control group than in the normal group (control: 8.43 ± 0.67 g vs. normal: 4.77 ± 0.67 g, p < 0.05; Figure 1D). The spleen index was significantly lower in the PN, HA, PN+HA, and DEX groups than in the control group (PN: 7.02 ± 0.61 g, HA: 7.00 ± 0.32 g, PN+HA: 6.42 ± 0.29 g, and DEX: 4.48 ± 0.45 g vs. control, p < 0.05). The serum IgE concentration was significantly higher in the normal group (control: 3.8 ± 0.2 µg/mL vs. normal: 0.4 ± 0.1 µg/mL, p < 0.05; Figure 1E). By contrast, the serum IgE concentration was significantly lower in the PN, HA, PN+HA, and DEX groups than in the control group (PN: 2.7 ± 0.2 µg/mL, HA: 2.6 ± 0.2 µg/mL, PN+HA: 2.0 ± 0.1 µg/mL, and DEX: 2.5 ± 0.4 µg/mL vs. control, p < 0.05). The serum IgE concentration was significantly lower in the PN+HA group compared to the PN group (p = 0.003).
3.2 Effect of PNs on TEWL in a DNCB-Induced AD Model
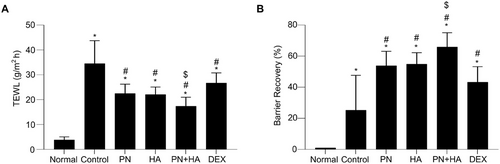
Changes in skin barrier function were observed by calculating TEWL in the DNCB-induced mouse model, because decreased skin barrier function leads to elevated TEWL. On Day 30, TEWL was significantly elevated in the control group than the normal group (control: 34.5 ± 3.8 g/m2h vs. normal 3.9 ± 0.5 g/m2h, p < 0.05; Figure 2A). TEWL was significantly lower in the PN, HA, PN+HA, and DEX groups than in the control group (PN: 22.5 ± 1.5 g/m2h, HA: 22.1± 1.2 g/m2h, PN+HA: 17.4 ± 1.5 g/m2h, and DEX: 26.7 ± 1.7 g/m2h vs. control, p < 0.05). Additionally, the rate of barrier recovery, indicated by a return of TEWL to baseline, was significantly higher in the PN, HA, PN+HA and DEX groups than in the control group (PN: 53.9 ± 3.8%, HA: 54.9 ± 3.0%, PN+HA: 65.9 ± 3.7%, and DEX: 43.3 ± 4.1% vs. control: 25.3 ± 9.1%, p < 0.05; Figure 2B). Reduction of TEWL and the rate of barrier recovery were significantly better in the PN+HA group than in the PN group (p = 0.034 and p = 0.047, respectively).
3.3 Effect of PNs on Epidermal and Dermal Thickness in a DNCB-Induced AD Model
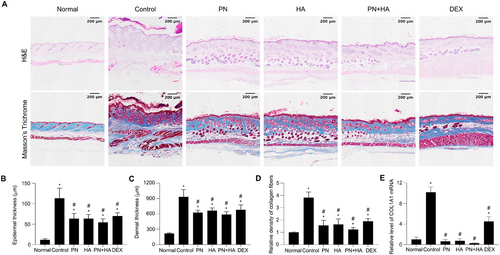
To confirm the histological effects of PNs on the DNCB-induced AD model, dermal and epidermal thickness were determined via H&E staining. The epidermis and dermis were abnormally thickened in the control group and pronounced parakeratosis was observed (Figure 3A, upper panel). Epidermal thickness was significantly increased in the control group compared to the normal group (control: 113.4 ± 10.1 µm vs. normal: 11.6 ± 1.2 µm, p < 0.05; Figure 3B) and was significantly reduced in the PN, HA, PN+HA, and DEX groups (PN: 63.5 ± 5.3 µm, HA: 63.5 ± 4.1 µm, PN+HA: 54.3 ± 3.6 µm, and DEX: 70.1 ± 3.2 µm vs. control, p < 0.05). Moreover, dermal thickness was significantly elevated in the control group compared to the normal group (control: 930.5 ± 58.4 µm vs. normal: 216.7 ± 5.2 µm, p < 0.05; Figure 3C) and was significantly reduced in the PN, HA, PN+HA, and DEX (PN: 624.3 ± 19.1 µm, HA: 661.1 ± 22.3 µm, PN+HA: 585.6 ± 23.6 µm, DEX: 680.4 ± 38.8 µm, vs. control, p < 0.05). Epidermal and dermal thickness was lower in the PN+HA group than in the PN group (p = 0.181 and p = 0.231, respectively); however, these differences were not statistically significant.
