Muscle metabolism and impaired sprint performance in an elite women’s football game
Abstract
The present study examined skeletal muscle metabolism and changes in repeated sprint performance during match play for n = 20 competitive elite women outfield players. We obtained musculus vastus lateralis biopsies and blood samples before, after, and following intense periods in each half of a friendly match, along with 5 × 30-meter sprint tests and movement pattern analyses (10-Hz S5 Global Positioning System [GPS]). Muscle glycogen decreased by 39% and 42% after an intense period of the second half and after the match, respectively, compared to baseline (p < 0.05). Post-match, 80% type I fibers and 69% type II fibers were almost empty or completely empty of glycogen. Muscle lactate was higher (p < 0.05) after the intense period of the first half and post-match compared to baseline (14.3 ± 4.6 (±SEM) and 12.9 ± 5.7 vs. 6.4 ± 3.7 mmol/kg d.w.). Muscle phosphocreatine was reduced (p < 0.05) by 16% and 12%, respectively, after an intense period in the first and second half compared to baseline. Blood lactate and glucose increased during the match and peaked at 8.4 ± 2.0 and 7.9 ± 1.2 mmol/L, respectively. Mean 5 × 30 m sprint time declined by 3.2 ± 1.7 and 7.0 ± 2.1% after the first and second half, respectively, and 4.7 ± 1.6% (p < 0.05) after an intense period in the first half compared to baseline. In conclusion, match play in elite female football players resulted in marked glycogen depletion in both fiber types, which may explain fatigue at the end of a match. Repeated sprint ability was impaired after intense periods in the first half and after both halves, which may be associated with the observed muscle metabolite perturbations.
1 INTRODUCTION
Competitive elite football for women has changed markedly during the last two decades.1, 2 The increased professionalism within the sport is represented by the increased athleticism of match play as total distances covered have increased from 8.52 to 10–11 km3, 4 with 1.5–2.0 km completed at high speed4,5 Differences in game load measures have also been identified between standards of competition,6 playing position,7 game types,7 and time-points within a match,8,7
Match performance characteristics of players competing at the highest competitive standard of European football differed markedly from their male counterparts.9 Moreover, elite players of both genders show indications of temporary fatigue during and toward the end of a game.5, 7, 10-12 Although research suggests that both female and male players tax oxidative and glycolytic energy systems significantly during a match,8, 11, 13 no information exists about the relationship between the decline in sprint performance during a women's game and alterations in skeletal muscle metabolites that are implicated in the onset of fatigue during intense activity, that is, lactate, pH, and glycogen. In male football players, glycogen reduction has been implicated in fatigue development during a game as its stores decline down to levels (~200 mmol/kg d.w.) that cannot sustain maximal glycolytic rate 14 and negatively affects sarcoplasmic reticulum (SR) Ca2+ handling.15 Furthermore, in male players, blood lactate is a poor indicator of muscle lactate metabolism during a football match and other types of intermittent exercise.10
However, gender differences do exist in muscle morphology and bioenergetics. Males and females may differ in fiber type composition and capillarization which in turn affect muscle's potential for mitochondrial vs. glycolytic energy production.16 During exercise, females appear to exhibit different energy substrate availability in the circulation and muscle and different patterns of glucose and fatty acid uptake by the muscle,17 lower glycolytic activity18 as well as different carbohydrate and fat oxidation rates than males.17, 19 Laboratory studies suggest that skeletal muscle adenosine triphosphate (ATP) breakdown, plasma catecholamines, muscle and blood lactate, and glycogen reduction in type I fibers are all lower in women than in men during sprinting.19-21 However, muscle metabolism has not yet been investigated during a football game in elite women players.
To determine the contribution of muscle glycogen, ATP, and phosphocreatine (PC) in the fatigue development during a football match of elite women players, we applied an experimental model with multiple muscle biopsies before, during, and after a game to examine the changes in muscle and blood metabolites along with changes in performance indicators, such as repeated sprint performance and match running performance. Thus, the goal of this investigation was to examine the skeletal muscle metabolic profile and relate it to repeated sprint performance during an elite women's football match. We hypothesized that repeated sprint performance and skeletal muscle metabolism would be affected after intense periods in both halves of an elite women's football match, and that muscle, but not blood, metabolites would be related to running performance indicators.
