New insight into prurigo nodularis: Proadrenomedullin N-terminal 20 peptide mediates mouse mast cell activation via Mrgprb2
Yixin Shao, Zijing Xiao and Yinghong Jin have contributed equally to this work.
Abstract
Background
Prurigo nodularis (PN) is a chronic inflammatory skin disorder that is characterized by extremely itchy nodules. Proadrenomedullin N-terminal 20 (PAMP) activates mast cell degranulation via Mas-related G protein-coupled receptor X2 (MRGPRX2), which is associated with pruritus in allergic contact dermatitis. However, the mechanisms underlying the action of PAMP and MRGPRX2 in PN remain unclear.
Objective
To determine the role of PAMP-induced mast cell activation via MRGPRX2 (mouse homologous Mrgprb2) in PN.
Methods
The expression of PAMP and the number of MRGPRX2-expressing mast cells in the skin biopsies of patients with PN, atopic dermatitis (AD), and healthy participants were analyzed using immunohistochemistry and immunofluorescence, respectively. The biphasic response of PAMP9-20 mediated by Mrgprb2 in mouse peritoneal mast cells (PMC) was validated in vitro using qRT-PCR, ELISA, flow cytometry, and siRNA techniques.
Results
PAMP expression and the number of MRGPRX2+ mast cells in lesional PN skin, but not in AD, were elevated compared to healthy skin. PAMP9-20 mediates the immediate and delayed phase responses of PMC, such as degranulation, histamine and β-hexosaminidase release, and secretion of inflammatory factors such as CCL2, TNF-α, and GM-CSF. These effects were inhibited when Mrgprb2 expression was silenced. Silencing Mrgprb2 did not affect the biphasic response of PMC that was induced by IgE-FcεRI activation.
Conclusions
The results show that PAMP mediates mouse mast cell activation via Mrgprb2, which may be involved in the pathogenesis of PN. The PAMP/ Mrgprb2 pathway, independent of classical IgE signaling, could be developed as a candidate drug target for treating PN.
1 INTRODUCTION
Prurigo nodularis (PN) is a chronic, intractable, inflammatory skin disease that is characterized by itchy nodules.1 The exact pathogenesis of the disease remains unclear. Immune and neuronal dysregulation that are caused by the “scratch-pruritus” cycle may play major roles.2 Interestingly, approximately half of all patients with PN also have atopy or atopic dermatitis (AD).1, 3 The pathogeneses of AD and PN partially overlap, including hyperkeratosis, dermal Th2 inflammatory cell infiltration, and increased expression of IL-31.2 However, PN is more similar to a neurological skin disease than AD, with a higher intensity of pruritus and worse quality of life.4 Treatment of PN has always been challenging, and patients with PN often resist or cannot tolerate adverse reactions to traditional drug treatments.5 Recently, dupilumab, an IL-4Rα monoclonal antibody, has been approved for the treatment of PN by targeting type 2 immunity.6 The development of more effective therapies for PN remains an unmet medical need, and the identification of potential novel drug targets is essential for therapeutic advancement.
Histamine-dependent pruritus has been studied extensively. However, most patients with PN do not respond to antihistamine, suggesting the existence of a histamine-independent pruritus pathway.7 These alternative pathways are currently popular topics in the field of chronic pruritus. MAS-related G protein-coupled receptor X2 (MRGPRX2) is a G protein-coupled receptor that is primarily expressed in mast cells8 and plays an important role in pruritus, inflammation, and pseudoallergic reactions.9, 10 The number of MRGPRX2-expressing cells in the lesional skin of patients with chronic prurigo is correlated with disease severity. MRGPRX2 serum levels in patients with chronic prurigo correlate with disease severity and impaired quality of life.11 A variety of basic secretagogs, such as compound 48/80 and substance P, mediate mast cell degranulation and release pruritogens and inflammatory mediators by activating MRGPRX2.12 These findings suggest that MRGRPX2 acts as a bridge between mast cell function and PN pathogenesis.