3.4 Effect of PNs on Collagen Deposition in a DNCB-Induced AD Model
MT staining was performed to assess collagen deposition and fibrosis in DNCB-induced AD lesions. Collagen fibers were distinctly stained blue, whereas the background appeared red. The normal group had a regular and dense collagen layer in the dermal layer, whereas the control group had an overly thickened dermal layer, and the collagen layer was stained dark blue due to unbalanced collagen fiber formation (Figure 3A, lower panel). The relative density of collagen fiber staining in AD skin lesions was significantly lower in the PN, HA, PN+HA, and DEX groups than in the control group (PN: 1.6 ± 0.2, HA: 1.6 ± 0.2, PN+HA: 1.2 ± 0.1, and DEX: 1.9 ± 0.1 vs. control: 3.8 ± 0.2, p < 0.05; Figure 3D). DNCB-induced collagen deposition was inhibited more in the PN+HA group than in the PN group (p = 0.092); however, this difference was not statistically significant. To further evaluate collagen deposition, the mRNA expression level of collagen (COL1A1) was measured by real-time RT-PCR (Figure 3E). Collagen mRNA expression was significantly elevated in the control group compared to the normal group, consistent with the histological observations (p < 0.05). Collagen mRNA expression was significantly lower in the PN, HA, and PN+HA groups than in the control group, mirroring the reductions observed in histological analysis of collagen deposition.
3.5 Effect of PNs on Mast Cell and Eosinophil Infiltration in DNCB-Induced AD Model
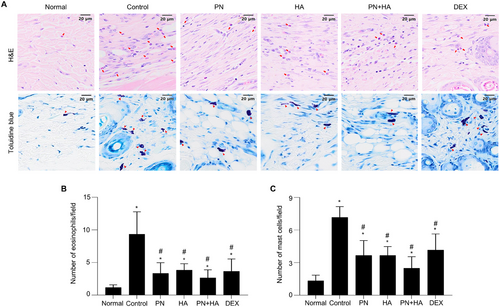
To assess the effect of PNs on immune cell infiltration in the skin of mice with DNCB-induced AD, dorsal skin sections were stained with H&E and TB to evaluate eosinophil and mast cell infiltration, respectively (Figure 4A). Immune cell infiltration into the dermal layer was significantly increased in the control group compared to the normal group (number of eosinophils per field: control: 9.2 ± 1.4 vs. normal: 1.2 ± 0.2, p < 0.05; number of mast cells per field: control: 7.1 ± 0.4 vs. normal: 1.3 ± 0.1, p <0.05). The number of eosinophils per field was significantly lower in the PN, HA, PN+HA, and DEX groups than in the control group (PN: 3.4 ± 0.6, HA: 3.8 ± 0.4, PN+HA: 2.6 ± 0.5, and DEX: 3.6 ± 0.7 vs. control, p < 0.05; Figure 4B). Similarly, the number of mast cells per field was significantly lower in the PN, HA, PN+HA, and DEX groups than in the control group (PN: 3.4 ± 0.3, HA: 3.8 ± 0.4, PN+HA: 2.6 ± 0.5, and DEX: 4.1 ± 0.6 vs. control, p < 0.05; Figure 4C). DNCB-induced mast cell and eosinophil infiltration was lower in the PN+HA group than in the PN group (p = 0.128 and p = 0.441, respectively); however, these differences were not statistically significant.
3.6 Effect of PNs on mRNA Expression of Inflammatory Cytokines in a DNCB-Induced AD Model
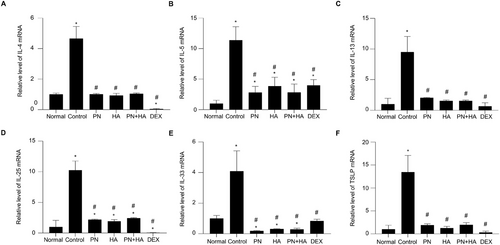
To determine whether PNs inhibit mRNA expression of AD-related cytokines, we measured mRNA expression of IL-4, IL-5, IL-13, IL-25, IL-33, and TSLP in skin tissue by real-time RT-PCR (Figure 5A–F). The mRNA expression of these inflammatory cytokines was significantly upregulated in the DNCB-induced group compared to the normal group, with fold increases in IL-4 (4.66 ± 0.32, p < 0.05), IL-5 (11.37 ± 0.90, p < 0.05), IL-13 (9.50 ± 1.04, p < 0.05), IL-25 (10.26 ± 0.61, p < 0.05), IL-33 (4.10 ± 0.54, p < 0.05), and TSLP (13.44 ± 1.49, p < 0.05). However, the PN, HA, PN+HA, and DEX treatments significantly reduced the mRNA levels of these cytokines: IL-4 (PN: 1.01 ± 0.02, HA: 0.93 ± 0.06, PN+HA: 1.04 ± 0.02, and DEX: 0.04 ± 0.01), IL-5 (PN: 2.82 ± 0.40, HA: 3.85 ± 0.60, PN+HA: 2.83 ± 0.56, and DEX: 3.98 ± 0.38), IL-13 (PN: 2.03 ± 0.03, HA: 1.53 ± 0.07, PN+HA: 1.53 ± 0.06, and DEX: 0.66 ± 0.23), IL-25 (PN: 2.19 ± 0.04, HA: 1.93 ± 0.11, PN+HA: 2.44 ± 0.04, and DEX: 0.05 ± 0.02), IL-33 (PN: 0.19 ± 0.01, HA: 0.32 ± 0.01, PN+HA: 0.29 ± 0.03, and DEX: 0.84 ± 0.05), and TSLP (PN: 1.93 ± 0.11, HA: 1.27 ± 0.15, PN+HA: 1.98 ± 0.19, and DEX: 0.32 ± 0.12), all compared to the control group (p < 0.05).