2 MATERIALS AND METHODS
2.1 Participants
A total of n = 30 elite women football players (representing all outfield positions) were approached, and n = 20 agreed to participate in the study (eight defenders, six midfielders, and six attackers), and the 17 players completing the full 90-min friendly match (three were injured during the match) had a mean (±SD) age of 23 ± 4 years, height: 166 ± 5 cm, body mass: 60.2 ± 7.5 kg, and body fat: 28.7 ± 6.3%. The number of players was selected in accordance with a previous study on muscle metabolism during football match play in men.22 Participation in the study was secured if volunteers (i) were division 1 players, (ii) practiced ≥5 times/week and participated in ≥1 games/week during the last five seasons, (iii) were free of recent injuries and/or illnesses (for ≥8 months prior to the study), (iv) were not consuming ergogenic supplements or medications (for ≥8 months prior to the study), (v) were not smokers, and (vi) had a regular menstrual cycle during the past 12 months. Participants were excluded from this study if they were using birth control agents, were anemic, or had irregular dietary intake. The experimental match was performed during participants’ mid-follicular phase of their menstrual cycle (days 5–10 of their cycle with the first day being the onset of menses).23 Participant's performance in Yo-Yo intermittent recovery level 1 (Yo-Yo IR1) and Yo-Yo intermittent endurance level 1 (Yo-Yo IE1) tests was 1104 ± 371 (range: 560–1720) and 2460 ± 786 (range: 1160–4000) m, respectively, had a VO2max of 51.2 ± 5.6 (40.1–59.2) ml/kg/min, and their peak 30-m sprint performance was 4.94 ± 0.28 (4.68–5.44) s. A maximal heart rate (HRmax) of 198 ± 6 (185–204) beats/min was recorded during Yo-Yo IR1, meeting the criteria for assessment of HRmax.24 Participants were fully informed of experimental procedures and possible risks and discomforts associated with the study before signing their written consent to participate. Procedures were in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki, as revised in 2013), and approval was obtained from the Institutional Ethics Committee (1078/2-2/10-2–2016).
2.2 Study design
The players participated in an experimental game on natural grass. During an adaptive period of 2 weeks, volunteers were familiarized with experimental procedures and participated in very light training (at local football facilities) aimed at developing match tactics and team cohesion. At the end of the adaptation period and before the game, participants underwent 3-day baseline performance testing at University facilities. Throughout the match, a number of physiological measurements were performed at fixed times (ie, at rest, pre-match, at half-time, and post-match) and after intense exercise periods within each half. Volunteers were randomly assigned, using the sealed envelope procedure, in two teams representing all field positions and played against each other in a full 90-minute match according to official regulations. A total of five additional players served as substitute players (from various playing positions), that could enter the pitch for some minutes when a player was leaving the pitch for physiological measurements or sprint testing. Ten players (full-match measurement group) had blood samples taken at rest, before, half, and after the game. These players had biopsies taken before and after the game. Another ten players (intense periods measurement group) had blood samples at rest, before and half of the game, and after an intense exercise period in each of the two halves. For these players, a muscle biopsy was taken after an intense exercise period in each half, identified as a period with a high number of high-intensity runs and sprints, evaluated by a UEFA Pro-License coach. An intense period in each half-included HR (>90%HRmax). To evaluate sprint performance during match play, a repeated sprint test was performed before the game (N = 20) as well as immediately after each half (N = 10) (full-match measurement group) or immediately after an intense period within each half (N = 10) (intense periods measurement group). The game started early in the afternoon (2–4 p.m.) under normal conditions with the temperature ranging from 17 to 19°C. A standardized 20-min warm-up period was performed before the game, with three phases of low, moderate, and high aerobic exercise intensity, with ball exercises and possession drills incorporated in the moderate and high-intensity exercise periods. Locomotor activities and exercise intensity of the match were recorded with 10-Hz S5 Global Positioning System (GPS) and heart rate monitors.
2.3 Preparation procedures
Prior to the matches, the players refrained from strenuous exercise and intake of alcohol for 48 h and from tobacco and caffeine for 12 h. Dietary intake was monitored with daily diet recalls 3 days before the experimental period and was standardized for all participants by a nutritionist as described in Draganidis et al.25 Meal composition was analyzed by a Hellenic food database “Science Fit Diet 200A” (Science Technologies, Athens, Greece). The prescribed food choices (ie, dairy products, bread, pasta, meat, fruits, vegetables, rice, cereals, etc.) were similar to athletes’ normal diet and contained physiological levels of antioxidants. On the day of the game, players arrived at the stadium 1.5 h before. Before the warm-up, a catheter (18 g, 32 mm) was placed in an antecubital vein and covered by a wrist bandage. In preparation for later obtainment of biopsies in m. vastus lateralis, an incision was made under local anesthesia (20 mg/ml lidocain without adrenalin) and covered by sterile band-aid strips and a thigh bandage. In addition, a GPS and heart rate monitor were placed on each subject.
2.4 Baseline measurements
During baseline testing, participants underwent a medical examination and had their body mass, height, and body composition and physical conditioning status measured. Body mass and height were measured on a beam balance with a stadiometer (Beam Balance-Stadiometer, SECA, Vogel & Halke) as previously described.26 Body mass index (BMI) was calculated as mass per height squared. Open-circuit spirometry was utilized for VO2max assessment using an automated online pulmonary gas exchange system (Vmax Encore 29, BEBJO296; Carefusion) via breath-by-breath analysis during a graded exercise testing on a treadmill (Stex 8025T) as previously described.27 Football-specific conditioning was measured using the Yo-Yo intermittent endurance test 1 (Yo-Yo IE1) and the Yo-Yo intermittent recovery test 1 (Yo-Yo IR1) with procedures previously described.24 The Yo-Yo and VO2max tests were performed on separate days. Additionally, 10-m sprint performance was recorded by infrared photocells with a precision of 0.01 s (Newtest Oy) 28 and countermovement jump height (CMJ) was measured on an Ergojump contact platform (Newtest Oy).26
2.5 Measurement of match locomotor activity pattern
Activity pattern characteristics during the match were quantified using a 10-Hz S5 GPS (Catapult Innovations). The GPS has previously been shown to provide a valid and reliable measure of instantaneous velocity during acceleration, deceleration, and constant motion.29 Time-motion characteristics were quantified as total distance (>0 km/h), standing/walking (0–2 km/h), jogging (2–13 km/h), running (13–16 km/h), high-intensity running (16–20 km/h), sprinting (>20 km/h), and accelerating and decelerating (1–2, 2–3, >3 m/s2).30 Heart rate was recorded during the entire game using a Team Polar Pro heart rate monitor (Polar Electro Oy).