Proadrenomedullin N-terminal 20 peptide (PAMP) is encoded by the adrenomedullin (ADM) gene and is involved in vasodilation, bronchiectasis, and angiogenesis.13 It is an agonist of human MRGPRX2 and has mouse homologous Mrgprb2.10, 12 Healthy controls experienced a distinct itch sensation and local erythema immediately after an intradermal injection of PAMP.14 Moreover, the expression of PAMP in the skin lesions of patients with allergic contact dermatitis (ACD) was elevated.9 ACD-associated itch in mice may be promoted by the activation of Mrgprb2 by PAMP9-20 (a more robust agonistic activity than PAMP), which induces the activation of mast cells to release tryptase and other pruritogens and excite a distinct itch-sensory neuron population.9 Our previous transcriptomic study found that ADM was significantly increased in prurigo-like lesions compared with eczematous lesions,15 suggesting that PAMP may play an important role in the pathogenesis of PN. However, its mechanism of action remains unclear.
This study investigated the properties of the PAMP/MRGPRX2 pathway and its role in the pathogenesis of PN. We demonstrated that the expression of PAMP and the number of MRGPRX2 expressing mast cells in lesional PN skin, but not in AD with severe itching, were increased compared with those in healthy skin. PAMP9-20 promoted mouse primary peritoneal mast cell activation via Mrgprb2 to induce a biphasic immune response, and these effects were independent of the IgE-FcεRI signaling. These findings provide evidence for a PN therapy based on PAMP/MRGPRX2 signaling.
2 METHODS
2.1 Participants’ recruitment, skin biopsies, and skin section preparation
Eighteen participants were enrolled at Huashan Hospital, Fudan University, Shanghai, China, with approval from the institutional ethics committee (No.2020-908). Written informed consent was obtained from all participants involved in the study. The inclusion criteria were age between 18 and 75 years, itch numerical rating scale (NRS) score ≥7, compliance with AD (Hanifin-Rajka criteria) or PN (European Prurigo Project criteria16) diagnosis, and the exclusion of severe concomitant illnesses. A 4 mm-diameter skin biopsy of the lower extremities was obtained from the lesional skin of six patients with PN, six patients with AD, and the normal skin of six age- and sex-matched healthy volunteers for both groups. All skin biopsy samples were fixed in 4% paraformaldehyde and embedded in paraffin. Four-micrometer-thick sections were dried and stored at room temperature. All sections were de-identified to avoid evaluation bias.
2.2 Reagents and antibodies
Rabbit anti-MRGPRX2 antibody (Cat# HPAD5520), Anti-Dinitrophenyl (DNP) mouse IgE (Cat# D8406), DNP-Human serum albumin (HSA), Bovine serum albumin, dimethyl sulfoxide, red blood cell lysis buffer, and p-nitrophenyl-N-acetyl-β-D glucosaminide were from Sigma-Aldrich (Darmstadt, Germany). Recombinant murine IL-3 and SCF were purchased from PeproTech (NJ). The rabbit anti-PAMP antibody (Cat# orb10050) was obtained from Biorbyt (Cambridge, UK). Alexa Fluor 488-conjugated avidin, fetal bovine serum, RPMI1640, Pen Strep, and DAPI were from Thermo Fisher (CA). The following reagents and antibodies were from Biolegend (CA): PE anti-mouse CD117(Cat# 105807), APC anti-mouse FcεRIα (Cat# 134315), and APC anti-mouse CD107a (Cat# 121613). PAMP9-20 was from Sangon Biotech (Shanghai, China).
2.3 Immunohistochemistry
All skin section slides were treated with 3% hydrogen peroxide at room temperature (RT) and then in a 100 °C water bath with Tris-EDTA antigen unmasking solution. After being blocked with a blocking solution (5% BSA, 0.05% Tween-20 in PBS) for 20 min, the slides were incubated overnight at 4°C in a humidified chamber with anti-PAMP antibody at 1:400 dilution. The slides were then incubated for 30 min at RT with horseradish peroxidase (HRP)-conjugated secondary antibody at a 1:2000 dilution. The slides were imaged using a Nikon Eclipse Ci-L microscope.