3.7 Effect of PNs on the Filaggrin Level in a DNCB-Induced AD Model
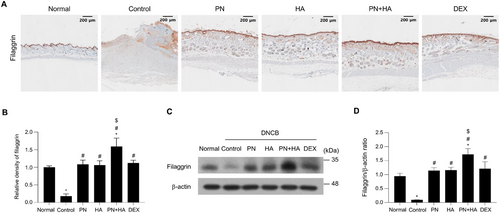
IHC staining of filaggrin was conducted to assess the effect of PNs on expression of skin barrier function-associated proteins in the epidermis of DNCB-induced AD mice because DNCB reduces production of proteins such as filaggrin [24]. The epidermal layer was complete and dense with high filaggrin expression in the normal group, however, in the control group, the epidermis was thinner with decreased expression of filaggrin (Figure 6A). Filaggrin expression was quantitatively analyzed by performing densitometry using ImageJ software within the same area, allowing precise evaluation of the staining intensity. The relative density of filaggrin staining was significantly lower in the control group than in the normal group (control: 0.18 ± 0.03 vs. normal: 1.00 ± 0.02, p < 0.05; Figure 6B) and was significantly increased in the PN, HA, PN+HA, and DEX groups (PN: 1.08 ± 0.05, HA: 1.06 ± 0.5, PN+HA: 1.59 ± 0.10, and DEX: 1.12 ± 0.03 vs. control, p < 0.05). Filaggrin expression was significantly higher in the PN+HA group than in the PN group (p = 0.001). To confirm these findings, Western blot analysis was conducted to evaluate filaggrin protein expression levels in dorsal skin tissue lysates (Figure 6C,D). These findings showed a similar trend as the IHC findings, with filaggrin expression significantly lower in the control group than in the normal group. The filaggrin protein level was significantly higher in the PN, HA, PN+HA, and DEX groups than in the control group and was highest in the PN+HA group, consistent with the results of IHC analysis.
4 Discussion
AD is a chronic disease characterized by skin dryness, skin barrier dysfunction, and cutaneous inflammation [25]. Regardless of the severity of AD, topical treatments, especially glucocorticoids, are the mainstay of AD therapy [26-28]. However, topical glucocorticoids have significant side effects and may worsen symptoms upon cessation, which often renders them unsuitable for long-term use [29]. Therefore, research is underway to identify novel topical drugs that are both effective and safe for long-term use.
PNs have been investigated in various medical fields for their anti-inflammatory and regenerative effects [8, 9]. In this study, we evaluated whether PNs downregulate immune responses in a DNCB-induced AD animal model. Our results showed that PN treatment decreased the development of AD skin lesions and positively affected other AD characteristics, such as increased spleen size, epidermal and dermal thickness, collagen deposition, and eosinophil and mast cell infiltration, in the skin of a DNCB-induced AD mouse model. In addition, PNs restored the mouse skin barrier function that was impaired by DNCB. Our study confirmed that HA also ameliorates AD symptoms in mice, which is consistent with previous reports [14]. It also demonstrated that the efficacy of HA was similar to that of PNs. Moreover, the therapeutic effects of PNs were further enhanced by adding HA. HA improves AD by regulating the PI3K/Akt pathway, a key inflammatory signaling pathway [14]. PNs and HA may have synergistic effects, regulating different inflammatory pathways. However, the molecular mechanisms through which PNs improve AD have not been reported; therefore, further studies are warranted to clarify why PN and HA have synergistic effects on AD. We acknowledge that the molecular basis for this is insufficiently explained in the current study. To address this limitation, we propose future investigations that aim to elucidate how PNs and HA interact with inflammatory pathways and contribute to skin barrier restoration at the molecular level. Such studies could provide deeper insights into the mechanisms underlying their combined effects and further validate their potential as a synergistic therapy for AD.
