Short-Term Severe Low Energy Availability in Athletes: Molecular Mechanisms, Endocrine Responses, and Performance Outcomes—A Narrative Review
Funding: The authors received no specific funding for this work.
ABSTRACT
Many athletes and coaches believe that reducing body mass can improve the power-to-body mass ratio and improve exercise performance. This narrative review aims to characterize the effects of short-term (days to weeks) severe (< 30 kcal/kg Fat Free Mass/day) low energy availability (LEA) on exercise performance and physiological parameters related to health and training adaptations in female athletes. The latter is based on the prevalence of LEA being higher among female athletes, and most of the research is conducted on this population. In addition, we briefly address emerging evidence on short-term severe LEA in male athletes to highlight potential sex differences in physiological responses and performance outcomes. Short-term severe LEA triggers energy-conserving responses, leading to disruption in several crucial physiological systems, including suppression of the hypothalamic–pituitary-ovarian axis, decrease in triiodothyronine and insulin-like growth factor I hormones, reduction in resting metabolic rate, and comprised protein turnover in collagen-rich tissues. If these detrimental effects of short-term severe LEA are not reversed, they can progress to long-term problematic LEA, resulting in hypothalamic amenorrhea, lowering of bone mineral density, increased injury risk, and impaired exercise performance. Recent studies further underscore the detrimental impact of short-term severe LEA in female athletes, revealing suppressed muscle protein synthesis, increased cortisol levels, altered immune function, enhanced fat utilization during exercise, and direct impairments in power, sprinting, and endurance exercise performances despite reductions in body mass. These findings highlight the concerns about the trade-offs between short-term severe LEA for body mass reduction and the ability to maintain optimal physiological function for exercise performance. Further, they challenge the widespread assumption that body mass reduction always improves exercise performance, emphasizing a need for case-by-case considerations within the sporting environment.
1 Introduction
A higher power-to-mass ratio may improve performance outcomes in many sport disciplines and may be achieved by altered body composition with increased lean mass and/or reduced body fat [1]. Although the power-to-mass ratio may have the greatest impact on performance in weight-bearing sports, such as cross-country skiing and running, the best professional cyclists also have higher power outputs relative to body mass during short intense work compared to their competitors [2]. A recent comprehensive review exploring the effects of body composition on performance variables in athletes (female and/or males) concluded that the higher the percent of body fat, the slower the race time [3]. However, other variables, such as training volume (duration and intensity), running pace during training, or previous athletic results were shown to be of equal or even greater importance for performance [3]. These findings raise the question: In the pursuit to improve sports performance in athletes, should the focus be on reducing fat mass? In this regard, it should be taken into account that an overemphasis on maintenance of body mass and/or a specific body composition can lead to body dissatisfaction, body image disturbances, and disordered eating and exercise training behaviors resulting in problematic low energy availability (LEA) [4]. Consequently, the common belief among athletes and coaches that reduced total body mass contributes to improved exercise performance may only be true in certain sporting contexts [5-8]. This is a critical issue, as the prevalence of restricted eating behaviors and eating disorders is high among athletes, especially female athletes [4]. While the potential health and performance risks associated with LEA have been emphasized in the International Olympic Committee's (IOC) consensus statement on Relative Energy Deficiency in sport (REDs) [4], the exact threshold at which EA becomes problematic remains debated. The commonly used cut-off of < 30 kcal/kg Fat Free Mass (FFM)/day is not a universal threshold but rather a suggested indicator of potential risk. Notably, emerging evidence now suggests that even short-term (days-to weeks) exposure to severe LEA—defined in this review as EA < 30 kcal/kg FFM/day that results in measurable physiological disruption—can negatively affect health and performance outcomes in athletes [9] Despite these risks, many male and female athletes continue to practice short-term severe LEA, particularly in the final weeks leading up to competition, aiming to reduce body mass by 2–3 kg [4, 9, 10]. For example, imagine athletes who 2–4 weeks before a competition are considering whether reducing body mass would benefit performance. Will a deliberate LEA exposure to decrease body and fat mass affect the quality of exercise training, metabolic responsiveness, injury and illness risk, and ultimately lead to a performance advantage on the day of competition?
In this narrative review, we specifically aim to characterize the potential effects of short-term severe LEA on performance, and physiological parameters related to health and adaptations to exercise training in female athletes. The focus on female athletes specifically is based on LEA being more prevalent among female athletes, including both sub-elite and elite female athletes, and most of the research is conducted on this population. Notably, much of the research within this specific area is primarily based on sub-elite female athletes. Further, while the focus is on female athletes, certain points of discussion are applicable to both sexes. In such cases, the term ‘athlete’ will be used without specifying sex.
2 Energy Availability in Female Athletes: Concepts and Adaptability
Elite athletes require optimal dietary energy and nutrient intake to support exercise performance, exercise adaptations, and essential physiological functions, including thermogenesis, reproductive function, and cellular maintenance. If total energy intake is insufficient, the body prioritizes energy allocation to vital processes over other functions [11]. To maintain a neutral energy balance, the energy intake must match the total energy expenditure.
During periods of negative energy balance, where adipose tissue and body proteins contribute to the body's energy needs, energy is prioritized for vital functions such as locomotion, thermoregulation, and cellular maintenance [11, 12]. Consequently, energy is diverted away from non-essential processes like reproductive function (situational energy priority “trade-off”) [13], leading to various normal and expected metabolic and energetic adaptations [13, 14], that reduce energy expenditure to prevent further loss of body mass [15].