2.6 Repeated sprint test
All players performed one repeated sprint test before and two during the game (see Ref.22) Each repeated sprint test consisted of five 30-m sprints, separated by a 25-s period of active recovery, during which the players jogged back to the starting line. Thus, each test lasted approximately 2 min. The test was started 20–30 s after obtainment of a muscle biopsy. Thus, the total time delay from match play to the sprint test was 35–60 s. The sprint times were recorded by infrared light sensors with a precision of 0.01 s (Newtest Oy).
2.7 Muscle and blood sampling
Muscle biopsies (~70–120 mg w.w. samples) were obtained from m. vastus lateralis in the dominant leg using the needle biopsy technique with suction. All biopsies were obtained with the subjects lying in the supine position on two beds standing 2 m from the sideline. Muscle biopsies were collected 15–30 s after cessation of match play (see Ref. 10) Blood samples were taken from an arm vein in the right arm using 5-ml syringes. Some of the blood samples were taken simultaneously with muscle samples, with the subjects lying on a bed. The remaining blood samples were collected within 30 s of match play, with the subjects sitting on a bed positioned 2 m from the sideline.
2.8 Muscle analysis
The muscle tissue was immediately frozen in liquid nitrogen (N2) and stored at −80°C. The frozen sample was weighed before and after freeze drying to determine water content. After freeze drying, the muscle samples were dissected free of blood, fat, and connective tissue. After extraction with perchloric acid (HClO4), neutralized extracts were analyzed for Lactate, ATP, PCr, and Cr (creatine) as previously described.31 In addition, the buffer solution was analyzed for Lac in the same way as the muscle extract in order to estimate the total muscle Lac production. Muscle glycogen content was determined spectrophotometrically (Beckman DU 650).32 Freeze-dried muscle tissue (1.5 mg) was boiled in 0.5 ml of HCL (1 mol/L) for 150 min before it was quickly cooled, whirl mixed, and centrifuged at 3500 g for 10 min at 4°C. Forty microliters of boiled muscle sample and 1 ml of reagent solution containing Tris buffer (1 mol/L), distilled water, ATP (100 mmol/L), magnesium chloride (1 mol/L), nicotinamide adenine dinucleotide phosphate (100 mmol/L), and glucose-6-phosphate dehydrogenase were mixed before the process was initiated by adding 10 μl of diluted hexokinase. Absorbance was recorded for 60 min before the glycogen content was calculated.32 Muscle glycogen was expressed as millimoles per kilogram of dry weight. Citrate Synthase (CS) activity was determined by the addition of oxaloacetate to a buffer solution containing muscle homogenate, DTNB buffer, acetyl-CoA, and H2O. Beta-hydroxyacyl-CoA dehydrogenase (HAD) activity was measured after the addition of acetoacetyl-CoA to a buffer solution containing imidazole, NADH, and EDTA. The absorbance of CS and HAD was recorded for 600 s, converted into enzyme activity rates, and expressed as micromoles per gram of dry weight per minute.32 Muscle pH was measured by a small glass electrode (Radiometer GK2801) after homogenizing a freeze-dried muscle sample of about 2 mg d.w. in a non-buffering solution containing 145 mmol/L KCl, 10 mmol/L NaCl, and 5 mmol/L iodoacetic acid.22 Myosin heavy chain (MHC) composition was analyzed using gel electrophoresis.33 Briefly, muscle homogenate (80 ml) was mixed with 200 ml sample buffer (10% glycerol, 5% 2-mercaptoethanol, 2.3% SDS, 62.5 mmol/L Tris-base, and 0.2% bromophenolblue at pH 6.8), boiled in a water bath at 100°C for 3 min, and loaded with three different amounts of protein (10–40 ml) on an SDS-PAGE gel [6% polyacrylamide (100:1, acrylamide:bis-acrylamide), 30% glycerol, 67.5 mmol/L Tris-base, 0.4% SDS, and 0.1 mmol/L glycine]. Gels were run at 80 V for at least 42 h at 4°C and MHC bands made visible by staining with Coomassie and three separate bands could be detected and characterized as MHC-1, MHC-2A, and MHC-2X. The gels were scanned (Linoscan 1400 scanner), and the MHC bands were quantified densitometrically (Phoretix 1D, nonlinear). MHC-2 was identified with Western blot using monoclonal antibody (Sigma M 4276) with the Xcell IITM protocol (Invitrogen). Analysis of skeletal muscle fiber size, capillarization, and glycogen content was performed on cross sections (8 µm) cut from tissue-tec embedded muscle samples.22 One section was stained with primary antibodies CD31, MHC fast, and laminin as well as secondary antibodies for visualization of capillaries, type II myofibers, and basal lamina. An addition section was stained with Periodic acid-Schiff stain and MHC fast for visualization of glycogen content and type II myofibers. Sections were analyzed for myofiber cross-sectional area and fiber-type distribution, capillary number and area, and glycogen content (density). Individual myofibers relative glycogen content was based on the average glycogen density of the 25 most full (100%) and empty (0%) type I and type II myofibers, from which the fiber type–specific relative glycogen content of individual myofibers was calculated.22, 34
