Therapeutic effects of mesoderm introduction of compound glycyrrhizin injection on the treatment of rosacea
Shanshan Li, Xu Zhao, and Yun Chen contributed equally to this work.
Abstract
Objectives
This study aims to introduce compound glycyrrhizin injection for the treatment of rosacea by mesoderm therapy, and further analyze the therapeutic and aesthetic effects of this treatment method and its impact on the dermatological quality of life index, which provides new ideas and methods for cosmetic dermatology treatment of rosacea.
Methods
The recruited rosacea patients were divided into Control group (n = 58) and observation group (n = 58) according to the random number table. The control group was treated with topical metronidazole clindamycin liniment, and the study group was additionally used mesoderm introduction of compound glycyrrhizin injection. The transepidermal water loss (TEWL), water content in corneum, and dermatology life quality index (DLQI) in rosacea patients were evaluated.
Results
Our results showed that the scores of erythema, flushing, telangiectasia, and papulopustule were significantly reduced in the observation group. In addition, the observation group significantly decreased TEWL and increased the water content of the stratum corneum. Furthermore, the observation group significantly reduced the DLQI of rosacea patients compared to the control group.
Conclusion
The use of mesoderm therapy combined with compound glycyrrhizic acid has a therapeutic effect on facial rosacea and improves patient satisfaction.
1 INTRODUCTION
Rosacea is a chronic skin syndrome characterized by a combination of different symptoms and signs in the raised midface region with characteristic recurrent remissions and exacerbations.1 The main clinical manifestations are fixed erythema in the middle of the face, sebaceous hyperplasia, flushing, papules and pustules, telangiectasia, and ocular manifestations with or without subjective symptoms such as burning, tingling, dryness or itching.2, 3 The etiology of rosacea is complex, and it may be a chronic inflammation induced by a variety of factors based on a certain genetic basis, mainly due to abnormal innate immunity and abnormal vasomotor function.4 Rosacea usually occurs in young and middle-aged women, and could be aggravated by sun exposure, causing significant physical discomfort and mental stress to patients.5 There is currently no cure for rosacea, and treatment options focus on controlling symptoms.6
The main components of compound glycyrrhizin are glycine, L-cysteine and glycyrrhizin, which could exert anti-inflammatory, liver protection, immune function regulation, steroid-like and anti-allergic effects.7 The active substance in compound glycyrrhizin licorice is glycyrrhizin, which is similar in structure to corticosteroids.8 However, when compound glycyrrhizin enters the human body, it does not have a significant effect on the hypothalamic-pituitary-adrenal axis.9 In addition, glycyrrhizic acid could significantly inhibit the over-activation effect of antigen cells on T cells, effectively regulate the release of cytokines from T cells, maintain the balance of Th1/Th2, and actively inhibit the release of hindering substances from mast cells.10
Mesotherapy refers to the injection of single or compound injections, including general drugs, enzymes, vitamins, antioxidants or hormones, in very small amounts into the epidermis, dermis, subcutaneous fat layer or intramuscular.11 In the application of dermatology and medical aesthetics, compared with other traditional noninvasive treatment or invasive treatment, mesoderm therapy has both the safety and comfort of noninvasive treatment and the efficacy of invasive treatment, so it is an excellent new technology worthy of promotion.12 The aim of this study was to investigate the clinical efficacy of mesoderm therapy by compound glycyrrhizin injection on the treatment of rosacea.
2 METHODS
2.1 Participants
The study was approved by the ethics committee of Dingzhou People's Hospital. The required sample size in the trail was calculated using power and sample size collection software. The limited sample size was 52 for each group. Therefore, a total of 116 patients were included in this study, and they were divided into the control group and the observation group according to the random number table, with 58 cases in each group. No participators dropped out during the treatment. The control group was treated with topical metronidazole clindamycin liniment. The research group used the treatment method of topical metronidazole clindamycin liniment + mesotherapy-compound glycyrrhizin injection. For mesotherapy, 0.5-mm roller microneedle was used to inject compound glycyrrhizin injection. All the participants gave their informed written consent.