In sports nutrition and exercise physiology, energy availability refers to the energy left to support energy demanding processes and functions after subtracting the energy expended during exercise from the total energy intake [4], and is expressed relative to fat-free mass (Loucks 2004) [16]. In the 2023 IOC consensus statement on REDs, LEA is conceptualized as either adaptable or problematic [4]. Adaptable LEA generally refers to mild short-term (days to weeks) lowered EA (30–40 kcal/kg FFM/day) that may trigger reversible physiological changes without adverse outcomes. In contrast, problematic LEA involves a more severe LEA (typically ≤ 30 kcal/kg FFM/day) over a short-term period (weeks) or long-term (months to years) periods, often resulting in measurable impairments in health and exercise performance. The physiological impact of LEA is influenced by multiple moderating factors like age, sport discipline, and training volume that might either mitigate or exacerbate risks of disturbances in female and male athletes as well as contextual factors that may influence the potential impact of LEA. These include the duration and severity of LEA; consistency (e.g., chronic or intermittent periods of LEA); the athlete's starting point regarding body composition and health issues; the nutrient composition of the diet; age and developmental stage; psychological stress which may exacerbate the impact of LEA, and genetic predisposition [4]. Therefore, no definitive or individualized clinical threshold of LEA or optimal energy availability (OEA) currently exists, particularly among female athletes across performance levels [14].
In female athletes as well as in male athletes, the underlying causes of short-term severe LEA may be inadvertent exposure during periods with high exercise training loads [17] in combination with poor or misinterpreted sport nutrition knowledge [18], low energy dense diets [19], insufficient appetite [20-22], and potential limits within the digestive system [23]. Short-term severe LEA may also occur intentionally to achieve a lower competitive body mass and body fat mass [15]. Emphasis on leanness or low body mass increases the risk of eating disorders, especially combined with other risk factors such as participation in weight-class and leanness sports, frequent dieting, and fluctuation of body mass [24], and use of pathological weight-control practices [25]. Pressure from social media and from coaches to achieve an optimal sport-specific body mass or shape is a significant risk factor [24, 26, 27], with more than 60% of elite female athletes reporting experiences of body-shaming pressure from their coaches [28].
3 Physiological Consequences of Short-Term Low Energy Availability
Exercise performance depends on a broad range of physiological parameters, with their relative importance varying across different sport disciplines.
Females, particularly young individuals, are highly sensitive to low energy and CHO availability [11, 29]. Short-term severe LEA has been shown to trigger a series of physiological responses aimed at conserving energy for vital processes in females (Figure 1) [30, 31]. These compensatory mechanisms adversely affect various aspects, including the endocrine, skeletal muscle, immune, and cognitive systems, ultimately compromising health and exercise performance. In the following sections, discussions of how many of these physiological parameters may be negatively affected by short-term severe LEA are presented (Figure 1).
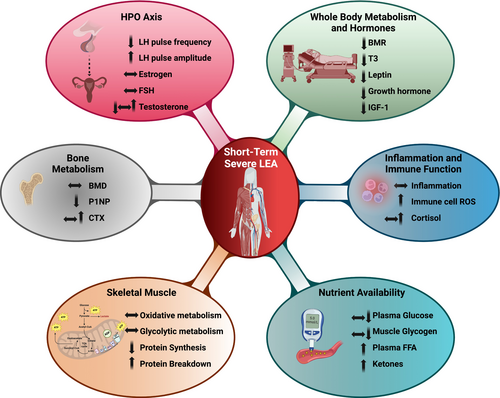
3.1 Short-Term Severe LEA Induces Changes in Luteinizing Hormone Pulsatility
Under conditions of OEA, the hypothalamus secretes gonadotropin-releasing hormone in a pulsatile manner, driving the cyclic release of luteinizing hormone (LH) and follicular stimulating hormone from the anterior pituitary. However, short-term severe LEA can significantly suppress LH pulsatility (i.e., serving as a proxy for gonadotropin-releasing hormone activity). In classic work illustrating this point, Loucks and associates [30], conducted a study where 14 regularly menstruating, habitually sedentary, young females participated in a 5 day protocol involving supervised exercise expended 15 kcal/kg of FFM. During the experimental period subjects were exposed to an energy availability of either 10, 20, 30, or 45 kcal/kg FFM. The findings revealed that when the energy availability was below 30 kcal/kg FFM, the LH frequency decreased, whereas the LH pulse amplitude increased. Similarly, Williams et al. [32], also reported a 21% reduction in LH pulse frequency during the mid-follicular phase following a short-term (4-day) increase in exercise training volume; but only when the increased energy expenditure was not compensated by a corresponding increase in energy intake to meet the energy demands of the higher exercise training volume. Furthermore, Loucks et al. demonstrated that a single day of recovery with increased energy intake resulted in a notable rise in LH pulse frequency in the females after the 5 days of LEA. Similarly, others have shown that restoring OEA after short-term LEA can reverse changes in LH pulsatility and restore estradiol levels [33]. However, further research is needed to clarify how recovery timelines may differ between individuals. These findings also underline the importance of OEA for maintaining reproductive function in females independently of their exercise training level. Conversely, if severe LEA is prolonged (weeks to months), it will trigger hormonal alterations, primarily by disrupting the hypothalamic-pituitary-ovarian (HPO) axis. The longer suppression of the HPO axis persists, the more likely the risk of functional hypothalamic amenorrhea (FHA) and long-term complications including infertility, particularly if left untreated for an extended period [34, 35].