2.9 Blood analysis
Within 20 s of sampling, blood samples were drawn into 5-ml heparin syringes, 200 μl of blood was hemolyzed, stored at −20°C, and later analyzed for lactate and glucose. The next heparin syringe with sample was rapidly centrifuged for 30 s. From this, plasma was collected and stored at −20°C until subsequent analysis. Plasma potassium concentration was measured using a flame photometer (Radiometer FLM3), with lithium as the internal standard.35 Plasma glycerol was determined on an automatic analyzer (Cobas Fara; Roche). Plasma-free fatty acid was measured fluorometrically using an enzymatic kit (WAKO Chemical), and plasma ammonia (NH3) was determined spectrophotometrically.22
2.10 Fluid loss and intake
To determine sweat loss during the game, the players were weighed wearing dry shorts immediately before the match, at half-time, and immediately after the match using a weight scale (Beam Balance-Stadiometer, SECA; Vogel & Halke), as previously described.22 The players were allowed to drink water ad libitum during the game, and their water intake was recorded.
2.11 Statistical analysis
A Shapiro-Wilk test was used to test data normality. Study's data sets were normally distributed for 41 out of 42 variables. Changes in performance of repeated sprints during the game were evaluated by two-way analysis of variance36 with repeated measures. Changes in blood metabolites before and during the game were determined by a one-way ANOVA with repeated measures. When a significant interaction was detected, data were subsequently analyzed using a Newman-Keuls post-hoc test. Differences in muscle metabolites between full game and intense periods were determined by Student's independent t-test, whereas differences for intense periods and full match were determined by Student's paired t-test. Differences in ratings of fiber type–specific glycogen content between rest and after the game were evaluated by a Wilcoxon signed rank test. Data are presented as means ± SEM. If a significant effect was found, pairwise comparison was tested using Bonferroni's post-hoc test. Differences were interpreted using effect sizes (ES) according to Hopkins, Marshall, Batterham, and Hanin37 as follows: trivial (ES < 0.2), small (ES = 0.2–0.6), moderate (ES = 0.6–1.2), large (ES = 1.2–2.0), very large (ES = 2.0–4.0), and huge (ES > 4.0). When 90% confidence intervals overlapped positive and negative values, the effect was deemed as unclear. Otherwise, the effect was deemed as the observed magnitude.38 The relationship between mean time during repeated sprint ability testing (RSAMT%) drop and glycogen% drop was evaluated using Pearson's correlation coefficient. The magnitude of the correlations was considered as trivial (r > 0.1–0.3), moderate (r > 0.3–0.5), large (r > 0.5–0.7), very large (r > 0.7–0.9), nearly perfect (r > 0.9), and perfect (r = 1.0) in accordance with Hopkins et al.37 Significance was set at p ≤ 0.05. Data analysis was performed using Statistical Package for Social Science statistical software (version 23, IBM SPSS Statistics).
3 RESULTS
3.1 Muscle glycogen
Muscle glycogen concentration was higher (p < 0.05) pre-match (409 ± 62 mmol/kg d.w.) than after intense period of the second half (248 ± 101 mmol/kg d.w., 39.4% reduction) and post-game (236 ± 86 mmol/kg d.w., 42.3% reduction) (ES = 2.0 [0.9;3.0] and 2.3 [1.2;3.4], respectively). Moreover, the intense period of the first half 318 ± 105 mmol/kg d.w. was significantly different from the intense period of the second half (p = 0.008; ES = 0.7 [0.2;1.6]) with a glycogen reduction of 22% (Table 1) (Figure 1). The fiber type–specific glycogen depletion analysis showed that pre-game, 78% and 70% of the type II and I fibers, respectively, were rated as completely or partly full with glycogen, whereas this value was lower (p < 0.05) after the game (21% and 31%, Figure 2). Post-match, a total of 69% and 80% of the type II and I fibers, respectively, were completely or almost empty of glycogen compared to 22% and 30% pre-match (Figure 2).