Inclusion criteria: (1) Patients who met the diagnostic criteria for rosacea: 1 diagnostic phenotype (fixed erythema in the mid-face, distributed in a specific pattern, with periodic aggravation; hypertrophic manifestations) or ≥2 major phenotypes (flushing; papules and pustules; telangiectasia; ocular manifestations). (2) patients with erythematocapillary type and papule-pustular type; (3) patients aged ≥18 years; (4) patients without other skin diseases in the treatment area; (5) patients who could receive regular treatment and complete follow-up; (6) patients who gave informed consent to the study treatment regimen.
Exclusion criteria: (1) Patients with systemic diseases such as diabetes, hypertension, HIV infection, and malignant tumors; (2) patients with neurological or psychiatric diseases; (3) pregnant or lactating women; (4) 3 months old Internal system application of glucocorticoids or immunomodulators; (5) patients with severe viral, bacterial, and fungal infections on the face; (6) patients with seborrheic dermatitis and facial disseminated lupus.
2.2 Intervention
In the control group, topical metronidazole clindamycin liniment was administered twice a day in the morning and evening. The treatment group received mesoderm therapy + compound glycyrrhizin injection once a week for a total of 8 weeks. All patients received follow-up visits after treatment, and the same camera was used to take facial photos of patients before treatment and after 8 weeks of treatment. The facial skin lesions of the two groups before and after treatment were compared according to the retained facial photos. The reduction of symptoms such as skin flushing, erythema, telangiectasia, papules, and pustules was mainly observed, and the therapeutic effect was judged accordingly. Patients with facial flushing, erythema and other symptoms alleviated by 90% or more were considered cured after treatment. A 60% to 89% reduction in the patient's facial flushing, erythema and other symptoms was considered a significant effect after treatment. Patients with facial flushing, erythema and other symptoms alleviated by 30% to 59% were considered effective after treatment. Patients with facial flushing, erythema, and other symptoms alleviated by less than 30% were considered ineffective after treatment.
2.3 Rosacea standard grading system score
Clinical symptom scores in rosacea were used to assess the severity of rosacea, including flushing, erythema, telangiectasia, and papulopustular scores. Flushing score: 0 points: no flushing; 1 point: mild (slight, transient, not obvious); 2 points: moderate (between mild and severe); 3 points: severe (persistent, frequent, obvious). Erythema score: 0 points: no erythema; 1 point: mild (no obvious erythema, limited to the center of the face or scattered on the face); 2 points: moderate (more obvious erythema, limited to the center of the face or scattered on the face); 3 score: severe (severe erythema distributed over the entire face). Telangiectasia score: 0 points: no telangiectasia; 1 point: mild (all small vessels, vessel diameter < 0.2 mm, covering area < 10% of the face); 2 points: moderate (several small vessels and/or or some large vessels, diameter > 0.2 mm, covering 10%–30% of face); 3 points: severe (many small and/or large vessels, covering > 30% of face).Papules and papules score: 0 points: no papules or pustules; 1 point: very few scattered papules and pustules, no plaques; 2 points: few papules and pustules, no plaques; 3 points: many papules and pustules, with plaque. Plaques are fused areas of inflammation that appear as red areas between papules and pustules with no abnormalities in the surrounding skin.
2.4 Determination of skin transepidermal water loss value
The transepidermal water loss (TEWL) value of the patient's cheek skin was measured by a noninvasive skin tester (CK Company, Germany). The room temperature of the test room was maintained at 20°C, and the skin was kept in a natural state for 20 min after cleaning. The measurement was repeated three times for each test site. TEWL is used to determine the state of the epidermal permeability barrier and to evaluate the skin barrier function.
2.5 Stratum corneum water content
Corneometer CM825, a skin moisture meter from COURAGE+KHAzAKA, Germany, was used to measure the moisture content of the stratum corneum of the patient's skin. Each site was measured three times, and the average value was taken. The higher the skin moisture content, the more robust the skin water barrier and the better the effect.
