Functional connectivity characteristics of the brain network involved in prickle perception of single fiber stimulation
Abstract
Background
The complex network connections, information transmission and organization play key roles in brain cognition on sensory stimulation. Previous studies showed that several brain regions of somatosensory, motor, emotional, cognitive, etc. are linked to fabric-evoked prickle. But the functional connectivity characteristics of the brain network involved in prickle perception is still unclear.
Materials and Methods
In the present study, resting state fMRI (functional magnetic resonance imaging) with functional connectivity analysis was adopted to build the initial brain functional network, and task fMRI with psychophysiological interaction analysis was employed to investigate modulation features of prickling task to functional connections in the brain network.
Results
The results showed that, in resting state, six groups or sub-networks can be identified in the prickle network, and when the subjects performed the prickling task, functional connectivity strength between some seed regions (e.g., somatosensory regions and precuneus, emotional regions and the prefrontal cortex, etc.) in the network increased.
Conclusion
Combining resting-state fMRI with task fMRI is a feasible and promising method to study functional connectivity characteristics of the brain network involved in prickle perception. It is inferred that the “itch” ingredient of prickle sensation was transmitted from somatosensory cortices to precuneus, and emotional attribute (e.g., pain) from somatosensory cortices to the prefrontal cortex and at last to emotional regions.
1 INTRODUCTION
Fabric-evoked prickle is a kind of complex sensation which is caused by the mechanical stimulation of fiber ends from fabric surface against to human skin.1 Many researchers studied the causes,2 evaluation methods,3 and elimination methods4 of it. In recent years, in order to figure out the neural mechanism of prickle perception, advanced technologies of neurosciences were used to study brain responses to cutaneous prickling stimulation.5 For example, EEG (electroencephalogram) and ERPs (event-related potentials) techniques, because of high temporal resolution, were often used by researchers to study the contact comfort of fabric.6 An EEG study7 showed that the percentages of θ, α, and β rhythm can be used as indexes to evaluate prickle sensation of fabric. But it is regrettable that the spatial resolution of EEG technology is not high enough,8 which leads to its limited application in the accurate localization of brain functional areas and brain network analysis.
The technology of MRI (magnetic resonance imaging) has been adopted by more and more researchers depending on its high spatial resolution and radiation-free performance. Structural MRI is usually employed to examine the tissue structure of human body for lesions, such as dermopathy.9, 10 In 1992, Ogawa et al.11 developed BOLD (blood oxygen level dependent) imaging method based on MRI, called functional MRI (fMRI), using which brain functional responses to sensory stimulation can be observed in various brain regions. Now fMRI has become one of the most widely used neuroimaging technology.12 Previously, task fMRI was used to detect brain responses when subjects received prickling stimulation to their skin,13 showing that brain regions involved in prickle perception are mainly the primary somatosensory cortex (S1), secondary somatosensory cortex (S2), motor cortex, precuneus, insular cortex (IC), anterior cingulate cortex (ACC), prefrontal cortex, and so on. However, studies have shown that the brain is a complex system with various networks, in which neural activities between various functional regions are not independent of each other, but there are specific connections, and the complex network connections, information transmission and organization play key roles in brain cognition on sensory stimulation.14, 15
Now in the present study, an experiment combining task fMRI and resting state fMRI was conducted to further investigate functional connections in the brain network involved in prickle perception of single fiber stimulation.
2 MATERIALS AND METHODS
2.1 Subjects
Ten healthy right-handed male subjects, with the age range of 22–34 years (average age of 27.4 years), took part in the fMRI experiment. Before the experiment, all the subjects were trained simply about the scanning procedure and gave written informed. The study was conducted in accordance with the Declaration of Helsinki, and the ethics committee of Shanghai Ruijin Hospital approved this study.
2.2 Experimental procedures
The fMRI experiment was performed at Shanghai Ruijin Hospital on a GE 3.0T clinical whole body MRI scanner with a standard head coil. The experiment mainly includes three stages, namely 3D T1, Run 1, and Run 2, as shown in Figure 1. The subjects were asked to keep their bodies (especially their heads) still throughout all the three stages. At the stage of 3D T1, the three-dimensional (3D) anatomical images (T1-weighted images) of their brains were collected using the scanning sequence of dimension-spoiled gradient recalled echo (T1WI). Then, at the stages of Run 1 and Run 2, BOLD (Blood Oxygen Level-Dependent) signals of their brains were gathered using the single-shot EPI (echo planar imaging) sequence to acquire the functional images.