2.4 Immunofluorescence
Immunofluorescence staining was performed using the multiplex fluorescent tyramide signal amplification (TSA) system, as previously described.17 Skin tissue sections embedded in paraffin were boiled in 1 mmol Tris-EDTA (PH9.0) at 100°C for 15 min for antigen repair. The samples were then blocked with 5% BSA for 20 min at RT and incubated overnight with the following primary antibodies at 4°C: 1:500 anti-MRGPRX2 and 1:100 Alexa488-conjugated avidin. The samples were counterstained with try-cy3 tyrinosamin conversion reagent and DAPI. Images were acquired using a TissueFAXS Plus automated acquisition system.
2.5 Cell culture
Specific pathogen-free C57BL/6 male mice that were purchased from the Shanghai Laboratory Animal center at the age of 6–8 weeks were used. For the culture of peritoneal mast cells (PMC), peritoneal cells were washed from the peritoneal cavity and cultured in RPMI1640 medium supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 10 ng/ml IL-3, and 30 ng/ml SCF at 37°C, 5% CO2, as previously described.18 PMC were used for experiments between 14 and 16 days of culture. For the culture of bone marrow-derived mast cells (BMMC), bone marrow cells were thoroughly flushed with RPMI1640 medium from both sides of the femur and tibia. After lysing red blood cells, the remaining cells were cultured in RPMI1640 medium supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 10 ng/ml IL-3, and 10 ng/ml SCF at 37°C,5% CO2. The suspension cells were split twice per week and cultured for 4−6 weeks prior to the experiments.18 The purity of mature mast cells was assessed by flow cytometry for at least 95% coexpression of c-Kit and FcεRIα.
2.6 Flow cytometry
Single-cell suspensions (5×105/ml) were blocked with FcBlock (anti-CD16/CD32) and subsequently stained with APC-CD107a for 30 min at 4°C in the dark. The live and dead cells were stained with DAPI. Data were collected using LSRFortessa (BD Biosciences, NJ) and analyzed using the FlowJo software v10.
2.7 RT-PCR and real-time quantitative RT-PCR analysis
Total RNA was purified from mast cells using the Cell RNA Purification Kit Plus (ESscience Biotech, Shanghai, China) and reverse transcribed using the Fast All-in-One RT Kit (ESscience Biotech), according to the manufacturer's instructions. qRT-PCR analyses were performed on a QuantStudio 7 Real-Time PCR System (Thermo Fisher) using 2x Super SYBR Green qPCR Master Mix (Bimake, TX). Gene expression was measured relative to that of GAPDH. The details of the primers that were used are listed in Supplementary Table S1.
2.8 β-hexosaminidase release assay
PMC were harvested and resuspended in Phenol red-free RPMI1640 containing 2% FBS. Next, 50,000 cells were seeded per well and stimulated with PAMP9-20, DNP-HSA, or PBS at the indicated concentrations for 30 min at 37°C and 5% CO2. Anti-DNP-IgE (200 ng/ml) was preincubated with PMC overnight at 37°C before being stimulated with 10 ng/ml DNP-HSA. After 30-minute incubation, the supernatants were collected and the cells were lysed using 0.1% Triton X-100. The supernatant and cell lysates were incubated with p-nitrophenyl-N-acetyl-β-D glucosaminide in 40 mM citrate buffer (pH 4.5) for 90 min at 37°C. The reactions were stopped by adding 0.4 M glycine (pH 10.7). The absorbance was measured at 405 and 570 nm using a Multiskan microplate reader (Thermo Fisher). The β-hexosaminidase release rate = [absorbance of supernatant / (absorbance of supernatant + absorbance of total lysate)] x100%.11
2.9 ELISA
GM-CSF, TNFα, and CCL2 release levels were measured using the corresponding ELISA Kit (MultiSciences, Zhejiang, China), according to the manufacturer's protocol. Briefly, PMC were stimulated for 24 h before the supernatants were harvested and stored at −80°C until they were used for ELISA. PMC supernatants were collected 30 min after stimulation, and histamine concentrations were detected using a histamine ELISA kit (Sangon Biotech). Data are representative of three independent experiments.