2.6 Dermatology life quality index
The dermatology life quality index (DLQI) is a relatively concise health-related quality of life indicator with a total of 10 questions. It is the patient's self-evaluation of their life experiences in the past week. The DLQI mainly includes the following 10 aspects: symptoms and degree of skin itching; whether skin problems have caused embarrassment and inferiority; whether skin problems have affected daily activities such as shopping and housework; whether skin problems have affected clothing; the impact of skin problems on social life and life; the impact of skin problems on sports; the impact of skin problems on work and study; the impact of skin problems on relationship with relatives and friends; the impact of skin problems on sexual life; whether skin problems increase the trouble and take up a lot of time. There are five alternative answers for each content. The scores corresponding to these five answers are: very many = 3 points, many = 2 points, a little = 1 point, not at all = 0 points, irrelevant = abstain or avoid this question. At the end the scores for all questions will be summed up to get the total score. The highest score is 30 points, and the higher the score, the greater the impact on life.
2.7 Statistical analysis
Data analysis was performed by SPSS 16.0 statistical software. Anderson-Darling test, D'Agostino & Pearson test, Shapiro-Wilk test, Kolmogorov-Smirnov test were used to test the normality of the data before analysis. Data were expressed as mean ± standard deviation (SD) or n (%), which was analyzed by Chi-square, Fisher's exact test, Mann Whitney test, or two-way analysis of variance (ANOVA) followed Turkey's multiple comparisons test. p < 0.05 was considered as statistically significant.
3 RESULTS
3.1 Baseline characteristics of the study subject
A total of 116 patients were included in this study, and they were divided into Control group (n = 58) and Observation group (n = 58) according to the random number table. The control group was treated with topical metronidazole clindamycin liniment, and the study group was treated with topical metronidazole clindamycin liniment combined with mesoderm therapy and introduced compound glycyrrhizin injection. The baseline data characterization of all participants was collected before treatment, including gender, age, duration of illness, classification, clinical symptom score (erythema/ flushing/ telangiectasia/ papulopustule). Our data indicated that there were no significant differences in baseline data between the two groups of patients (Table 1). It was particularly noteworthy that there was no significant difference in the scores of erythema, flushing, telangiectasia and papulopustule between the two groups of patients, indicating that the severity of the two groups of patients was the same before treatment.
| Study group | |||
|---|---|---|---|
| Characteristics | Control (n = 58) | Observation (n = 58) | p-Value |
| Gender | |||
| Male | 17 (29.3 %) | 14 (24.1 %) | 0.675 |
| Female | 41 (70.7 %) | 44 (75.9 %) | |
| Age (years) | 37.53 ± 11.17 | 38.29 ± 12.04 | 0.274 |
| Duration of illness (years) | 8.42 ± 2.49 | 8.91 ± 2.78 | 0.126 |
| Classification | |||
| Capillary dilated rosacea | 45 (77.6 %) | 40 (68.9 %) | 0.402 |
| Papulopustular rosacea | 13 (22.4 %) | 18 (31.1 %) | |
| Clinical symptom score-erythema | |||
| 0 | 1 (1.7 %) | 0 (0 %) | 0.431 |
| 1 | 20 (34.5 %) | 14 (24.1 %) | |
| 2 | 28 (48.3 %) | 32 (55.2 %) | |
| 3 | 9 (15.5 %) | 12 (20.7 %) | |
| Clinical symptom score-flushing | |||
| 0 | 0 (0 %) | 0 (0 %) | 0.581 |
| 1 | 6 (10.3 %) | 3 (5.2 %) | |
| 2 | 38 (65.5 %) | 40 (68.9 %) | |
| 3 | 14 (24.1 %) | 15 (25.9 %) | |
| Clinical symptom score-telangiectasia | |||
| 0 | 0 (0 %) | 0 (0 %) | 0.756 |
| 1 | 11 (18.9 %) | 13 (22.4 %) | |
| 2 | 31 (53.5 %) | 27 (46.6 %) | |
| 3 | 16 (27.6 %) | 18 (31.0 %) | |
| Clinical symptom score-papulopustule | |||
| 0 | 19 (32.8 %) | 16 (27.6 %) | 0.434 |
| 1 | 16 (27.6 %) | 21(36.2 %) | |
| 2 | 11 (18.9 %) | 6 (10.3 %) | |
| 3 | 12 (20.7 %) | 15 (25.9 %) | |
- Note: Values were expressed as n (percentage, %) or mean ± SD. p values were derived from Fisher's exact test or chi-square test or Mann Whitney test.
