RETRACTED: Cosentyx alleviates psoriasis-induced podocyte injury by inhibiting the tlr/nf-κb signaling pathway
Abstract
Background
Pathological studies have shown an association between psoriasis and renal podocyte injury, and the specific mechanism of podocyte injury in psoriasis remains unclear, with no effective treatments currently available. This study aimed to investigate the underlying mechanisms of podocyte and epidermal cell injury in psoriasis and evaluate the therapeutic effect of Cosentyx.
Materials and methods
A psoriasis-like mouse model was established using BALB/C mice, and Cosentyx treatment was administered via intraperitoneal injection. Various parameters, including skin lesions, urinary protein, kidney/serum inflammatory cytokines, kidney function, podocyte membrane proteins, and Toll-like receptors/nuclear factor kappa-b (TLR/NF-κB) pathway-associated proteins, were analyzed to explore the mechanisms of podocyte and epidermal cell injury in psoriasis and the potential ameliorative effects of Cosentyx.
Result
Treatment with Cosentyx significantly reduced the increased levels of urinary protein, creatinine, and blood urea nitrogen caused by psoriasis. Cosentyx inhibited the upregulation of kidney/serum inflammatory factors (IL-17, IL-1β, IL-6, TNF-α, and IL-22) and TLR/NF-κB-related proteins (TLR2, TLR4, MyD88, and NF-κBp65) in both psoriatic skin and kidney tissues, while also reducing the accumulation of oxidative products. Moreover, Cosentyx treatment suppressed podocyte apoptosis and promoted epidermal cell apoptosis. The experimental data demonstrated that psoriasis-like inflammation impaired renal podocytes through the TLR/NF-κB signaling pathway.
Conclusion
Cosentyx treatment effectively inhibited the expression of TLR/NF-κB-related proteins, providing a therapeutic effect for psoriasis-induced kidney and skin injuries.
1 BACKGROUND
Psoriasis is a common chronic inflammatory skin disease with complex etiology and pathogenesis, approximately 2%–3% of the general population.1 Although the exact mechanisms underlying its development are not fully understood, genetic factors, immune dysregulation, and environmental influences are believed to play significant roles in the disease. Genetic factors play an important role in the pathogenesis of psoriasis. Family studies have demonstrated a higher risk of psoriasis in individuals with affected relatives, indicating a genetic contribution to disease susceptibility. Several genes have been implicated in the occurrence and progression of psoriasis, including HLA-C, IL-23R, IL-12B, IL-23A, among others.2-5
Immune abnormalities are also recognized as key mechanisms in the development of psoriasis.6 Dysregulation of the immune system leads to aberrant activation and interaction of T cells, dendritic cells, and inflammatory cells, resulting in sustained cutaneous inflammation.7, 8 Specifically, an abnormal increase of Th17 cells and their associated cytokine IL-17 is closely associated with psoriasis pathogenesis.9, 10 Environmental factors are considered triggers for the onset or exacerbation of psoriasis. Infections, trauma, medications, and psychological stress have been implicated as environmental factors that can induce or aggravate psoriasis. Infections such as pharyngitis, upper respiratory tract infections, and skin infections can activate immune responses, leading to exacerbation of psoriasis.
Cosentyx (generic name: secukinumab) is a medication that belongs to a class of drugs known as biologic therapies.11 It is specifically designed to target and inhibit the activity of a protein called interleukin-17A (IL-17A), which plays a crucial role in the development of psoriasis and related inflammatory conditions.12 Cosentyx is primarily used for the treatment of moderate to severe plaque psoriasis, a common form of psoriasis characterized by raised, red, and scaly patches on the skin.13, 14 It is also approved for the treatment of psoriatic arthritis, a chronic inflammatory joint disease associated with psoriasis.