TEWL is a direct indicator of skin barrier function, and successful treatment of AD reduces TEWL [30]. In this study, TEWL was higher in DNCB-induced AD mice than in the normal group. However, PN-treated mice had significantly lower TEWL, indicative of improved skin barrier function. This effect was much more pronounced in the PN+HA group than in those treated with HA and PN groups. Although TEWL varies depending on the hydration status of skin, our findings suggest the potential role of PNs and HA in restoring skin barrier function in AD.
Cytokine dysregulation is a critical feature of AD. In this study, the levels of IL-4, IL-5, IL-6, IL-25, IL-33, and TSLP were significantly elevated in DNCB-induced AD mice, consistent with the roles of these cytokines in promoting inflammation and impairing skin barrier function. IL-4 and IL-5 are hallmark Th2 cytokines that amplify immune responses in AD, while IL-6 is a key pro-inflammatory mediator involved in activating downstream pathways, such as mitogen-activated protein kinase and nuclear factor-κB pathway, to promote cytokine production [31, 32]. As alarmins, IL-25 and IL-33, are secreted in response to epithelial damage and activate Th2 inflammation by interacting with immune cells, such as mast cells and eosinophils [31-33]. TSLP, which is secreted by keratinocytes, is a crucial upstream cytokine that transforms naive T cells into Th2 cells and contributes to the pathogenesis of AD through STAT pathway activation [31]. Our results show that PN and PN+HA treatment significantly reduced the expression levels of these cytokines, indicating their potential role in modulating inflammatory signaling and restoring immune homeostasis. Notably, the reduction of the TSLP levels suggests that PN and HA may mitigate TSLP-mediated pruritus and inflammation, further supporting their therapeutic potential.
An Elevated serum IgE level due to various environmental allergens and autoantibodies is often observed in patients with AD [5, 34]. In our DNCB-induced AD mouse model, the serum IgE concentration was reduced after PN treatment. As the largest secondary lymphoid organ, the spleen indicates the severity of AD via the proliferation of its cells which is considered an index of the hypersensitivity reaction. In the present study, the spleen index was significantly increased in the DNCB-induced AD mouse model, but was markedly reduced after PN treatment, indicative of the immunomodulatory effect of PNs. The absence of immunosuppression with such effects makes PNs a promising treatment for AD. These findings were also supported by histopathological analysis. Infiltration of immune cells, such as mast cells, is a representative symptom of AD [35]. Eosinophils are key immune cells that collaborate with mast cells and play a central role in eliciting allergic responses [36]. Both mast cells and eosinophils are recruited to inflamed dermis, where their activation exacerbates allergic skin conditions [37, 38]. PNs inhibited immune cell infiltration in AD skin lesions, and PN+HA inhibited immune cell infiltration more than PNs alone, although the difference was not statistically significant. Previous reports indicated that the number of mast cells and eosinophils in skin tissue of patients with AD decreases after therapy [39, 40]. Based on our results, topical therapy using PNs and HA is likely to be clinically effective.
Filaggrin is a key structural protein crucial for maintaining skin barrier integrity. Under inflammatory conditions, its expression decreases, compromising the skin barrier and contributing to the development of allergic skin disorders, including dermatitis and psoriasis [41, 42]. According to the IHC assay, filaggrin protein expression was effectively restored by the application of PNs and PN+HA. Previous reports indicated that filaggrin expression, which has been reduced by various stimuli, increases with improvement of AD symptoms [43-46]. Hence, increased expression of filaggrin upon treatment with PNs and PN+HA provides valuable molecular evidence that these substances are candidates for topical AD treatments.
One limitation of this study is the relatively small group size (n = 6), which reduces the statistical power to detect significant differences between groups. Although many of the observed trends were consistent and aligned with the hypothesized effects, some results did not achieve statistical significance. This limitation underscores the need for future studies with larger sample sizes to confirm and strengthen the findings presented in this study.
In conclusion, PNs efficaciously restore the skin barrier by recovering filaggrin expression, exert anti-inflammatory effects, and normalize immune dysregulation in the DNCB-induced AD mouse model. Moreover, PN in combination with HA showed superior efficacy than PNs alone in terms of enhancing filaggrin expression and overall therapeutic outcomes. These findings suggest that PN treatment may aid in developing more reliable therapeutic strategies for patients with AD. Additional comprehensive in vitro and clinical studies are warranted to further elucidate the therapeutic potential of PNs for AD.
Consent
All authors have read and approved the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
No data are availible for this study as no datasets were generated or analyzed during the current research.