3.2 Comparison of clinical effectiveness between the control and observation group
Before treatment and after 8 weeks of treatment, the same camera was used to take the facial photos of the two groups of patients, and the changes of facial skin lesions of the two groups of patients before and after treatment were compared. The treatment effect was judged based on the improvement of symptoms such as skin flushing, erythema, telangiectasia, papules, and pustules and was divided into recovery, effectual, effective, and ineffective, and the data were shown in Table 2. Recovery and effective in observation group were 17 (29.3%) and 21 (36.2%), respectively. The recovery and effective were 9 (15.5%) and 17 (29.3%) in the control group. The recovery and effective in observation group were significantly higher than those in control group. In addition, effective and ineffective in observation group were 13 (22.4%) and 7 (12.1%), respectively, were significantly lower than the control group, which were 20 (34.5%) and 16 (27.6%). Taken together, our results suggested that rosacea patients treated with topical metronidazole clindamycin liniment and mesoderm-introduced compound glycyrrhizin injection had better therapeutic effects.
| Study group | p-Value | ||
|---|---|---|---|
| Control (n = 58) | Observation (n = 58) | ||
| Recovery | 9 (15.5 %) | 17 (29.3 %) | 0.025 |
| Effectual | 13 (22.4 %) | 21 (36.2 %) | |
| Effective | 20 (34.5 %) | 13 (22.4 %) | |
| Ineffective | 16 (27.6 %) | 7 (12.1 %) | |
- Note: Values were expressed as n (percentage, %). p-Value was derived from chi-square test.
3.3 Comparison of clinical symptom scores between the control and observation group
After treatment, the two groups of patients were scored in four aspects including erythema, flushing, telangiectasia, and papulopustule. The lower the score, the more significant the treatment effect. Our results showed that the scores of erythema, flushing, telangiectasia, and papulopustule were significantly reduced in the observation group (Table 3), indicating that mesoderm therapy-introduced compound glycyrrhizin injection could more effectively improve facial symptoms in patients with rosacea.
| Study group | |||
|---|---|---|---|
| Control (n = 58) | Observation (n = 58) | p-Value | |
| Erythema | |||
| 0 | 13 (22.4 %) | 26 (44.8 %) | 0.019 |
| 1 | 25 (43.1 %) | 23 (39.7 %) | |
| 2 | 17 (29.3 %) | 9 (15.5 %) | |
| 3 | 3 (5.2 %) | 0 (0 %) | |
| Flushing | |||
| 0 | 15 (25.8 %) | 28 (48.3 %) | 0.018 |
| 1 | 20 (34.5 %) | 21 (36.2 %) | |
| 2 | 16 (27.6 %) | 6 (10.3 %) | |
| 3 | 7 (12.1 %) | 3 (5.2 %) | |
| Telangiectasia | |||
| 0 | 10 (17.2 %) | 21 (36.2 %) | 0.029 |
| 1 | 24 (41.4 %) | 26 (44.8 %) | |
| 2 | 19 (32.8 %) | 8 (13.8 %) | |
| 3 | 5 (8.6 %) | 3 (5.2 %) | |
| Papulopustule | |||
| 0 | 23 (39.7 %) | 30 (51.7 %) | 0.038 |
| 1 | 21 (36.2 %) | 25 (43.1 %) | |
| 2 | 9 (15.5 %) | 2 (3.5 %) | |
| 3 | 5 (8.6 %) | 1 (1.7 %) | |
- Note: Values were expressed as n (percentage, %). p-Values were derived from chi-square test.