Insulin-like growth factor I (IGF-I) is another hormone that is markedly suppressed during severe short-term LEA exposure [30]. This reduction in IGF-I has profound effects on the HPO axis, as IGF-I is known to enhance ovarian responsiveness to gonadotropins, promoting follicular development and estrogens production [36]. Consequently, diminished IGF-I levels impair ovarian follicle growth, further contributing to menstrual irregularities associated with severe LEA if prolonged over weeks/months. Additionally, leptin acts both as a peripheral signaling molecule of energy availability, which is suppressed after short-term LEA [37] and as a stimulating effector on GnRH secretion [38, 39]. Therefore, lowered leptin levels during short-term severe LEA may contribute to the suppression of the HPO axis and potentially to FHA, if maintained.
3.2 Thyroid Hormones and Resting Metabolic Rate (RMR)
Resting metabolic rate (RMR) represents the energy required to maintain essential physiological processes while the body is at rest. RMR accounts for 50%–70% of total energy expenditure, depending on the physical activity level [40]. RMR supports basal cellular metabolism, body temperature, brain activity, and the functions of vital organs, such as the heart, liver, and kidneys, which are responsible for cognitive function, blood circulation, and waste elimination. A reduced RMR, adjusted for body composition, may indicate that the body is compromising essential body functions to conserve energy under LEA exposure. A lower measured RMR compared to calculated RMR using standard equations based on sex, age, and FFM (RMR ratio) has been observed in female athletes with FHA [19, 41-44] and has been suggested as a proxy indicator of LEA-induced metabolic adaptations [44, 45]. A study by Oxfeldt et al. demonstrated that just 10 days of severe LEA (25 kcal/kg FFM) resulted in a significant decrease of 4% in RMR, which was not fully restored after 2 days of refueling [46]. The latter suggests that there is an increased risk of rapid fat mass gain after a period of LEA, but also indicates that general physiological functions are partially suppressed which may hamper the recovery processes and, consequently, impairing exercise performance.
One of the proposed primary endocrine responses to LEA is a reduction in thyroid hormone production, particularly triiodothyronine (T3), which plays a critical role in regulating RMR [47]. A decrease in T3 lowers RMR but may also impair exercise performance through various mechanisms such as the muscle contraction-relaxation cycle [48, 49], expression of mitochondrial biogenesis [50, 51] and mitochondrial uncoupling protein 3 (a mediator of thermogenesis), and working economy [52]. However, to date, there is limited evidence on T3 after short-term severe LEA exposure, although periods of caloric restriction of even a short duration are well known to reduce T3 [53, 54].
3.3 Catabolic Influence of Hormonal Changes on Muscle Mass
In an energy-deficient state, muscle growth is not prioritized. After just 5 days of LEA at 30 kcal/kg FFM/day, muscle protein synthesis was observed to be 27% lower compared to an OEA diet [55]. A high protein intake and resistance exercise may help mitigate muscle mass loss during periods of LEA [55]. However, in a randomized controlled study in moderately trained female athletes with 10 days of severe LEA at 25 kcal/kg FFM/day, the accumulated daily muscle protein synthesis rate remained lower than baseline and lower than a control group with OEA at 52 kcal/kg FFM/day, even though both groups performed resistance exercise 8 out of 10 days and consumed the same amount of protein (2.2 g protein/kg FFM/day) [56]. Consistent with these findings, nitrogen balance was negatively affected by LEA and was paralleled by a significant loss of FFM as measured by DXA, highlighting that short-term severe LEA can lead to measurable declines in muscle mass even under optimal protein intake and training conditions. In contrast, the control group exhibited an increase in both muscle protein synthesis rate and FFM following the same exercise training program [56]. Consistent with these findings, our recent study further demonstrated a significant reduction in FFM after 14 days of LEA (22 kcal/kg FFM/day and 2.4 g protein/kg FFM/day) in highly trained female athletes, as assessed by DXA, a decrease in which could not be attributed to the reduction in skeletal muscle glycogen, which was maintained [57, 58]. These results emphasize the need to carefully consider the risk of FFM loss when short-term severe LEA is used as a strategy for weight or body composition management.
The reduction in muscle protein synthesis rate during short-term severe LEA is likely related to the observed downregulation of key anabolic hormones such as insulin and T3 [56], or IGF-I [59], which play crucial roles in stimulating muscle protein synthesis [60]. Furthermore, the increased cortisol levels associated with LEA [57] may exacerbate the effects of reduced insulin, IGF-I, and T3 by further inhibiting anabolic signaling pathways, such as mTOR, and promoting protein degradation, intensifying the catabolic environment in skeletal muscle [61, 62].
While changes in estrogen levels are typically a consequence of long-term rather than short-term LEA, recent data suggest that short-term severe LEA may have a direct effect on the ability of estrogen to act on skeletal muscle. That is, in a recent study [57, 58], we observed that although estrogen concentrations remained unaffected by 14 days of short-term severe LEA (22 kcal/kg FFM/day), skeletal muscle estrogen receptor content was reduced. Specifically, LEA led to a 16% reduction in the skeletal muscle protein content of estrogen receptor beta, and although the reduction in the estrogen receptor alpha was not statistically significant, it decreased by 15% (unpublished data). Although these findings are based on a small sample size (10 highly trained females), future studies should confirm and further investigate this and their implications for health, adaptations to training, and exercise performance in female athletes.