2.10 siRNA transfection of PMC
Mrgprb2 expression was downregulated using siRNA against Mrgprb2 and a nonspecific siRNA as a negative control (GenePharma, Jiangsu, China). PMC were suspended at 5 × 105 cells per well and transfected with 10 nM Mrgprb2 siRNA and control siRNA in serum-free RPMI1640 medium using siRNA-Mate (GenePharma), according to the manufacturer's instruction at 37°C, 5% CO2.
2.11 Statistical analysis
Statistical tests were performed using GraphPad Prism 9. Results are expressed as the mean ± SEM. Two-tailed unpaired Student's t-tests and two-way ANOVA, followed by Šídák's multiple comparison tests, were used to assess significant differences between two experimental groups and several experimental groups, respectively. The results were considered statistically significant at p < 0.05.
3 RESULTS
3.1 PAMP expression and the number of MRGPRX2+ mast cells in the skin lesions of PN with severe pruritus were significantly increased
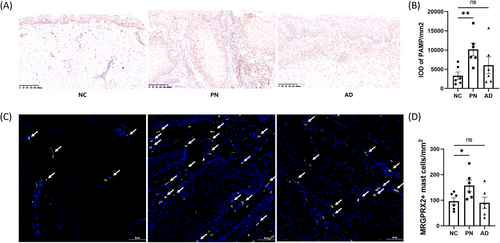
Compared with normal control (NC) skin, the protein expression of the MRGPRX2 agonist PAMP was significantly increased in PN lesions with severe itching, but similar results were not observed in AD lesions with severe itching (Figure 1A-B). PAMP was detected in numerous cell types scattered within the skin, especially in keratinocytes and perivascular inflammatory cells. Immunofluorescence staining showed that the number of MRGPRX2-expressing mast cells was elevated in itchy PN skin but not in itchy AD skin (Figure 1C-D).
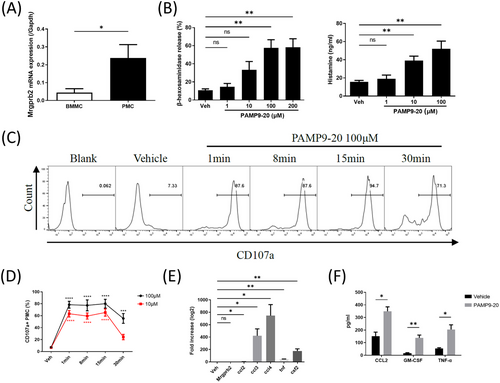
3.2 PAMP9-20 induced the immediate and delayed immune responses of mast cells through Mrgprb2
The mRNA expression of Mrgprb2 in PMC was significantly higher than that in BMMC (Figure 2A), which is consistent with a previous report.19 PAMP9-20 induced the release of β-hexosaminidase and histamine in a dose-dependent manner after 30 min of stimulation (Figure 2B). PAMP9-20 activated PMC in a dose-dependent manner to express CD107a. One minute after PAMP9-20 stimulation, the percentage of CD107a+PMC reached its peak, remained at 8 and 15 min, and began to decrease 30 min after stimulation (Figure 2C). The percentage of CD107a+ PMC was significantly higher than that of PBS (Figure 2D). Compared with PBS, the mRNA relative expression of Ccl2, Ccl3, Ccl4, Tnf, and Csf2 in PMC significantly increased after 100 μM PAMP9-20 administration for 30 min, but the gene expression of Mrgprb2 was comparable (Figure 2E). The concentrations of CCL2, TNF-α, and GM-CSF in the supernatant of PMC increased significantly compared with PBS after incubation with 100 μM PAMP9-20 for 24 h (Figure 2F). Compared with the control siRNA, after transfection of Mrgprb2 siRNA and PAMP9-20 incubation, the proportion of CD107a+ PMC, β-hexosaminidase release rate, relative mRNA expression of Ccl2, Ccl3, Ccl4, Tnf, and Csf2, as well as the CCL2, TNF-α, GM-CSF, and histamine concentrations in the PMC supernatant were all significantly reduced (Figure 3).