In psoriasis, glomerular podocytes play a crucial role. Glomerular podocytes form the filtration barrier within the glomerulus and are responsible for filtering and clearing waste and excess substances from the blood while retaining beneficial substances such as proteins and red blood cells.15 In psoriasis, podocytes may suffer damage and dysfunction. Psoriasis patients often exhibit renal impairment (RI), although the specific mechanisms are not fully understood. Research suggests that psoriasis may induce podocyte injury and apoptosis by activating inflammatory and oxidative stress responses.16 This could be associated with elevated levels of inflammatory cytokines and aberrant autoimmune reactions in the body. Podocyte damage may lead to increased permeability of the filtration barrier, causing excessive leakage of large molecules, such as proteins, into the urine, resulting in proteinuria.17 Additionally, podocyte injury can lead to decreased filtration function, affecting waste clearance and fluid balance. Therapeutic drugs like Cosentyx may alleviate psoriasis-induced podocyte injury to some extent by inhibiting the TLR/NF-κB signaling pathway, thereby improving renal function and ameliorating the disease.18 However, the underlying mechanisms of this action require further research and exploration to better understand the relationship between psoriasis and glomerular podocytes.
Various treatment approaches are available for psoriasis, including topical therapies, phototherapy, systemic medications, and biologic agents. However, a fully effective and minimally invasive treatment remains elusive. In this context, Cosentyx, as a novel biologic agent, has shown significant therapeutic potential. By targeting psoriasis-related inflammatory cytokines and modulating immune responses, Cosentyx effectively alleviates renal and cutaneous damage caused by psoriasis. This study aims to investigate the efficacy and underlying mechanisms of Cosentyx in improving renal and cutaneous damage in patients with psoriasis, providing novel insights and treatment strategies for psoriasis management.
2 MATERIALS AND METHODS
2.1 Modeling of psoriasis and Cosentyx treatment methods
Male SPF grade BALB/C mice (10−12 weeks old; weighing 25–30 g) were obtained from Cyagen Biosciences (Suzhou, China). The mice were individually housed in cages with a 12-h light/dark cycle and maintained at a temperature of 24−26°C with a humidity of 40%. The mice had ad libitum access to water and standard ordinary feed. All animal experiments were conducted following approval from the Animal Ethics Committee. In this study, psoriasis-like skin inflammation was induced in male SPF grade BALB/C mice by removing the hair from their backs using a hair removal cream. Subsequently, the mice were subjected to daily topical application of Miquimod cream on the depilated area. The control group received Vaseline application on their backs. For the treatment group, Canakinumab, a monoclonal antibody injection, was diluted in 0.9% saline and administered intradermally at a dilution ratio of 1:200, with each mouse receiving 0.3 mL of the diluted Canakinumab once a week. The mice in both groups underwent weekly treatments. This experimental approach was employed to investigate the effects of Canakinumab in the psoriasis model on the mice's skin.
2.2 Cell culture and inflammatory factor detection
HaCaT cell, MPC-5 cell and macrophage purchased from Hunan Fenghui Biotechnology Co., Ltd. The cells were cultured and divided into four groups: the control group, the model group, the model group + macrophages, and the model group + Cosentyx treatment group. After 24 h of co-culture, the cell culture supernatants were collected for the detection of inflammatory factors. The test kits used for measured inflammatory factors included human IL-6 kit (Elabscience Biotechnology Co., Ltd.#E-EL-H6156), mouse IL-6 kit (Elabscience Biotechnology Co., Ltd. #E-EL-M0044c) human IL-10 kit (Elabscience Biotechnology Co., Ltd. #E-EL-H6154), mouse IL-10 kit (Elabscience Biotechnology Co., Ltd. #E-EL-M0046c) human IL-17 kit (Elabscience Biotechnology Co., Ltd #E-EL-H5812c), mouse IL-17 kit (CUSABIO # CSB-E04608m), human IL-22 kit (Elabscience Biotechnology Co., Ltd #E-EL-H0106c), mouse IL-22 kit (Elabscience Biotechnology Co., Ltd # E-EL-M2446c), and human TNF-α kit (Elabscience Biotechnology Co., Ltd #E-EL-M3063).