Since performance in most strength and power-related sports disciplines is closely linked to muscle mass, the potential performance benefits of a period of LEA to promote loss in body mass should be carefully considered relative to the potential disadvantages, particularly the high risk of loss of FFM (i.e., principally muscle mass) during the LEA period.
3.4 LEA-Induced Hormonal Changes Challenge Collagen-Rich Tissues
Collagen, the main structural protein in bones, is furthermore the main structural protein in skin, ligaments, tendon, and connective tissue, making it the most abundant protein in the human body. Thus, the consequence of LEA likely extends beyond bone health to other collagen-rich tissues. However, given the limited evidence on these broader complications, further research is warranted.
Among the most concerning physiological consequences of problematic LEA is its detrimental impact on bones, leading to an elevated risk of reduced bone accrual, low bone mineral density, changes in bone microarchitecture and strength with increased risk of bone stress injuries and osteoporosis. Even short-term severe LEA has been shown to negatively affect bone turnover [63, 64]. Papageorgiou et al. examined the effect of short-term severe LEA on bone turnover markers [64]. They recruited active, eumenorrheic females who were exposed to 3 days of severe LEA (15 kcal/kg FFM) through either dietary restriction or increased exercise energy expenditure. The results showed that dietary restriction reduced the bone formation marker P1NP by 17%, whereas no changes were observed in bone resorption marker CTX. In contrast, exercise-induced LEA did not affect either bone formation or resorption markers even though both LEA conditions led to reductions in IGF-I. This suggests that in the group with the increased exercise stimuli, exercise mitigated the negative effects of LEA on bone turnover. Nevertheless, in a study in elite male walkers exposed to 6 days of LEA (10–25 kcal/kg FFM), 25 km of race walking enhanced the level of the bone resorption marker CTX, whereas the CTX level was unchanged in the control group [63]. Additional studies are needed to investigate the effects of LEA in elite female athletes. Noteworthy, reduced carbohydrate (CHO) intake is also reported to negatively influence markers of bone turnover independently of EA in male and female athletes [63, 65, 66]. Moreover, cross-sectional studies consistently demonstrate a higher risk of low bone mineral density in female athletes categorized with LEA/eating disorders and/or FHA compared to controls [19], which underlines that the protective effect of load-bearing exercise does not seem to counteract the negative effects of problematic LEA. However, no controlled, long-term studies have directly examined the effect of recurrent short-term periods of problematic LEA on bone health in female athletes, largely due to ethical concerns about introducing LEA in female athletes over a prolonged period [67]. Nonetheless, the LEA-induced reduction in key hormones, such as estrogen, T3, and IGF-I, regulate collagen turnover and bone remodeling [68, 69], raises concerns regarding impaired tissue responsiveness to exercise training stimuli and an increased risk of injury, even after short-term severe LEA.
In the context of sport performance, stress fractures and tendon or ligament injuries often necessitate prolonged periods of cessation of exercise training, resulting in the loss of both strength and endurance adaptations. The importance of consistency in the training of athletes (males and females) is often overlooked but is essential for improving exercise performance and maintaining the ability to perform at peak levels. Beyond the increased risk of injuries associated with severe LEA, a high prevalence of illness can certainly disrupt consistency in training, potentially affecting both exercise performance and physiological training adaptations in athletes [70].
3.5 Immunological and Inflammatory Changes by LEA and Low Carbohydrate Availability
There appears to be a direct link between LEA and the immune system. Observational studies suggest that periods of high training volumes are associated with an increased risk of illness among female and male athletes compared to periods of lower training volume [71]. Notably, this elevated risk of illness seems particularly prevalent among female athletes [72, 73]. Symptoms of LEA have also been linked to illness in Olympic male and female athletes [73], which according to the IOC consensus statement, may be due to impaired immune function as a consequence of problematic LEA [4]. However, the effects of short-term severe LEA on immunological parameters have remained unclear.
Several studies have explored the impact of LEA on immune function and the inflammatory response to acute exercise and have suggested a potential link [74-78]. Sarin et al. examined female physique athletes during a 21-week period intended to reduce body mass and found that severe LEA (self-reported dietary diaries: 25 kcal/kg FFM/day) and exercise training were associated with changes in several molecular pathways related to suppressed immune cell proliferation and function [76]. Furthermore, Jeppesen et al. [57] showed that 14 days of severe LEA (25 kcal/kg FFM/day) in highly trained female athletes led to alterations in isolated immune cells and elevated cortisol levels, though systemic inflammation markers remained unchanged. This latter study is the first study to demonstrate that short-term severe LEA directly affects immune cells, inducing a change in their redox state, specifically by increasing the capacity to produce reactive oxygen species (ROS) and, thereby, oxidative stress. Additionally, short-term severe LEA was associated with lower mobilization of immune cells in response to acute exercise and with changes in several plasma proteins, with isolated immune cells showing higher ROS formation compared to conditions of OEA.