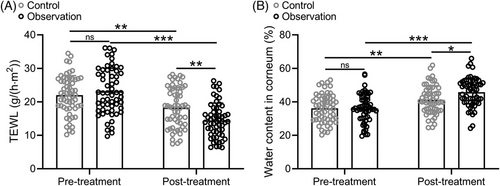
3.4 Comparisons of TEWL and water content in corneum between the control and observation group
After treatment, skin physiology tests were performed on both groups, mainly including skin transepidermal water loss and stratum corneum water content. Our results showed that both the control and observation groups significantly decreased TEWL, but the observation group decreased more significantly (Figure 1A). In addition, both the control and observation groups significantly increased the water content of the stratum corneum, but the observation group increased more significantly (Figure 1B). In conclusion, the two groups of treatment effectively improved the physiological state of the skin, but the observation group had better effect.
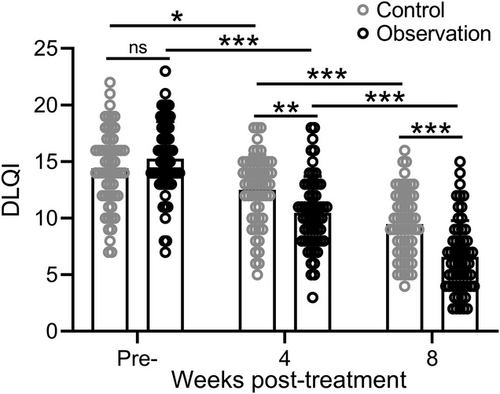
3.5 Comparisons of DLQI between the control and observation group
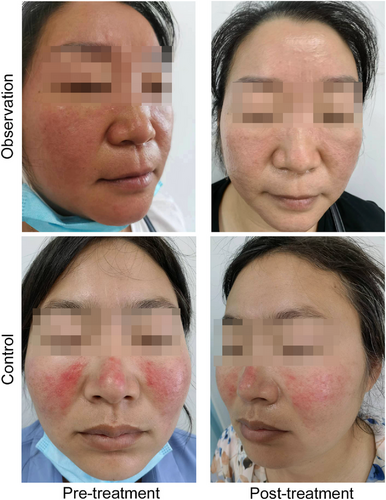
The DLQI before and after treatment was compared between the two groups to assess the extent to which the skin condition affects the patient's life. Our results showed that DLQI scores gradually decreased as treatment progressed, indicating that the degree of rosacea's impacted on patients' lives gradually decreased. The observation group decreased more obviously, and the improvement degree was better than that of the control group (Figure 2). The representative photography of patients between the two groups before and after treatment were shown in Figure 3.
4 DISCUSSION
Rosacea is a chronic relapsing inflammatory disease. The etiology and pathogenesis of rosacea are still unclear.13 At present, some studies suggest that rosacea is associated with abnormal neurovascular regulation, neuroimmune interaction, microbial imbalance, abnormal activation of skin innate immunity.14 It has been reported that inflammatory responses are present in all phenotypes of rosacea, and even rhinophyma, which is not clinically inflamed, has abnormal inflammation-related genes at the level of transcriptome studies.15 Vascular abnormalities in rosacea are mainly manifested in telangiectasia and perivascular inflammatory cell infiltration in the superficial and middle dermis.16 The dilated capillaries are mainly reflected in the dilation of the diameter of the capillaries and the abnormal morphology of the blood vessels. Inflammatory cell infiltration in rosacea is dominated by monocytes including lymphocytes, histiocytes and plasma cells.17 Treatment of rosacea patients becomes tricky based on the above pathological and physiological processes of blood vessels and inflammation.18 Although topical metronidazole, azelaic acid, and systemic tetracyclines have been shown to be effective in rosacea, there is still no effective treatment for persistent facial erythema.19 At present, many scholars have also proposed the use of laser or combined laser treatment of rosacea, which can achieve a certain clinical effect.20
Compound glycyrrhizin is a compound extracted from licorice, and its main component is glycyrrhizic acid.21 Compound glycyrrhizin can inhibit the production of antibodies.22 It has been reported that the use of compound glycyrrhizin resulted in multi-target inhibition of the complement system and downregulation of the production of inflammatory factors and mediators.23 Compound glycyrrhizin can also produce corticosteroid-like effects by down-regulating the number of glucocorticoid receptors and competitively inhibiting various metabolisms of glucocorticoids, thereby inactivating enzymatic activity.24 Compound glycyrrhizin can play an anti-inflammatory effect by inhibiting high mobility group box protein 1. Therefore, compound glycyrrhizin is commonly used in the treatment of inflammatory and allergic skin diseases.25 The most common clinical administration of compound glycyrrhizin is oral and intravenous infusion in traditional compound glycyrrhizin injection drug therapy. Studies by scholars at home and abroad have found that due to the first-pass effect of oral drugs and the decrease of blood drug concentration in intravenous drip, the systemic application of compound glycyrrhizin can increase the probability of adverse reactions.26 The adverse reactions of oral and intravenous infusion of compound glycyrrhizin mainly