2.3 Tissue immunofluorescence
Immunofluorescence is performed using tissue sections prepared from different experimental groups. The sections are fixed with 4% paraformaldehyde and treated with Triton X-100 for permeabilization. Blocking with bovine serum albumin (2%) is followed by incubation with primary antibodies against IL-4 and IFN-γ overnight. After washing with PBS, the sections are incubated with secondary antibodies. Finally, the sections are mounted with anti-fade mounting medium containing DAPI and observed using a fluorescence microscope. The specific reagents and materials used include 4% paraformaldehyde (Beyotime Biotechnology, #P0099), PBS buffer (Wuhan Doctor De Biological Engineering Co, LTD, #AR0030), secondary antibody dilution buffer (Beyotime Biotechnology, LTD, #P0108), IL-4 monoclonal antibody (Proteintech, #66142-1-Ig), anti-IFNγ antibody (#bs-0480R), anti-Podocin antibody (Merck, # SAB4200810), anti- SYNPO Polyclonal antibody (Proteintech, # 21064-1-AP), goat anti-mouse IgG H&L (abcam, #ab150115), goat anti-rabbit IgG H&L (abcam, #ab150079), anti-fade mounting medium with DAPI (Solaibao Technology Co., LTD, #S2110), Triton X-100 (Solaibao Technology Co., LTD, #T8200), fluorescence microscope (OLYMPUS, #BH2-RFCA), and Decolorizing Shaker.
2.4 FlowCytometry
The cells from each group mentioned above were digested with 0.25% trypsin (Solaibao Technology Co., LTD, #T1300), and 5 × 104–105 resuspended cells were collected. After centrifugation at 1000 rpm for 5 min, the supernatant was discarded, and 500 μL of 1 × Binding Buffer was added to gently resuspend the cells. Then, 5 μL of Annexin V-FITC (Multi sciences, # AP101-100) was added and gently mixed. Subsequently, 10 μL of propidium iodide (PI) staining solution (Multi sciences, # AP101-100) was added and gently mixed. The mixture was incubated at room temperature (20–25°C) in the dark for 10–20 min and then placed on ice, optionally using aluminum foil for light avoidance. During the incubation, the cells were gently resuspended 2–3 times to improve the staining effect. Flow cytometry (BD Biosciences, #E97501093) was used for detection.
2.5 Detection of M1 and M2 polarization
Collect 2 × 105∼1 × 106 cells and resuspend them in culture medium. Centrifuge at 1000 rpm, 4°C for 5 min, and discard the supernatant. Add 1 mL of PBS and centrifuge at 1000 rpm, 4°C for 5 min. Resuspend each tube of cells in 100 μL of PBA and add F4/80 (Abcam, #ab237026), CD86 (Abcam, #ab256270), and CD206 antibodies (Santa Cruz Biotechnology, #sc-58986 PE) to each tube. Incubate at 4°C in the dark for 1 h. Add 1 mL of PBS, centrifuge at 1000 rpm, 4°C for 5 min, and discard the supernatant. Resuspend the cells in 400 μL of PBS and analyze the results using FlowJo l0 by detecting PerCP F4/80 in FL3-H channel, Alexa Fluor 488 CD86 in FL1-H channel, and PE CD206 in FL2-H channel on a flow cytometer (BD Biosciences, #E97501093).