| Muscle variables | Pre-match | Intense period in 1st half | Intense period in 2nd half | End of the match |
|---|---|---|---|---|
| Glycogen (mmol/kg d.w.) | 409 ± 62 | 318 ± 105*** | 248 ± 101* | 236 ± 86* |
| Lactate (mmol/kg d.w.) | 6.4 ± 3.7 | 14.3 ± 4.6* | 9.8 ± 3.7 | 12.9 ± 5.7* |
| ATP (mmol/kg d.w.) | 24.0 ± 1.5 | 22.6 ± 1.2 | 22.1 ± 1.1* | 23.8 ± 2.7 |
| PCr (mmol/kg d.w.) | 79.1 ± 9.8 | 66.6 ± 8.9* | 69.8 ± 2.8* | 80.2 ± 11.8** |
| Creatine (mmol/kg d.w.) | 44.3 ± 4.4 | 54.1 ± 7.9* | 49.2 ± 9.3 | 42.9 ± 3.5** |
| pH (-log H+) | 7.25 ± 0.10 | 7.20 ± 0.04 | 7.21 ± 0.07 | 7.17 ± 0.13 |
Note
- Values are means ± SEM. Pre- and post-match ATP, PCr, creatine, and lactate (N = 8); glycogen (N = 8); pH (N = 6); CS-HAD activity (N = 7); MHC (N = 4). Intense exercise periods ATP, PCr, creatine, and lactate (N = 7); glycogen (N = 7); pH (N = 7).
- * Significantly different (p < 0.05) from before match.
- ** Significantly different from the pre-match.
- *** Significantly different (p < 0.05) from intense period 2nd half.
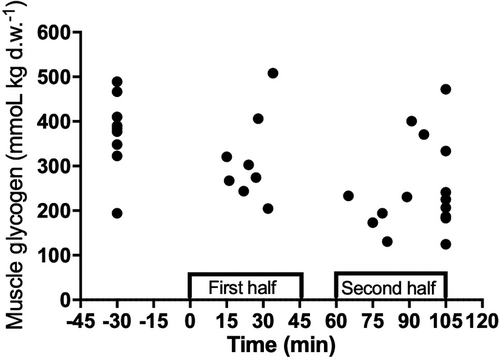
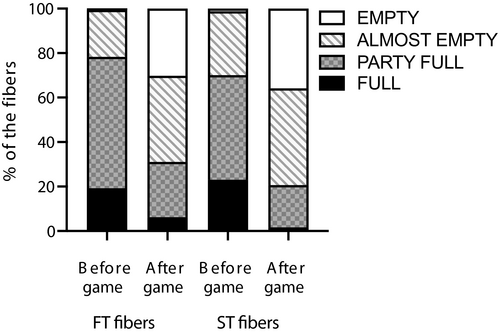
3.2 Muscle ATP, PCr, lactate, and pH
A detailed representation of muscle metabolites is reported in Table 1 and Figure 3. Muscle ATP pre-match was (24.0 ± 1.5 mmol/kg d.w.) and was lowered (p = 0.017; ES = 1.4 [0.5;2.4]) after an intense period in the second half (22.1 ± 1.1 mmol/kg d.w.). Muscle PCr pre-game and after the match was higher than after an intense period first (p < 0.05; ES = 1.3 [0.4;2.3] and 1.3 [0.3;2.2], respectively) and second half (p < 0.05; ES = 1.3 [0.3;2.2] and 1.2 [0.2;2.1], respectively). Furthermore, muscle lactate was higher (p < 0.05) post-match (12.9 ± 5.7 mmol/kg d.w.) and after an intense period in the first half (14.3 ± 4.6 mmol/kg d.w.; ES = 1.3 [0.4;2.3] and 1.9 [0.9;2.9], respectively) compared to pre-match (6.4 ± 3.7 mmol/kg d.w.). In addition, muscle creatine was higher (p < 0.05) after an intense period in the first half than pre- and post-match (ES = 1.6 [0.6;2.5] and 1.9 [0.9;2.9], respectively). Muscle pH remained unaltered during the match.