include shock and anaphylactic shock, mainly manifested as decreased blood pressure, unconsciousness, dyspnea, cardiopulmonary failure, flushing, facial edema, etc.27 In addition, there are reports that unreasonable application of compound glycyrrhizin may lead to pseudo aldosteronism. Patients who increase the dose or use compound glycyrrhizin continuously for a long time may experience symptoms such as high hypokalemia, increased blood pressure, sodium and body fluid retention, edema, and weight gain. Adverse reactions and medication strategies of compound glycyrrhizin can easily lead to problems such as reduced patient compliance.28 Therefore, in order to improve the therapeutic effect, it is necessary to explore other modes of administration of compound glycyrrhizin.
In this study, we additionally used mesoderm therapy injection compound glycyrrhizin as the observation group on the basis of the control group who received topical metronidazole clindamycin liniment. Our results showed that the study group was able to significantly relieve the symptoms of flushing, erythema, telangiectasia, papules and pustules in patients with rosacea. The reason may be that the active ingredient of compound glycyrrhizin injection is glycyrrhizin, which can relieve local inflammation. The erythema is formed after the stimulation of skin inflammation, which is caused by the continuous expansion of blood vessels or the proliferation of blood vessels. The latest research found that compound glycyrrhizin can reduce the level of IL-37, an antimicrobial peptide product in patients.29 It can be seen that the application of compound glycyrrhizin in the treatment of rosacea can effectively control the inflammatory progression of rosacea by reducing the level of IL-37 in patients. In addition, we found that the symptoms of telangiectasia in patients with rosacea in the experimental group were significantly relieved than those in the control group. The reason may be that compound glycyrrhizin plays an important role in improving microcirculation and blood rheology. Compound glycyrrhizin can achieve the curative effect of receding erythema by improving the regulating function of neurovascular and relieving telangiectasia.
Skin barrier function plays an important role in the pathogenesis of rosacea. The skin barrier function mainly refers to the physical barrier of the stratum corneum. When skin is damaged, its barrier function is bound to be compromised, resulting in higher water loss. TEWL and skin stratum corneum water content are important indicators for evaluating skin barrier integrity in vivo.30 Decreased TEWL and increased skin stratum corneum content are considered indicators of skin barrier integrity or restoration.31
The results of this study showed that both treatments were effective in improving the physiological state of the skin, but our proposed injection therapy was more effective. However, our research still has some shortcomings. First, due to time and resource constraints, we only recruited 116 participants in our study. The limited research sample may reduce the accuracy and generalizability of the research results. We plan to conduct multiple heart studies enrolling as many patients as possible in future studies to further identify the role of mesoderm injection therapy. Furthermore, our study only reveals the adjunctive effect of mesoderm introduction of compound glycyrrhizin injection treatment for the treatment of rosacea. However, the biological mechanism by which mesoderm introduction of compound glycyrrhizin injection treatment improves skin inflammation remains unclear. We plan to further study the regulation mechanism of mesoderm introduction of compound glycyrrhizin injection treatment on skin inflammation in future research.
5 CONCLUSIONS
In conclusion, our data showed that the use of mesoderm therapy combined with compound glycyrrhizic acid had a therapeutic effect on facial rosacea and improved patient satisfaction.
CONFLICT OF INTEREST STATEMENT
The authors have no financial interest to declare in relation to the content of this article.
FUNDING INFORMATION
The authors received no specific funding for this work.
Open Research
DATA AVAILABILITY STATEMENT
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.