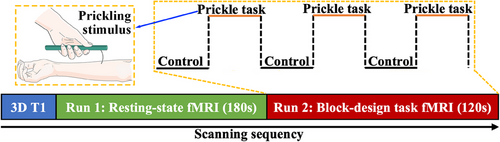
In particular, Run 1 is a resting-state fMRI stage lasting 180 s, in which, the subjects were asked to just close their eyes and keep awake, and Run 2 is a block-design task fMRI stage (120 s) same as the previous study13, including three blocks, and every block contains two conditions, Control (20 s) and Prickle task (20 s). In the condition of control, the subjects were also asked to just close their eyes and keep awake, same as Run 1 stage. While in the condition of Prickle task, as shown in Figure 1, the specified skin sites of the subjects’ right volar forearm (hairy skin) were pricked by a single-fiber stimulus, with the frequency of two times per second, and the subjects were required to concentrate on perceiving the stimulation.
2.3 Neuroimaging data processing
The acquired functional and anatomical data were processed using CONN16, 17 and SPM.18 The data were firstly preprocessed including realignment,19, 20 slice timing correction,21 outlier detection, direct segmentation and MNI-space normalization,22-24 and smoothing, of which, functional data were smoothed using spatial convolution with a Gaussian kernel of 4 mm full width half maximum (FWHM). Then, the functional data were denoised25, 26 including the regression of potential confounding effects characterized by white matter timeseries, CSF (Cerebrospinal Fluid) timeseries, motion parameters and their first order derivatives,27 outlier scans,28 session and task effects and their first order derivatives, and linear trends, followed by bandpass frequency filtering of the BOLD timeseries29 between 0.008 and 0.09 Hz.
After that, 30 seed regions or ROIs (regions of interest) involved in prickle perception of single fiber stimulation30 were selected from the Harvard-Oxford cortical structural Atlas (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases). For the resting-state condition (Run 1), ROI-to-ROI connectivity matrix was estimated characterizing the patterns of functional connectivity between the 30 ROIs, to form a brain functional network of prickle perception which could be called the prickle network. At last, for the prickle-task condition (Run 2), generalized psychophysiological interaction (gPPI) analysis31, 32 was used to study the changes of functional connectivity among the 30 ROIs in the prickle network when the subjects performing the prickle perceiving task.
3 RESULTS AND DISCUSSION
3.1 Functional connectivity within the prickle network in resting state
Results were reported after group-level analysis, which is based on Functional Network Connectivity (FNC) multivariate parametric statistics.33 The group-averaged ROI-to-ROI functional connectivity matrix (unthresholded) of the prickle network in resting state (Run 1) is shown in Figure 2, containing the selected 30 ROIs involved in prickle perception of single fiber stimulation. As shown in Figure 2, functional connectivity strength is represented by Fisher-transformed bivariate correlation coefficients (Fisher Z scores) estimated separately for each pair of ROIs, characterizing the association between their BOLD signal timeseries.
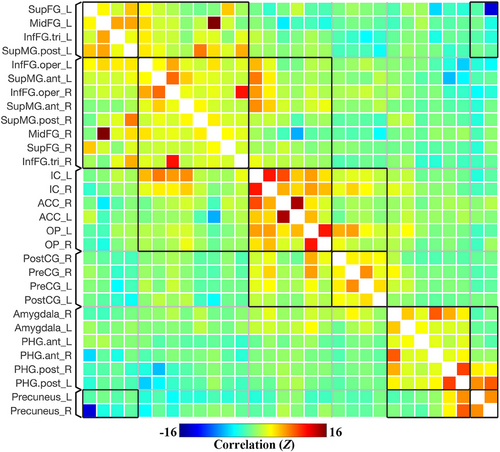
The results were thresholded using a combination of a p < 0.05 connection-level threshold, and a familywise corrected p-FDR < 0.05 cluster-level threshold (MVPA omnibus test).34 The connections (thresholded results of functional connectivity) among all 30 ROIs of the brain network in resting state is shown in Figure 3.