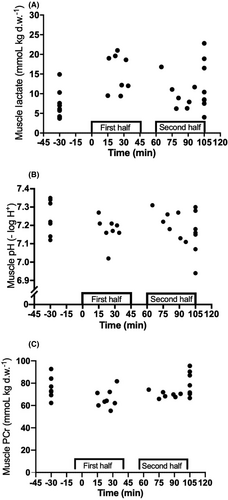
3.3 Blood variables during match play
Blood variables are shown in Tables 2 and 3. Blood lactate at half-time was higher than at rest and pre-match (p < 0.05; ES = 2.6 [1.5;3.8] and 2.2 [1.2;3.3], respectively). Likewise, blood lactate post-match was higher than at rest and pre-match (p < 0.001; ES = 4.4 [6.0; 2.8] and 3.8 [5.3; 2,4], respectively). Furthermore, blood glucose at half-time and post-match was higher compared to rest and pre-match (p < 0.05; ES = 0.7;3.9). Plasma glycerol at rest was significantly different from pre-match, half-time, and post-match (p < 0.05; ES = 1.9 [1.0;2.9], 3.2 [2.0;4.5] and 2.9 [1.6;4.1], respectively). Moreover, plasma FFA at pre-match was higher compared to rest and post-match (p < 0.05; ES = 1.5 [0.6;2.4] and 1.8 [0.8;2.8], respectively). (Table 2). In the group with the intense periods, blood lactate at rest was higher (p < 0.05) than pre-match, intense period of first half, half-time, and intense period of second half (ES = 4.3 [3.0;5.7], 6.9 [4.7;9.2], 2.3 [1.3;3.4], and 2.0 [1.0;3.0], respectively). In addition, blood glucose at pre-match was higher than rest, half-time, and intense period at second half (p < 0.05; ES = 2.7 [1.6;3.7], 1.3 [0.3;2.2], and 1.7 [0.7;2.6], respectively). In plasma variables, plasma glycerol at rest was significant lower from pre-match, half-time, and intense period at second half (p < 0.05; ES = 1.8 [0.9;2.7], 2.1 [1.1;3.2], and 1.8 [0.8;2.7], respectively). Moreover, plasma FFA at pre-match and intense period at second half was significant higher compared to rest (p < 0.05; ES = 1.4 [0.6;2.3] and 1.6 [0.7;2.5], respectively). Lastly, plasma NH3 at pre-match was significant higher than rest, intense period of first half, half-time, and intense period of second half (p < 0.05; ES = 2.1 [1.2;3.1], 1.8 [0.7;2.8], 1.9 [0.8;3.0], and 1.8 [0.8;2.8], respectively).
| Full game group | Rest | Pre-match | Half-time | End of match |
|---|---|---|---|---|
| Blood lactate (mmol/L) | 1.3 ± 0.4 | 1.9 ± 0.7 | 6.4 ± 3.0*,** | 8.4 ± 2.0*,** |
| Blood glucose (mmol/L) | 4.7 ± 0.4 | 4.7 ± 0.4 | 6.7 ± 2.1*,** | 7.9 ± 1.2*,** |
| Plasma glycerol (µmol/L) | 63.1 ± 24.7 | 140.3 ± 51.2* | 189.9 ± 52.6* | 146.2 ± 34.9* |
| Plasma FFA (µmol/L) | 509 ± 217 | 809 ± 184* | 724 ± 163 | 521 ± 106** |
| Plasma NH3 (μmol/L) | 62.3 ± 21.1 | 128.8 ± 80.3 | 155.4 ± 98.0 | 115.3 ± 49.9 |
| Plasma Na+ (mmol/L) | 126.1 ± 4.2 | 128.6 ± 5.4 | 131.4 ± 3.5 | 129.3 ± 4.7 |
| Plasma K+ (mmol/L) | 3.9 ± 0.5 | 4.7 ± 1.2 | 4.9 ± 1.4 | 4.6 ± 1.1 |
Note
- Values are means ± SEM. Rest (N = 9); Pre-match (N = 9); Half-time (N = 7); Full-match (N = 6).
- * Significantly different (p < 0.05) from rest.
- ** Significantly different (p < 0.05) from pre-match.
| Intense periods group | Rest | Pre-match | Intense period in 1st half | Half-time | Intense period in 2nd half |
|---|---|---|---|---|---|
| Blood lactate (mmol/L) | 1.2 ± 0.3 | 5.5 ± 1.4* | 4.2 ± 0.7* | 5.1 ± 2.7* | 3.7 ± 1.9* |
| Blood glucose (mmol/L) | 4.7 ± 0.3 | 7.3 ± 1.4* | 5.9 ± 1.4 | 5.8 ± 0.9** | 5.4 ± 0.7** |
| Plasma glycerol (µmol/L) | 95.0 ± 48.6 | 218.8 ± 88.1* | 138.8 ± 51.7 | 207.7 ± 85.7* | 208.1 ± 80.2* |
| Plasma FFA (µmol/L) | 559 ± 275 | 992 ± 335* | 623 ± 150 | 758 ± 233 | 1052 ± 360* |
| Plasma NH3 (μmol/L) | 61.2 ± 17.3 | 111.0 ± 28.8* | 62.8 ± 24.0** | 63.6 ± 16.0** | 60.9 ± 25.7** |
| Plasma Na+ (mmol/L) | 130.9 ± 3.4 | 136.3 ± 5.6 | 129.7 ± 4.1 | 135.4 ± 3.9 | 133.0 ± 3.2 |
| Plasma K+ (mmol/L) | 4.3 ± 0.4 | 4.0 ± 0.7 | 3.9 ± 0.7 | 4.5 ± 1.0 | 4.1 ± 0.2 |
Note
- Values are means ± SEM. Rest (N = 10); Pre-game (N = 9); Intense period in the first half (N = 5); Half-time (N = 6); Intense period in the second half (N = 7).
- * Significantly different (p < 0.05) from rest.
- ** Significantly different from pre-match.
3.4 Fluid loss and intake
The net fluid loss during a match was 0.8 ± 0.9 (range: 0.0–4.1) L, or 1.2 ± 1.1 (0.0–5.3)% of body mass. The fluid intake was 0.52 ± 0.28 (0.00–1.25) L. Thus, the total fluid loss during the match was 1.28 ± 0.89 (0.48–4.60) L, corresponding to 2.0 ± 1.1 (0.7–5.9)% of the player's individual body mass.