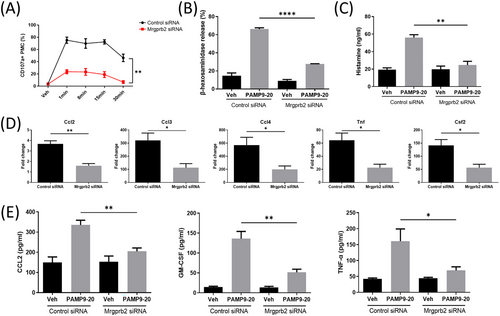
3.3 Mrgprb2 pathway is independent of the classic IgE signaling pathway
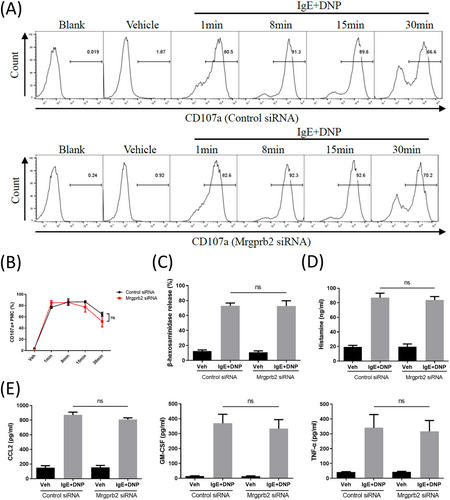
After transfection with Mrgprb2 siRNA, PMC were pre-incubated with 200 ng/ml anti-DNP-IgE overnight and then stimulated with 10 ng/ml DNP-HSA for 30 min. We found that the proportion of CD107a+ PMC, β-hexosaminidase release rate, relative mRNA expression of Ccl2, Ccl3, Ccl4, Tnf, and Csf2, as well as the CCL2, TNF-α, GM-CSF, and histamine concentrations in the PMC supernatant were not significantly different compared with those transfected with negative control siRNA (Figure 4).
4 DISCUSSION
The ADM gene encodes pre-adrenomedullin, which is cleaved into two biologically active peptides, PAMP and adrenomedullin (AM).20 However, only PAMP cause itching in humans.13 The expression of PAMP in the skin remains unclear. The current study confirmed, for the first time, that the protein expression of PAMP in PN, but not in AD skin lesions with severe itching, was significantly higher than that in the NC skin. This is consistent with the transcriptional results that were obtained in our previous study.15 These suggest a unique role for PAMP in the pathogenesis of PN. It has been reported that the expression of PAMP in ACD lesions is elevated and is mainly expressed in keratinocytes.9 Tajima et al. found that neuroendocrine cells around blood vessels stained positively for PAMP and AM, similar to the expression of serotonin-positive cells and that both PAMP and AM were always co-localized in secretory granules in rat gastric mucosa.21 We found that PAMP were widely expressed in various skin cells, mainly in keratinocytes and perivascular inflammatory cells. The significantly proliferated epidermis and increased infiltration of immune cells around the blood vessels in PN may be the reasons for the increased expression of PAMP.
PAMP and PAMP9-20 (with greater muscular agonistic activity) are newly discovered ligands of the histamine-independent pruritus receptor, MRGPRX2, which is mainly expressed in mast cells.12 Therefore, we investigated the expression of MRGPRX2 in patients with severe itching and found a significant increase in the number of MRGPRX2+ mast cells in lesional PN skin compared with healthy skin. Previous research has also demonstrated that the number of MRGPRX2+ mast cells in the skin lesions of patients with chronic prurigo is positively correlated with disease severity.11 The role of MRGPRX2 in AD has recently received considerable attention. RNA-seq analysis showed that the gene expression levels of MRGPRX2 in lesional AD and psoriatic skin in Caucasians with severe pruritus were significantly higher than those in non-lesional skin without pruritus.22 However, in our previous study, we did not detect an increase in the transcript levels of MRGPRX2 in the lesional skin of Chinese patients with AD.15 Similar results were obtained at the protein level in this study, suggesting that the expression of MRGPRX2 may be influenced by racial differences. However, further sample size expansion is required to confirm this hypothesis.