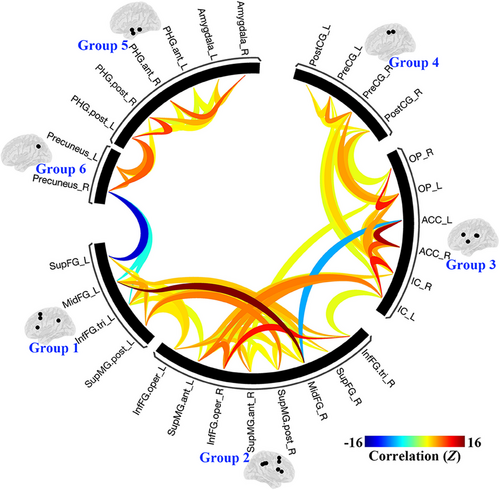
Overall, as shown in Figures 2 and 3, six groups or sub-networks were identified in the prickle network, and there are more connections and higher connectivity strength within than between each pair of sub-networks. Obviously, it can be explained by the process of FNC statistics in which the groups or sub-networks were identified using a complete-linkage hierarchical clustering procedure35 based on ROI-to-ROI anatomical proximity and functional similarity metrics.
Specifically, ROIs in Group 3 are mainly distributed near the insula, including the bilateral insular cortex (IC), parietal operculum cortex (OP), and anterior cingulate cortex (ACC), mainly involved in high-level somatic sensation36 and modulation of emotional responses.37, 38 Group 4 is made up of the bilateral precentral gyrus (PreCG) and postcentral gyrus (PostCG), the primary regions of the motor system and somatosensory system. Group 5 contains the bilateral amygdala and parahippocampal gyrus (PHG), which are parts of the limbic and paralimbic system and play important roles in emotion processing.39, 40 Group 6 only contains the bilateral precuneus, a particular brain region which plays a role in itch sensations and their brain processing,41 and is uniquely activated with itch and not pain.42
In addition, the difference between Group 1 and Group 2 seems hard to discern, because, in one hand, both contains brain regions of the prefrontal cortex and supramarginal gyrus (SupMG), and in the other hand, there are more connections between them than any other pairs of groups. Therefore, the two groups can be combined in this study, named Group 1&2, mainly involved in cognitive functions.43-45
3.2 Modulation of prickle task to connections within the prickle network
After group-level analysis in which cluster-level inferences were based on parametric statistics from Gaussian Random Field theory,46 the gPPI results of Seed-Based Connectivity (SBC) modulation during prickling task were thresholded using a combination of a cluster-forming p < 0.001 voxel-level threshold, and a familywise corrected p-FDR < 0.05 cluster-size threshold.47 Distribution of the SBC modulated clusters is shown in Table 1 and displayed in MNI brain template in Figure 4. If one SBC modulated cluster is discovered through gPPI analysis, it means that functional connectivity between the selected seed ROI and the cluster is modulated by the prickling task.
| Seed ROIs | SBC modulated brain regions | MNI coordinates (x, y, z) | Cluster size |
|---|---|---|---|
| S1 (PostCG) | L, SupOC, Precuneus | −18, −76, 38 | 31 |
| R, PreFC, SupFG | 20, 20, 46 | 17 | |
| R, SupMG | 54, −46, 34 | 16 | |
| S2 (OP) | R, PreFC, FP | 26, 44, 18 | 15 |
| R, Precuneus | 12, −40, 46 | 13 | |
| R, MedOC, Cuneal | 16, −86, 18 | 13 | |
| R, InfOC | 50, −68, −16 | 13 | |
| IC | R, PreFC, SupFG | 0, 10, 68 | 22 |
| L, SupOC | −12, −70, 58 | 19 | |
| R, PreFC, MidFG | 34, 14, 32 | 14 | |
| R, PreFC, FP | 28, 38, 38 | 13 | |
| L, PreFC, SupFG | −28, 0, 62 | 12 | |
| ACC | R, PreFC, InfFG | 44, 16, 20 | 12 |
- Abbreviations: FP, Frontal Pole; InfOC, Inferior Occipital Cortex; S1, Primary Somatosensory Cortex; S2, Secondary Somatosensory Cortex; PreFC, Prefrontal Cortex; SupOC, Superior Occipital Cortex; MedOL, Medial Occipital Lobe.