It is important to note that glucose is a crucial macronutrient for immune cell function [79], and that reduced carbohydrate intake is a common consequence of LEA. Therefore, the immunological changes observed with short-term severe LEA may be more closely related to low carbohydrate availability rather than LEA per se. Indeed, high carbohydrate availability during exercise has been shown to attenuate transient immune perturbations, inflammation, and oxidative stress following acute exercise [80].
Consistently, a low carbohydrate diet has been associated with altered responses to acute exercise, including changes in adrenaline and cortisol levels, neutrophil and lymphocyte mobilization, and an enhanced pro-inflammatory response [80, 81]. Interestingly, this pro-inflammatory response is diminished with high carbohydrate intake (70% of daily intake) [80, 82]. Similarly, Burke et al. showed that low carbohydrate intake (0.5 g/kg/day), rather than LEA alone, caused a greater increase in exercise-induced interleukin-6 after 6 days [83]. However, further studies determining the consequences of short-term LEA on immune function are warranted.
3.6 Cognitive and Neurological Effects
Cognitive function is intricately linked to energy availability, as the brain relies on a continuous supply of glucose to meet its metabolic demands. In conditions of LEA, reduced glucose availability and related hormonal changes may contribute to cognitive and neuromuscular impairments, particularly in areas related to concentration, reaction time, and decision-making. LEA may also impair neuromuscular coordination by reducing the energy available for efficient motor unit recruitment and muscle fiber firing patterns, thereby potentially impairing performance in sports that demand quick reflexes and complex movement patterns, such as ball sports. Supporting these findings, Tornberg et al. [84] conducted a cross-sectional study that demonstrated slower reaction times in elite endurance female athletes with clinically verified FHA, as a surrogate marker for LEA, and reduced blood glucose concentrations compared to their eumenorrheic counterparts. Furthermore, the reaction time was negatively associated with T3 [84], which is reported to influence neurotransmitter regulation and synaptic signaling, both of which are crucial for cognitive and motor function [85]. While mechanistic insights into the T3-cognition relationship in athletes are still emerging, relevant evidence from the general population supports a role for energy status in modulating T3 levels. For example, Weiss et al. (2008) conducted a 12-month randomized controlled trial and found that individuals undergoing caloric restriction, but not those who lost a similar amount of fat mass through exercise, experienced significant reductions in circulating T3 [86]. These findings suggest that energy intake itself, independent of fat loss, can influence thyroid hormone levels. This raises the possibility that athletes undergoing intentional dietary restriction may experience similar hormonal shifts, which could, in turn, influence cognitive and neuromuscular performance though direct links in this population remain to be established.
Taken together, while current data suggest plausible pathways through which LEA could impair brain and neuromuscular function, direct evidence in athletic populations is limited, and more controlled studies are needed. This remains a promising and important area for future research.
4 Short-Term Low Energy Availability and Exercise Performance
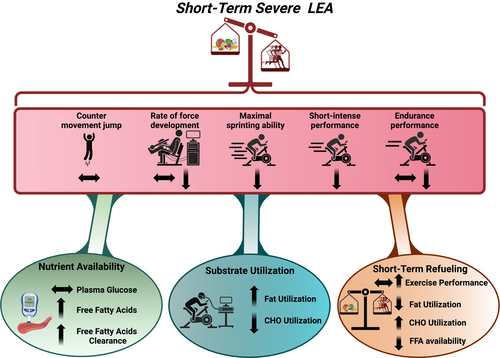
Short-term LEA may improve exercise performance in weight-bearing sports if the negative physiological consequences of energy deficiency do not outweigh the potential benefits of body mass reductions. However, the impact of short-term LEA on exercise capacity is complex and depends on the specific type of physical activity, such as power, strength, short high-intensity efforts, and endurance-based performances. Recent highly controlled studies indicate that short-term severe LEA is detrimental to a female athlete's exercise capacity [46, 57, 87], challenging the assumption that lower body mass guarantees improved exercise performances. In the following section, we will discuss the effects of short-term severe LEA on maximal muscular strength and power, short-intense (1–4 min) exercise performance, and endurance capacity (Figure 2).
4.1 Maximal Muscular Strength and Power
The ability to generate maximal force or power is primarily determined by neuromuscular factors, such as motor unit recruitment and the synchronization of muscle fibers during maximal and repeated contractions. Previous studies suggest that short-term severe LEA impairs these processes, leading to reduced maximal force production, explosive power, and power-dominated performances, including impaired maximal cycling capacity [74, 84, 88-92]. A similar observation was recently made by Oxfeldt et al. in a highly controlled study, in which the effects of 10 days of severe LEA were examined in moderately trained females [46]. In this study, LEA was associated with a 13% reduction in the rate of force development of knee extensors, which may be linked to the observed reduction in T3, as discussed earlier [84] Additionally, a 5% reduction in repeated sprint ability (5 × 6 s) was observed in the LEA group, while maximal isometric strength and counter movement jump remained unaffected by the intervention. It is important to note that the females increased their total exercise training volume (duration and intensity) during the study, which enhanced the repeated sprint performance in the group receiving OEA. These findings suggest a direct link between short-term severe LEA and impaired performance and highlight the crucial role of OEA to support exercise adaptations (described previously).
4.2 Short-Intense Exercise Performance (1–4 Min)
Performance at an intensity near V̇O2max relies heavily on glycolytic energy turnover, which is closely related to carbohydrate availability, as evidenced by reductions in muscle glycogen of up to 25% following 2–4-min all-out performance [93-96]. Whereas short-term LEA is often associated with lower carbohydrate intake, two recent studies demonstrate that lower carbohydrate availability is not related to impaired short-intense and endurance exercise performance following short-term severe LEA [46, 57].