2.6 Western blot
Cell or tissue proteins were extracted and their concentrations were determined. An appropriate amount of protein samples was loaded and subjected to SDS-PAGE using a 10% polyacrylamide gel until the target protein was effectively separated. After the electrophoresis, the gel was removed and transferred onto a PVDF membrane using a constant current of 350 mA at 4°C for 2 h in transfer buffer. The membrane was then blocked with 5% skim milk at room temperature with gentle shaking for 2 h. Primary antibodies used were Anti-TLR4 antibody (Abcam, #ab218987), Anti-MyD88 antibody (Abcam, #ab133739), Anti-p-NF-kB antibody (Abcam, #ab76302), Anti-NF-kB antibody (Abcam, #ab32536), Anti-Podocin antibody (Abcam, #ab181143), Anti-Synaptopodin antibody (Abcam, #ab259976), Anti-Caspase-3 antibody (Abcam, #ab32351), Anti-PCNA antibody (Abcam, #ab92552), Anti-Bcl-2 antibody (Abcam, #ab32124), and Anti-GAPDH antibody (Abcam, #ab9485). The primary antibodies were incubated with the membrane at 4°C overnight with gentle shaking. The membrane was then washed with 1 × TBST solution for 5 min, four times. Subsequently, the membrane was incubated with corresponding secondary antibodies (1:8000) at room temperature for 1.5 h. After the secondary antibody incubation, the membrane was washed with 1 × TBST solution for 5 min, four times, followed by visualization and analysis of the results.
2.7 Detection of SOD, ROS, and MDA
SOD levels were determined using an Elisa kit (Shanghai Jinyan Biological, #F12172) according to the manufacturer's instructions. ROS levels were measured using an Elisa kit (Shanghai Jinyan Biological, #F06102) following the manufacturer's instructions. MDA levels were detected using an Elabscience Biotechnology Co., Ltd Elisa kit (#E-EL-0060c) as per the manufacturer's instructions.
2.8 Statistical analysis
Data are presented as mean ± standard deviation and analyzed with at least three replicates. Normality and homogeneity of variance are assessed using the Shapiro-Wilk test and Levene's test, respectively. For comparisons between two groups, independent samples t-test or Mann–Whitney U test is used. For comparisons among multiple groups, one-way analysis of variance (ANOVA) or Kruskal-Wallis H test is employed, followed by Tukey's HSD or Dunn's multiple comparisons for post hoc analysis. A P-value less than 0.05 is considered statistically significant. Statistical analyses are performed using SPSS software (IBM Corp., USA). The experimental results are presented in graphical and tabular forms and plotted using GraphPad Prism software (GraphPad Software, USA).
3 RESULTS
3.1 Result 1: Cosentyx effectively mitigates renal and cutaneous damage caused by psoriasis
Cosentyx treatment significantly reduced the elevated levels of urinary protein, creatinine, and urea nitrogen, which are indicators of psoriasis-induced renal damage (Figure 1A–C). Histological examination of mouse skin and kidneys revealed that Cosentyx treatment led to a reduction in both skin and kidney damage at 3 and 14 days (Figure 1D). ELISA analysis of skin and kidney tissues demonstrated that Cosentyx treatment increased the expression of the anti-inflammatory cytokine IL-10 while decreasing the expression of pro-inflammatory cytokines IL-6, TNF-α, IL-17, and IL-22 (Figure 1E). In kidney tissue, there were no significant changes in inflammatory factors after 3 days of Cosentyx treatment, but after 14 days, there was an increase in the expression of anti-inflammatory cytokine IL-10 and a significant decrease in the expression of pro-inflammatory cytokines IL-6, TNF-α, IL-17, and IL-22. These findings indicate that Cosentyx treatment effectively reduces skin and kidney inflammation associated with psoriasis modeling.