Mast cells release a series of endogenous pruritogens after activation, which can be divided into two phases: immediate and delayed. The rapid phase response refers to the release of various pre-existing particles, including histamine, tryptase, β-hexosaminidase, etc., in the cytoplasm of mast cells to the outside of the cell after encountering a stimulus. The late-phase response is defined as mast cells encoding and synthesizing cytokines, chemokines, and other inflammatory mediators and releasing them later to promote the recruitment and activation of lymphocytes and other adaptive immune cells.23 The biphasic response of mast cells is essential for chronic pruritus development. The immediate-phase response, known as degranulation, is an important feature of mast cell activation.24 Studies have shown that PAMP can cause degranulation and release of histamine from peritoneal mast cells in mice and rats.9, 25 Mrgprb2 knockout in mice significantly reduced the number of activated peripheral sensory neurons that were stimulated by PAMP9-20 and inhibited scratching behavior in an ACD mouse model.9 Consistent with these findings, we used siRNA technology to silence Mrgprb2 expression in PMC, which also proved that PAMP9-20 mediated Mrgprb2-dependent mast cell degranulation and the release of histamine and β-hexosaminidase.
A recent study used a high-resolution real-time laser confocal microscope and fluorescent dye-labeled avidin probes to observe the mast cell degranulation within seconds of stimulation. Single-cell video recordings revealed the all-or-nothing phenomenon of mast cell degranulation.26 CD107a is a membrane surface marker of mast cell degranulation.27 We found that mast cell degranulation peaked within 1 min of PAMP9-20 stimulation and began to decline at 30 min; this effect was inhibited after Mrgprb2 was silenced. This indicates that Mrgprb2-dependent mast cell degranulation mediated by PAMP9-20 is extremely time-sensitive, which is in line with the report that itch sensation was immediately evoked after intradermal injection of PAMP in healthy volunteers.
Chronic pruritus is closely related to the inflammatory cascade that is mediated by cytokines and chemokines and is mutually causal. The current study showed that PAMP9-20, through Mrgprb2, mediated the in vitro release of various inflammatory mediators by murine mast cells. The delayed phase immune response of mast cells that are mediated by PAMP9-20 may be associated with the chronicity of PN; however, this hypothesis requires confirmation through in vivo studies.
The IgE-FcεRI pathway-mediated mast cell activation is the classic signaling pathway of pruritus, which is involved in the pathogenesis of a variety of skin diseases.23 We used siRNA interference technology to explore the interaction of the MRGPRX2 pathway and the IgE-FcεRI pathway in mast cell activation in vitro. The results showed that silencing Mrgprb2 did not affect the immediate or delayed phase response of mast cells after activation of the IgE-FcεRI pathway. Mrgprb2 knockout did not affect the number of mast cells, histamine concentration, or IgE-mediated allergic reactions in mice; however, CP48/80-induced histamine release, inflammation, and airway contraction in Mrgprb2 knockout mice were significantly reduced.12 This suggests that the MRGPRX2 and classic IgE-FcεRI pathways are independent of each other. The existence of IgE-FcεRI-independent pathways explains the ineffectiveness of conventional antihistamines in treating PN, which can be used as candidate drug targets.
ACKNOWLEDGMENTS
We wish to thank all dermatologists in the Department of Dermatology, Huashan Hospital (Jinran Lin, Yiyi Gong, Shangshang Wang, etc.) for performing skin biopsies and dermatopathologists (Lianjun Chen, Qiao'an Zhang, Jiewen Pan) for making the pathological diagnosis. We thank all patients and healthy volunteers who agreed to participate in this study. This work was funded by the National Natural Science Foundation of China (No. 81803130 and 82003348), Shanghai Association for Science and Technology (No. 21Y11905100), Shanghai Municipal Commission of Health and Family Planning (No. 2023ZZ02018), Shanghai Municipal Key Clinical Specialty (No-shslczdzk01002), and Beijing Bethune Charitable Foundation (No. JJXYZ-LC-02).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS APPROVAL
The current study was conducted according to the guidelines of the Declaration of Helsinki and approved by the ethics committee of Huashan Hospital, Fudan University (No. 2020-908).
Open Research
DATA AVAILABILITY STATEMENT
The data of the current study are available from the corresponding author on reasonable request.