Oxfeldt et al. [46], observed a 2.5% decrease in power output during a 4-min time trial (TT) on a bike ergometer after 10 days of severe LEA. Although LEA was associated with a modest reduction in muscle glycogen, the levels did not reach critically low values, nor were females hypoglycemic prior to the test [46, 56]. Interestingly, while LEA was associated with impaired short-intense performance, OEA for 10 days enhanced 4-min TT performance by 2.3%. Similarly, Jeppesen et al. [57], observed a 19% reduction in time to exhaustion (TTE) at 100% of V̇O2max on a bike in highly trained female endurance athletes after 14 days of severe LEA, despite a lower total work performed during a 20-min TT performed immediately prior (see below). Similar to Oxfeldt et al. the impaired performance capacity in 20-min TT and the following TTE at 100% of V̇O2max was not associated with lower carbohydrate availability, as resting and post-exercise blood glucose levels before, during, and following exercise, as well as skeletal muscle glycogen at rest, remained unchanged with LEA [58].
4.3 Aerobic Performances and Endurance Capacity
Loss of body mass can potentially enhance endurance performance in weight-bearing sports, as endurance performance has been found to be negatively correlated with body fat [3]. However, since glycogen is the primary energy substrate at intensities above 75% of V̇O2max [97], reduced carbohydrate intake during periods of LEA may lower glycogen availability and thereby impair prolonged endurance performance [98]. Surprisingly, the effects of short-term LEA on endurance capacity have not been well established under highly controlled conditions. However, a recent study demonstrated impaired endurance capacity after 14 days of severe LEA, with an 8% impairment of 20-min TT performance [57]. The observed performance impairment could not be attributed to changes in exercise training volume, which was maintained throughout the study, nor to reduced carbohydrate availability. However, short-term severe LEA induced a metabolic adaptation that led to an increase in fat oxidation during submaximal exercise and a large increase in the clearance of free fatty acids with exercise, suggesting a larger utilization of fat [58]. While these changes may partly explain the performance impairment, it is unlikely that they fully explain the entire impairment, as 3 days of refueling restored substrate utilization to pre-LEA levels while performance remained impaired by 7% [58].
Conversely, not all studies have reported impaired performance with short-term LEA. Kojima et al. [99], investigated the effect of 3 days of LEA (~17 kcal/kg FFM/day) in seven trained male distance runners and observed that performance in a predominantly aerobic exercise test (~20 min at 90% V̇O2max) was statistically unchanged. However, it is important to interpret these findings with caution due to the low number of participants and the numerically ~16% impairment in the LEA condition, although this did not reach statistical significance (p = 0.39). The possibility of a type II error cannot be excluded, and performance may have been affected to a relevant degree. The apparent preservation of performance in this study might reflect the greater resilience of male athletes to short-term LEA, as suggested by others [100]. Another consideration is the impact of body mass on weight-bearing performance; running economy may transiently benefit from reduction in body mass, potentially offsetting performance impairments due to LEA. In contrast, in cycling, where body mass is less influential, Jeppesen et al. showed that endurance performance remained impaired even after adjusting for body mass [57]. Additionally, differences in the duration and the degree of LEA exposure may explain the conflicting findings between studies, suggesting that endurance performance deteriorate progressively with prolonged LEA and the degree of LEA.
It should also be emphasized that even though high quality evidence for the effects of long-term LEA exposure (i.e., months to years) on performance is sparse, a few studies have suggested that performance may be impaired [84, 92, 101-103]. Although the exact effects remain unknown, it is likely that long-term LEA exposure, like short-term severe LEA, negatively impacts exercise performance, given the well-documented physiological consequences of severe LEA and its adverse effects on exercise training adaptations (as described previously).
5 Psychological Impacts of Short-Term Low Energy Availability
Four-week LEA interventions among female athletes have been linked to psychological indicators, including increased negative mood states, specifically greater levels of tension and confusion [104, 105]. However, our two studies with 10–12 days of severe LEA in female athletes did not report any changes in mood states [46] or motivation (readiness) to complete a 20-min TT (Jeppesen et al. 2024, unpublished data).
Early performance improvements following loss of body mass may compel female athletes to maintain the nutritional and exercise training approaches, reducing their psychological readiness to increase energy availability and prevent REDs [106]. Emphasis on leanness or low body mass whether arising from internal pressures or external influences (e.g., from coaches [28]), increases the risk of eating disorders, especially combined with other risk factors such as participation in weight-class and leanness sports, frequent dieting, and fluctuation of body mass [24], and use of pathological weight-control practices [25].
5.1 Influence of Refeeding Following Short-Term Severe LEA on Exercise Performance
Female athletes, whether aware or unaware of their exposure to short-term severe LEA, should ideally implement a subsequent refueling period to restore any reduced endogenous carbohydrate stores and optimize physiological function. However, those unaware of their LEA risk may fail to take the necessary steps to recover, potentially impacting both their health and performance despite the potential benefit of reduced body mass. The concept of refueling is gaining scientific attention as a potential strategy for athletes. In a well-controlled study by Burke et al. [87], highly trained male and female racewalkers underwent a 24 h carbohydrate-rich refueling period, following 9 days of severe LEA while intensifying exercise training. This refueling improved endurance performance (10 km race walk times) by 3.5%. However, it is important to note that the group receiving a carbohydrate-rich OEA had a similar improvement by 4.5% [87]. Similarly, in the study by Oxfeldt et al. [46] performance impairments induced by 12 days of severe LEA were largely restored after 48 h of refueling, although performance remained impaired compared to females who had maintained OEA throughout the study.