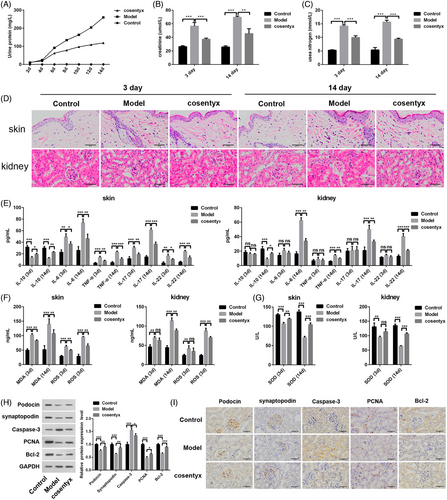
Psoriasis is characterized by elevated levels of reactive oxygen species (ROS) and compromised antioxidant activity.19, 20 This is evidenced by increased levels of superoxide dismutase (SOD) and malondialdehyde (MDA) products.7, 21 Cosentyx treatment was able to decrease ROS and MDA levels induced by psoriasis modeling in both mouse skin and kidney tissues (Figure 1F). Furthermore, Cosentyx treatment upregulated the expression of SOD in skin and kidney tissues, thereby enhancing their antioxidant capacity (Figure 1G). Psoriasis often leads to podocyte injury in renal glomeruli. To assess this, podocyte-related proteins, as well as proteins associated with cell proliferation and apoptosis, were examined. After Cosentyx treatment, the expression of podocyte membrane protein (Podocin) and synaptopodin in human renal glomeruli was increased, along with an elevation in the proliferation marker protein PCNA, while the apoptosis-related protein Caspase-3 was decreased (Figure 1H). Immunohistochemical analysis of mouse kidneys yielded results consistent with the protein analysis (Figure 1I). These findings suggest that Cosentyx promotes podocyte proliferation, inhibits podocyte apoptosis, and effectively mitigates kidney damage caused by psoriasis.
3.2 Result 2: Cosentyx promotes M2 polarization of macrophages and reduces the expression of inflammatory cytokines in psoriasis
Cosentyx treatment resulted in elevated levels of the anti-inflammatory cytokine IL-4 and decreased expression of the pro-inflammatory cytokine INF-γ in skin and kidney tissues (Figure 2A). Fluorescence imaging of kidney and skin tissues revealed a reduction in red fluorescence, indicating INF-γ, and an increase in IL-4 fluorescence in the Cosentyx group compared to the Model group (Figure 2B,C). Immunofluorescence co-localization analysis showed that the secretion of inflammatory cytokines was mainly localized in the interstitial tissue, suggesting the presence of immune cell infiltration, and Cosentyx may reduce immune cell infiltration in the skin and kidneys, thereby alleviating the inflammatory damage caused by psoriasis.
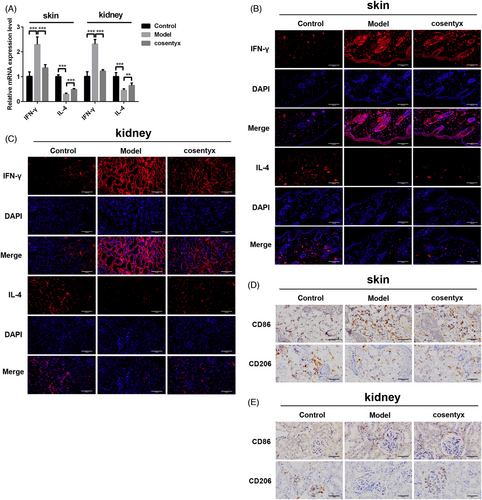
Macrophages play a crucial role in promoting immune responses in psoriasis through the secretion of inflammatory cytokines, and their increased numbers contribute to disease progression.8, 22, 23 Macrophages can be classified into pro-inflammatory M1 phenotype and anti-inflammatory M2 phenotype, with CD86 as a marker for M1 macrophages and CD206 as a marker for M2 macrophages. To investigate the impact of Cosentyx on macrophages in psoriatic skin and kidney tissues, immunohistochemistry was performed to assess the expression of CD86 and CD206. The results demonstrated that Cosentyx treatment reduced CD86 expression and increased CD206 expression in both skin and kidney tissues compared to the Model group (Figure 2D). These findings suggest that Cosentyx promotes the M2 polarization of macrophages, thereby reducing the secretion of inflammatory cytokines and improving the inflammatory response in psoriatic skin tissues.