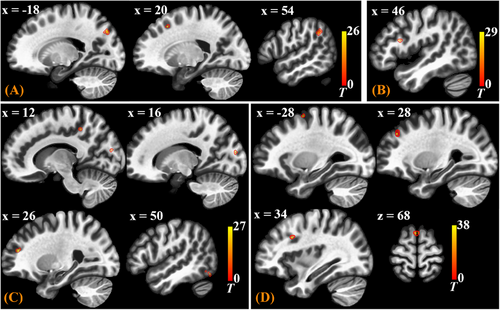
As shown in Table 1 and Figure 4, for the seed ROI of S1 (Primary Somatosensory Cortex, PostCG), there are three clusters discovered, mainly located in brain regions of the precuneus, prefrontal cortex (PreFC), and supramarginal gyrus (SupMG), meaning that when the subjects performed the task of perceiving the prickling stimulation from the single fiber against to their forearm skin, the functional connectivity strength between S1 and these brain regions increased. Notably, cluster with the largest size is in the precuneus. It is interesting that for the seed ROI of S2 (Secondary Somatosensory Cortex, OP), clusters were also discovered in the two regions, PreFC and precuneus, with the largest cluster in PreFC.
Prickle sensation evoked by mechanical irritation of fiber ends against to human skin is a combination of pain and itch, and is dominated by itch.30, 48 According to the previous analysis, as shown in Figure 3, at the resting state (Run 1), there was no connection survived after corrected between S1 (PostCG in Group 4) and precuneus (Group 6), and between S2 (OP in Group 3) and precuneus. It indicates that the precuneus plays an important role in perceiving prickle stimulation. Considering the special role of the precuneus in itch sensation, it can be boldly inferred that when the subjects performed the task of perceiving the prickling stimulation, the “itch” ingredient of prickle sensation was transmitted from S1 and S2 to precuneus.
Then for the situation of IC (Insular Cortex) and ACC (Anterior Cingulate Cortex) as seed ROIs, SBC modulated clusters were both mainly discovered in PreFC (Prefrontal Cortex), as shown in Table 1 and Figure 4. There were some connections between Group 3 and Group 1&2 at the resting state (Figure 3). Now the prickle task made the connections even stronger. Therefore, it can also be inferred that emotional attribute (e.g. pain) of the prickle sensation is transmitted from S1 and S2 to PreFC and at last to IC and ACC.
4 CONCLUSION
It is a feasible and promising method to study functional connectivity characteristics of the brain network involved in prickle perception by combining resting-state fMRI with task fMRI. In resting state, six groups or sub-networks can be identified in the prickle network. When the subjects performed the task of perceiving the prickling stimulation from the single fiber against to their forearm skin, functional connections between some seed regions (e.g., somatosensory regions and precuneus, emotional regions and the prefrontal cortex) in the network were modulated by the prickling task. It is inferred that the “itch” ingredient of prickle sensation was transmitted from somatosensory cortices to precuneus, and emotional attribute (e.g., pain) from somatosensory cortices to the prefrontal cortex and at last to emotional regions (e.g., insular cortex and anterior cingulate cortex). However, further studies and targeted experiments are needed to support the inference.
ACKNOWLEDGMENTS
This research was funded by the Shandong Provincial Natural Science Foundation (grant number ZR2020QF115), Scientific Research Fund of Zhejiang Provincial Education Department (grant number Y202354012, 113129A4F21075, 22200112-F), and Research Foundation of Zhejiang Sci-Tech University (grant number 21202299-Y).
CONFLICT OF INTEREST STATEMENT
None of the authors have any potential financial conflict of interest related to this manuscript.
ETHICS STATEMENT
The study was conducted in accordance with the Declaration of Helsinki, and the ethics committee of Shanghai Ruijin Hospital approved this study. Before the experiment, all the subjects were trained simply about the scanning procedure and gave written informed.
Open Research
DATA AVAILABILITY STATEMENT
Research data are not shared.