In contrast, Jeppesen et al. [57], found that a 72 h refueling period after 10 days of severe LEA only partially restored short-intense performance, whereas endurance performance remained impaired, compared to pre-LEA levels. This supports the notion that other mechanisms, beyond carbohydrate availability and changes in substrate oxidation, contribute to the compromised exercise capacity with short-term severe LEA in female athletes.
In summary, while short-term severe LEA followed by brief refueling periods may restore performance in certain situations, the lack of superior performance benefits compared to maintaining OEA observed in our studies [46, 57], suggest that maintaining OEA remains the better approach for female athletes at least in the short run.
6 Practical Strategies for Managing LEA in Female Athletes
The adaptive response to exercise training is influenced by a combination of factors, including the duration, intensity, type, and frequency of exercise training, as well as the quality, quantity, and timing of nutritional intake [107]. Therefore, female athletes need to tailor their energy and nutrient intake to their goals and workloads across different exercise training periods to ensure optimal recovery, exercise training adaptation, performance, and overall health [108, 109]. However, many female athletes report insufficient energy and carbohydrate intake [110, 111]. In a qualitative study by Langbein et al. including sub-elite endurance athletes (10 females, 2 males), half of the athletes reported unintentionally increasing their exercise training volume without increasing energy intake [101]. Compared to male athletes, females seem to have more difficulties periodizing dietary intake and instead maintain a similar energy intake during periods of training and competition [112]. This highlights the importance of being aware of an enhanced risk of unintentional LEA exposure in female athletes when the training volume is increased, for example, during a training camp [17]. Athletes and coaches need to be vigilant to the signs and symptoms that can increase the risk of LEA and REDs development. Risk factors associated with the development of problematic LEA and disordered eating behaviors in female athletes are (e.g., high competitiveness), early sport-specific exercise training, weight class rules and pressures from the sport environment to attain a certain body mass or shape [18, 113]. In pursuit of a lower body mass or ideal physique, female athletes often engage in unhealthy weight-control behaviors, which can precipitate REDs with and without disordered eating behavior or eating disorders [106]. Furthermore, female athletes who perceive performance improvements following loss of body mass may be compelled to maintain the nutritional and exercise training approaches, reducing their psychological readiness to reestablish OEA and prevent REDs [106]. Therefore, to prevent the potential negative effects of short-term severe LEA as well as problematic LEA and eating disorders in female athletes it is crucial for the sporting environment to ensure safe practices and carefully consider why, who, and how potential manipulation of body mass and body composition in female athletes should be addressed [3]. According to the latest best practice recommendations, assessment and manipulation of body composition should only be considered for adult female national level/elite athletes above 18 years (≥ Tier 3 [114]) without concerns around eating behaviors or physique/body image anxiety [3]. Furthermore, periods of manipulation of body composition should be well-planned and include structured and individualized nutrition plans that optimize recovery and reduce time spent in a catabolic state, followed by nutritional and mental support to reestablish OEA to mitigate potential psychological or physiological adverse effects [108].
Increased knowledge in sports nutrition and access to qualified dietitians and other health professionals can mitigate the risk of unintentional LEA exposure [18, 108]. However, the high prevalence of body dissatisfaction, unhealthy dieting, and eating disorders among female athletes calls for a cultural shift in attitudes and behavior in the sporting environment [3, 115]. This necessitates altering specific subcultural attitudes, behaviors, communication, as well as competition rules that contribute to detrimental practices concerning the regulation of body mass and body composition in female athletes [115]. Educational programs should be initiated early, targeting adolescent female athletes, their parents, and their coaches. Relevant educational topics include psychological development during adolescence, balanced nutrition for health and performance, and avoidance of negative comments and pressure on body mass in the context of sports and family environments.
7 Emerging Evidence in Male Athletes: Implications for Sex Differences
While the primary focus of this narrative review is on female athletes due to the higher prevalence of LEA and greater representation in the existing literature, emerging evidence in male athletes offer valuable insights into potential sex-based differences in physiological responses to short-term severe LEA. Although research in males remains relatively scarce and mainly limited to small-scale interventions, observational data, or case reports, recent studies have documented physiological disturbances at energy availability levels below ~20–25 kcal/kg FFM/day in male athletes.
In a short-term, randomized crossover study, Koehler et al. (2016) exposed six exercising males to 4 days of either LEA (15 kcal/kg FFM/day) or OEA (40 kcal/kg FFM/day), with and without daily exercise [116]. The study demonstrated significant reductions in leptin and insulin with LEA, whereas no changes were observed in testosterone, IGF-1, T3, or ghrelin. These findings suggest that while energy-sensing hormones respond rapidly to LEA, the reproductive and metabolic axes in males may exhibit greater resistance to short-term perturbations compared to females. Similarly, a short-term crossover study by Papageorgiou et al. (2017) assessed the effects of 5 days of LEA (15 kcal/kg LBM/day) on bone turnover in both physically active males and females [100]. In females, LEA led to an increase in bone resorption (β-CTX) and decreases in bone formation (P1NP). In contrast, group-level changes in males were not significant; however, the average changes in bone turnover markers (~12% increase in β-CTX and ~14% decrease in P1NP) were directionally similar to those observed in females. Notably, 6 of the 11 males displayed altered bone turnover, suggesting inter-individual susceptibility despite a lack of significant group-level effects in the males. These findings highlight the need for larger or longer-term studies to elucidate sex-specific thresholds, dose–response, or timelines of LEA-induced physiological disruptions.