3.3 Result 3: Cosentyx promotes apoptosis of HaCaT cells
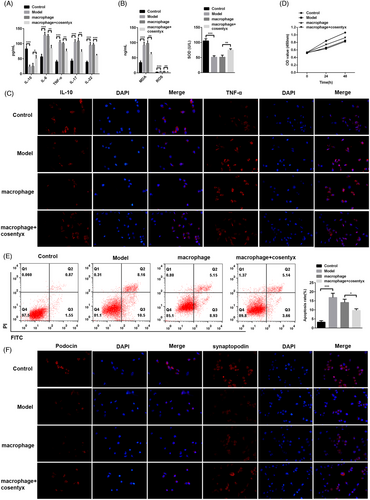
To further investigate whether Cosentyx acts through epidermal cells, HaCaT cells (epidermal cells) were co-cultured with macrophages and treated with Cosentyx. The subsequent analysis focused on examining the changes in relevant inflammatory cytokines and oxidative stress levels. Consistent with the findings from animal experiments, ELISA results demonstrated that the macrophage + Cosentyx treatment group exhibited elevated levels of the anti-inflammatory cytokine IL-10 and reduced expression of pro-inflammatory cytokines including IL-6, TNF-α, IL-17, and IL-22 in the cell culture supernatant (Figure 3A). Similarly, the macrophage + Cosentyx treatment group showed increased expression of the antioxidant enzyme SOD and a concomitant decrease in ROS and MDA levels in the cell culture supernatant (Figure 3B). Immunofluorescence analysis of HaCaT cells further confirmed these findings, as the macrophage + Cosentyx treatment group showed intensified green fluorescence for IL-10, an anti-inflammatory cytokine, while exhibiting reduced fluorescence for TNF-α, a pro-inflammatory cytokine (Figure 3C). These alterations were accompanied by decreased cell viability of HaCaT cells (Figure 3D). Additionally, flow cytometry analysis revealed that the macrophage + Cosentyx treatment promoted apoptosis of HaCaT cells compared to the macrophage group, thereby alleviating psoriasis symptoms (Figure 3E). Furthermore, Cosentyx was found to promote the M2 polarization of macrophages while reducing the M1 polarization and thereby decreasing the secretion of inflammatory cytokines. In summary, these results suggest that Cosentyx alleviates psoriasis damage by promoting apoptosis of HaCaT cells, inhibiting macrophage inflammation, and reducing oxidative stress levels.
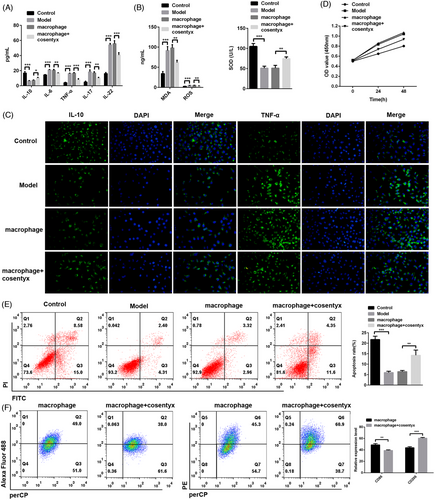
3.4 Result 4: Cosentyx suppresses apoptosis and inflammation in MPC5 cells
To elucidate the specific mechanism of Cosentyx on podocytes, mouse renal glomerular podocyte MPC5 cells were cultured to establish a psoriasis model, and subsequent experiments were conducted in combination with macrophages. Consistent with the findings in HaCaT cells, ELISA results demonstrated that the macrophage + Cosentyx treatment group exhibited increased levels of the anti-inflammatory cytokine IL-10 and decreased levels of pro-inflammatory cytokines IL-6, TNF-α, IL-17, and IL-22 in the cell culture supernatant (Figure 4A). Similarly, Cosentyx treatment resulted in elevated expression of the antioxidant enzyme SOD, accompanied by a reduction in ROS and MDA levels in the cell culture supernatant (Figure 4B). Immunofluorescence analysis of MPC5 cells confirmed these findings, as the macrophage + Cosentyx treatment group exhibited enhanced red fluorescence for IL-10, an anti-inflammatory cytokine, while showing reduced fluorescence for TNF-α following Cosentyx treatment (Figure 4C). These changes were accompanied by increased cell viability of MPC5 cells (Figure 4D). Additionally, flow cytometry analysis revealed that the macrophage + Cosentyx treatment suppressed apoptosis of MPC5 cells compared to the macrophage group (Figure 4E). The fluorescence intensity of the secreted proteins podocin and synaptopodin by podocytes was also elevated (Figure 4F). These results indicate that Cosentyx can inhibit apoptosis of MPC5 cells, suppress inflammation and accumulation of oxidative products in MPC5 cells, and enhance the expression of podocin and synaptopodin proteins.