3.5 Repeated sprint performance during match play
All five sprints and repeated sprint ability mean time (RSAMT) at rest and before the match were faster (p < 0.05; ES = [0.1;2.9]) compared to half-time and after the match with RSA being 3.2 ± 1.7% and 7.0 ± 2.1% slower in the first half and after the match (Figure 4A). After an intense period in the first half, all five sprints and mean sprint time (4.7 ± 1.6%) were slower than before game (p < 0.05; ES = [0.6;1.2]). After an intense period in the second half, the fifth sprint was 9.9 ± 3.1% slower than the fifth sprint before the match (p = 0.029; ES = 1.7 [0.7;2.8]) (Figure 4B).
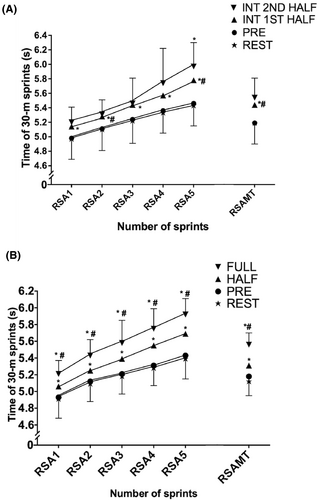
3.6 Heart rate loading and activity pattern during match play
Mean heart rate during the match was 163 ± 9 bpm, and peak heart rate was 190 ± 8 bpm. The players covered a total distance of 8.5 ± 1.2 km during the match, of which distances of 903 ± 275 m were high-intensity running and 307 ± 166 m were sprinting. Distances covered in acceleration and deceleration above 2 m/s2 were 233 ± 52 m and 172 ± 40 m, respectively. No significant differences were observed in neither mean and heart rate nor in activity profile between the first and second half.
3.7 Repeated Sprint Performance and muscle variables correlations
From pre- to post-match and intense periods testing, the RSAMT percentage decrease was highly correlated (p < 0.05) with the percent decrease of muscle glycogen (r = 0.604) (Figure 5), decrease in muscle pH (r = 0.672), and increase in muscle lactate (r = 0.567), whereas no significant correlations were observed between RSAMT percentage decrease and match-induced increase in blood lactate (r = 0.133, p > 0.05) or absolute blood lactate concentration (r = 0.216, p > 0.05).

4 DISCUSSION
The present study is the first to examine muscle metabolism during a women's football match using elite players. The principal findings were that muscle glycogen values were lowered to values below critical levels during the second half, and that a majority of the slow and fast twitch fibers were very low on glycogen after the match. Moreover, muscle ATP and PCr levels were lowered, creatine levels were elevated, and muscle lactate was doubled during intense match periods. Finally, repeated sprint ability was impaired after intense periods, as well as after both halves compared with baseline, which may be associated with the observed muscle metabolite perturbations.
The vastus lateralis muscle glycogen levels decreased by ~175 mmol/kg d.w. during the match reaching 235 mmol/kg d.w. after the match. Thus, considering that the resting glycogen levels were in the lower range of normal baseline levels,39 the match-induced glycogen utilization resulted in end-game glycogen levels that were nearly comparable to what has been reported in competitive male players.22, 40, 41 The observed average end-game glycogen level of 235 mmol/kg d.w. is below levels of 250 mmol/kg d.w. which has been shown to negatively affect SR Ca2+ handling 15 and close to the levels where glycolytic rate is compromised.14 Moreover, we observed that both fast and slow twitch fibers displayed a large muscle glycogen depletion with 70%–80% being rated low on glycogen, which is in accordance with findings in male football players during friendly matches 22 and simulated football,40 as well as other team sports like ice hockey.42 The role of glycogen for muscle performance is yet not fully understood,however, in the present study, a progressive decline in repeated sprint ability was observed during the match, as well as a correlation between drop in muscle glycogen and decline in sprint performance, whereas no correlation was observed between blood metabolites and the impaired sprint performance. Using transmission electron microscopy, glycogen has been shown to be deposited in specific compartments within the myocytes, which may be associated with specialized regulatory and metabolic functions.43 After a high-level male football match, all three subcellular compartments have been shown to be markedly affected,44 which may impair different steps in the excitation-contraction coupling. For example, a possible link between SR calcium (Ca2+) handling and the intermyofibrillar glycogen pool has been identified,15 which may also play a role during a football match.34 Finally, the intramyofibrillar glycogen located in connection with the contractile filaments close to triad junctions within the t-tubules and the subsarcolemmal glycogen, which is stored below the cell surface membrane, may have an impact on muscle function potentially by down-regulation of sodium-potassium (Na+-K+)-ATPase activity, since these ATPases in the transverse tubular system, where ionic perturbations are likely to be most severe during exercise,45 preferentially utilize ATP derived from glycolysis.46 The ability to regulate Na+ and K+ in skeletal muscle appears to be of importance in football since the Na+−K+ ATPase is the best predictive muscle variable for high-speed running during the most intense periods of a match.27 Thus, during a women football match, different steps in the E-C coupling may be affected by the progressive decline in muscle glycogen, which may be linked to the gradual or accumulative fatigue response observed in the present study.