Evidence for functional impairments in male athletes in response to LEA has been reported by Jurov et al. (2021, 2022). In the 2021 study 12 trained male endurance athletes (VO2max ≥ 55 mL/min/kg) completed a 14-day LEA protocol involving a 50% reduction in EA. The reduction in EA was associated with impaired performance in an incremental test to exhaustion, reduced explosive power as measured by countermovement jump, altered lactate metabolism, and decreased self-reported well-being—despite no measurable endocrine suppression or reduction in resting energy expenditure [117]. In a subsequent study by Jurov et al. 2022, six recreationally active males were exposed to 4 days of LEA (15 kcal/kg FFM/day) with or without exercise [118]. Similarly to the previous study, explosive power was reduced following LEA. Additionally, changes in lactate metabolism and declines in subjective well-being were observed. These findings support the notion that short-term LEA can impair physiological function and perceived health in males, even in the absence of classic endocrine disruptions.
Broader insights into the effects of LEA in males are provided by Fagerberg (2018), who reviewed studies involving male bodybuilders undergoing prolonged calorie restriction [119]. This work suggests that notable endocrine disturbances, such as suppression of testosterone and thyroid hormones, typically occur after sustained exposure to EA levels below 20–25 kcal/kg FFM/day, often persisting for several weeks or months. Muscle loss, mood disturbances, and cardiovascular issues were also reported, particularly as athletes approached extremely low body fat levels (~4–5%). Similarly, Keay et al. (2018) employed a sport-specific clinical interview and biomarker assessment (SEAQ-I) in 50 competitive male cyclists and reported that LEA was present in 28% of the cyclist [120]. The study suggested that EA was the most significant determinant of lumbar spine bone mass density and LEA, besides low bone mass density, was also associated with reduced body fat % and lower testosterone levels. These findings highlight how endocrine and skeletal disturbances can manifest subclinically in males, particularly in the context of sustained low EA and non-weight-bearing sports, and preventative actions are recommended before irreversible clinical changes manifest.
Overall, the current body of evidence supports the idea that sex differences may exist in the threshold, time course, and physiological systems most vulnerable to LEA. However, functional consequences, such as reduced power output, anemia, and impairments in well-being, can occur even in the absence of overt hormonal changes, raising important questions about the timing and nature of adaptation. Despite these emerging insights, individual variability remains high, and methodological limitations including small sample sizes, heterogeneous study designs, and inconsistent EA quantification limit broad generalization. Future well-controlled studies including both male and female groups are needed to clarify sex differences related to the need for sex-specific screening, prevention, and intervention strategies for LEA across a range of sports and athletic populations.
8 Conclusion
- Energy-conserving responses affecting multiple physiological systems, including endocrine, skeletal muscle, immune, and cognitive functions, and thereby compromising recovery and training adaptations.
- Reduced muscle protein synthesis rates and FFM, even with high protein intake and resistance exercise
- Suboptimal changes in various performance parameters, including cycling sprint and endurance performance and rate of force development in strength test.
Thereby, the review questions the trade-offs between reducing body mass and maintaining optimal physiological function, health, and performance of the female athlete.
9 Perspective
Short-term severe LEA in female athletes presents a multifaceted challenge with significant implications for both health and performance. While optimizing the power-to-mass ratio is often a goal in weight-sensitive sports, accumulating evidence suggests that the physiological costs of short-term severe LEA may outweigh its short-term potential performance benefits. Future research should prioritize understanding individual variability in response to LEA, considering factors such as genetics, sex, training status, and sport-specific demands.
Importantly, progress in this area is currently limited by several key methodological challenges. These include the difficulty of accurately assessing energy availability in free-living athletic populations, a lack of standardized and validated protocols for quantifying EA and diagnosing LEA, as well as a scarcity of well-controlled longitudinal or cross-over intervention studies. Moreover, inconsistencies in how LEA is operationalized in cross-sectional studies hinder direct comparisons and make it challenging to determine the exact point at which LEA begins to negatively affect physiology or performance. Addressing these limitations will be essential for developing a more systematic and translational research framework to guide clinical and performance decisions. There is also an urgent need for the development of practical tools and validated biomarkers that allow for real-time monitoring of energy availability. Such advances could help athletes and practitioners detect and prevent both unintentional and strategic LEA and guide timely interventions. In parallel, research should explore novel strategies to mitigate the adverse effects of severe short-term LEA when weight manipulation is unavoidable in the lead-up to competition.
From a practical standpoint, educating female athletes, coaches, and their support staff on the risks associated with severe short-term LEA is critical. This education should promote evidence-based approaches to body composition management that encouraged to adopt body composition management strategies that prioritize long-term health and performance sustainability. Ultimately, a paradigm shift may be required in the sporting community, moving away from short-term weight-cutting strategies and toward a model that values physiological integrity, athlete health, and performance outcomes.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