3.5 Result 5: Cosentyx mitigates psoriasis-induced MPC5 cell injury by suppressing the TLR/NF-κB signaling pathway
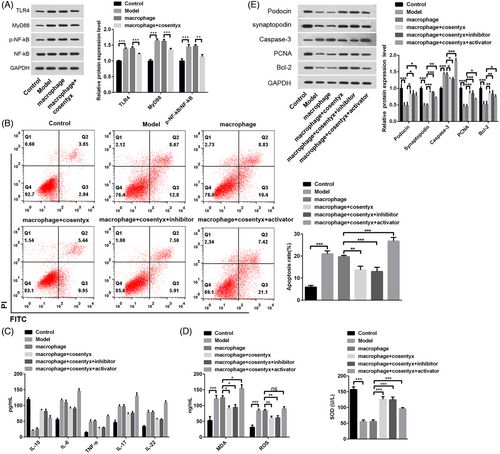
Pathological studies have suggested a correlation between psoriasis and renal injury (RI), but the underlying mechanisms linking RI to psoriasis remain unclear.24, 25 The experimental results indicated that psoriasis could lead to foot cell dysfunction, while treatment with Cosentyx improved psoriasis-induced foot cell dysfunction. However, the specific mechanism of action is not fully understood. Psoriasis-induced RI has been associated with the TLR/NF-κB pathway,18 prompting us to investigate whether the NF-κB signaling pathway is involved in foot cell injury. Through the analysis of TLR4, MyD88, NF-κB, and p-NF-κB, proteins related to the NF-κB pathway, we found that protein expression in the Model group was significantly elevated, indicating the activation of the NF-κB signaling pathway in psoriasis. However, in the macrophage + Cosentyx group, the NF-κB signaling pathway-related proteins were reduced (Figure 5A). Flow cytometry results showed a significant increase in apoptotic cells in the Model group, whereas the macrophage + Cosentyx and macrophage + Cosentyx + inhibitor groups displayed a substantial decrease in apoptotic cells due to NF-κB pathway inhibition. Conversely, the macrophage + Cosentyx + activator group exhibited an induction of cell apoptosis upon NF-κB activation (Figure 5B). As expected, the macrophage + Cosentyx + activator group showed elevated levels of inflammation and MDA (Figure 5C-D), indicating that NF-κB signaling pathway activation promotes inflammation and oxidative stress in psoriasis. Additionally, WB analysis revealed that the expression of Podocin and synaptopodin proteins was influenced by the NF-κB signaling pathway, with the NF-κB activator inhibiting their expression. Furthermore, the NF-κB activator stimulated cell apoptosis by upregulating Caspase-3 and inhibiting PCNA and Bcl-2 (Figure 5E). The use of NF-κB inhibitor and activator confirmed that psoriasis-induced foot cell injury is facilitated by activating the NF-κB signaling pathway, leading to glomerular podocytes apoptosis, inflammation, and oxidative damage, whereas NF-κB inhibition alleviates glomerular podocytes injury.