The large decline in muscle glycogen observed in this and other team sports studies 10, 34, 41, 42 may be linked to a high glycolytic activity in relation to the most intense periods of a match. In this investigation, muscle lactate increased almost threefold following individual high-intense periods in the first half, which is comparable to males,22 and blood lactate concentrations of 6 and 8 mmol/L were measured after the two halves, respectively, which is slightly higher than previous findings in women players.11 Thus, glycolytic activity is high during periods of an elite women football match. Muscle ATP was lowered after an intense period in the second half compared to baseline, indicating a disturbed energy status in a myocytes, especially when taking into account the fast re-synthesis of ATP and the 20–30 s delay in muscle biopsy sampling during a football match. Moreover, muscle PCr declined from around 80 to 65 and 70 mmol/kg after an intense period in the first and second half, respectively. However, using a previously reported re-synthesis rate of 0.5 mmol·kg d.w./s in relation to our ~30-s muscle biopsy sampling delay,22 the actual concentrations may have been around 50–55 mmol/kg immediately after an intense period in the match. These values are markedly higher than what has been measured during football-specific exhaustive testing such as the Yo-Yo IR2 test,10 which speaks against low muscle PCr as a cause of fatigue in women's football.
Although, the RSAMT decline from pre-post game and intense periods testing was highly correlated with muscle pH, acidity was not changed during the match suggesting that a drop in muscle pH is not the cause of fatigue observed during the match. These results are in agreement with those reported by intense intermittent studies.35, 47 It must be noted that compared to a modest pH decline observed during a males’ game,10 such a decline was not observed during a women's game. Potassium does not appear to be implicate in fatigue development during the game since it remained unchanged throughout the match, which, however, is likely to be caused by the rapid recovery of extracellular potassium and delayed blood sampling. The fact that ammonia remained unchanged during the match suggests that, in contrast to males,22 the adenosine mono-phosphate deaminase reaction was not adequately activated.
The present study was conducted as an experimental match, which is the closest exercise scenario to a real competitive match. The heart rate data confirm that the cardiovascular loading was at a similar level as previously reported in elite women during a competitive match.8, 11 The analysis of the match revealed that the players covered in a total distance of 8.5 km with 900 m classified as high-intensity running and 300 m as sprinting. These tracking results equal the values from the UEFA Champions League for women 9 but lower than other reports from high-level women's football.11, 48 However, the sprint test results revealed that the intensity of the match was sufficient to cause pronounced fatigue, with sprint decrements similar to what has been observed after competitive elite women's matches.11 Thus, the physiological loading during the experimental match is likely to resemble the demands in a competitive situation.
The net fluid loss was approximately 1% of the body mass, which only has played a minor role for the performance decrement at the end of the match.49 Moreover, the blood glucose concentrations peaked at the end of the match which rules out hypoglycemia as a cause of post-match fatigue.
This study is the first to investigate muscle metabolism and performance during an elite women football match, and therefore gives us new insight into potential gender differences in muscle energetics and exercise tolerance during team sport match play. Compared to previous studies with males, the muscle glycogen utilization was lower in the present investigation,22, 40, 41 which partly may relate to gender differences.16, 17 Women have been shown to have lower muscle glycogen concentrations compared to males,19 which may be associated with lower training status. However, in the present study, the players were well-trained high-level players, and relatively speaking, more well trained than the male populations in the studies by Krustrup et al.22 and Nybo et al.41 During the intense periods, which may reflect peak periods50 and the sequences with highest glycolytic activity, muscle lactate accumulation and PCr utilization tended to be lower than shown in males,22 which may be caused by an inferior anaerobic capacity in women compared to men.18, 19 Future studies should address possible gender differences in muscle metabolism during intermittent sports.
A potential limitation of this investigation is that participants’ menstrual cycle phase was verified only by participant self-reporting and not through the measurement of circulating estrogen and progesterone concentration. Nevertheless, it has been postulated that there is limited evidence available in the literature to describe how exercise performance fluctuates across the various phases of the menstrual cycle.51
In conclusion, average muscle glycogen breakdown is moderate during an elite women football game with a large portion of both fast and slow twitch fibers being nearly depleted of glycogen, which may cause fatigue during a game. Anaerobic energy production is markedly elevated during intense periods of a women football game but may be lower than in males. These findings will provide novel insight not only to exercise scientists but also to practitioners regarding the physical and nutritional preparation of a women's game.
ACKNOWLEDGEMENTS
The authors are grateful to all participants and their coaches and clubs for their tremendous effort, work ethic, and support. Furthermore, the help from Dr Jens Jung Nielsen at the University of Copenhagen is greatly appreciated. The study was supported by the “Exercise and Health” program of the Department of Physical Education of the University of Thessaly (program number 5854), as well as by the Novo Nordisk 570 Foundation grant to Team Denmark.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