4 DISCUSSION
In this study, we investigated the relationship and mechanisms underlying psoriasis-induced podocyte injury in BALB/C mice. Our pathological findings confirmed an association between psoriasis and RI, highlighting the need to explore the specific mechanisms involved. Currently, effective treatments for psoriasis-induced podocyte injury are lacking. Therefore, we aimed to elucidate the molecular pathways responsible for podocyte injury in psoriasis and explore the therapeutic potential of Cosentyx in this context. Through the use of imiquimod-induced psoriasis-like skin lesions in mice, we demonstrated that psoriasis-like inflammation resulted in significant podocyte injury, as evidenced by increased urine protein, elevated creatinine and blood urea nitrogen levels, and impaired kidney function. Histological examination further revealed an augmented positive area of hematoxylin-eosin and periodic acid-Schiff staining in the kidney, indicating renal damage induced by psoriasis. Additionally, we explored the role of the TLR/NF-κB signaling pathway in psoriasis-induced podocyte injury. The expression of TLR2, TLR4, MyD88, and NF-κBp65, as well as pro-inflammatory cytokines IL-17, IL-1β, IL-6, TNF-α, and IL-22, were significantly upregulated in psoriasis model mice. Moreover, oxidative stress markers, such as MDA, were increased, while the antioxidant enzyme SOD was decreased, suggesting oxidative damage in psoriasis-induced podocyte injury.
Cosentyx, an inhibitor of interleukin-17A,26 has been reported to effectively alleviate Ankylosing Spondylitis.27 and psoriasis.28 In our study, we found that Cosentyx treatment resulted in reduced urine protein levels, improved creatinine and blood urea nitrogen levels, and ameliorated histological changes in both skin and kidney tissues. Moreover, Cosentyx treatment suppressed the upregulation of TLR/NF-κB pathway-related proteins and inflammatory cytokines, as well as mitigated the accumulation of oxidative products in the kidney and skin tissues. Furthermore, our results indicated that Cosentyx treatment inhibited podocyte apoptosis and promoted keratinocyte apoptosis. This observation suggested that Cosentyx might protect podocytes from psoriasis-induced injury and have potential therapeutic effects on skin lesions.
Previous studies have reported the association of the TLR4/NF-κB signaling pathway with psoriasis. Bioinformatics analysis combined with luciferase reporter gene assays in HaCaT cells revealed that TLR4 is a direct target of miR-489-3p. Overexpression of miR-489-3p inhibited the TLR4/NF-κB signaling pathway and reduced cell proliferation. Silencing TLR4 alleviated the impact of miR-489-3p, leading to enhanced cell proliferation and secretion of inflammatory cytokines.29 This study also confirmed the efficacy of inhibiting the TLR4/NF-κB signaling pathway in the treatment of psoriasis. However, other research has found that stimulation of certain foods (SF) can exacerbate psoriasis, and the expression of Notch and TLR-2/NF-κB p65 signaling pathway proteins in the psoriatic lesions is abnormally reduced, which is related to the severity of the skin lesions.30 Therefore, changes in the expression of the TLR4/NF-κB signaling pathway also need to be interpreted dialectically.
The study revealed an upregulation of BLACAT1 expression in psoriatic tissues. Overexpression of BLACAT1 exacerbated clinical manifestations in psoriasis and increased epidermal thickness in Imiquimod (IMQ)-induced mice. BLACAT1 promoted keratinocyte proliferation and suppressed apoptosis. Further investigations indicated that BLACAT1 positively modulated AKT1 expression by sequestering miR-149-5p, thus promoting psoriasis development.31 In line with our findings, existing literature suggests that Etanercept alleviates IMQ-induced pathological changes and inflammation, reduces the protein expression of High Mobility Group Box 1 (HMGB1), receptor for advanced glycation end-products, and Toll-like receptor 4, ultimately improving inflammation in a psoriasis-like mouse model.32 Through a comprehensive analysis of these research outcomes, a deeper understanding of the intricate nature of psoriasis is attained, offering more precise directions for future research and treatment strategies.
In conclusion, our findings provide evidence that psoriasis-like inflammation damages podocytes through the TLR/NF-κB signaling pathway. Cosentyx treatment showed promise in mitigating psoriasis-induced podocyte injury by inhibiting TLR/NF-κB-related protein expression. This study sheds light on potential therapeutic interventions for podocyte injury in psoriasis and highlights the importance of targeting the TLR/NF-κB signaling pathway as a novel therapeutic approach. However, further studies are warranted to fully understand the underlying mechanisms and potential long-term effects of Cosentyx treatment in psoriasis-induced renal injury.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.