Endurance exercise training volume is not associated with progression of coronary artery calcification
Funding information
This work was supported by an operating grant from the North Sea Race (“Nordsjørittet”), Abbott Diagnostics (Abbott Diagnostics, IL, USA), the Laerdal Foundation (Stavanger, Norway), Stavanger University Hospital, the Norwegian Health Association (Oslo, Norway), ConocoPhillips and the Simon Fougner Hartmann Trust.
Abstract
Background
Recent cross-sectional studies have suggested a dose-dependent relationship between lifelong exposure to physical activity and the burden of calcified coronary artery disease (CAD). No longitudinal studies have addressed this concern.
Hypothesis
Exercise volume is associated with progression of coronary artery calcium (CAC), defined as ≥10 units increase in CAC score.
Methods
Sixty-one recreational athletes who were assessed by coronary computed tomography angiography (CCTA) as part of the NEEDED 2013/14 study were re-assessed 4-5 years later, in 2018.
Results
Subjects were 45.9 ± 9.6 years old at inclusion, and 46 (74%) were male. Between 2013 and 2018, the participants reported median 5 (range: 0-20, 25th-75th percentile: 4-6) hours of high-intensity exercise per week. None of the included subjects smoked during follow-up. At inclusion, 21 (33%) participants had coronary artery calcifications. On follow-up CCTA in 2018, 15 (25%) subjects had progressive coronary calcification (≥10 Agatston units increase in CAC). These subjects were older (53 ± 9 vs 44 ± 9 years old, P = .002) and had higher levels of low-density lipoprotein at baseline (3.5 (2.9-4.3) vs 2.9 (2.3-3.5) mmol/L, P = .031) as compared to subjects with stable condition. No relationship was found between hours of endurance training per week and progression of coronary artery calcification. In multiple regression analysis, age and baseline CAC were the only significant predictors of progressive CAC.
Conclusion
No relationship between exercise training volume and the progression of coronary artery calcification was found in this longitudinal study of middle-aged recreational athletes.
1 INTRODUCTION
The presence of coronary artery calcification is associated with increased risk of adverse cardiovascular events.1, 2 Despite beneficial effects of physical activity on cardiovascular (CV) morbidity and mortality, recent studies have demonstrated a surprisingly high prevalence of coronary artery calcium (CAC) in 40%-68% of recreational athletes.3-5 These studies have suggested a dose-dependent relation between lifelong exposure to physical activity and calcified coronary artery disease (CAD).3-6 This relationship, however, may seem contra intuitive, based upon the health and longevity of athletic subjects.
No previous study has used repeated coronary computed tomography angiography (CCTA) to monitor changes in CAC in recreational athletes. The present study aimed to assess the impact of exercise volume on the progression of CAC, defined as ≥10 units increase in CAC score (Agatston units) during >4-year follow-up in middle-aged recreational athletes without known CV disease prior to baseline CCTA assessment.
2 MATERIAL AND METHODS
The present analysis is a sub-study of the North Sea Race Endurance Exercise Study (NEEDED) 2018. We assessed the change in CAC between the baseline CCTA acquired in either the NEEDED 2013 or 2014 study 7, 8 and the follow-up CCTA assessment in 2018 (Figure S1). NEEDED 2018 was approved by the Regional Ethics Committee (REK 2013/550 and REK 2018/63), in compliance with the Declaration of Helsinki. All participants signed informed consent forms prior to enrollment into the studies.
In order to be included in the NEEDED study program, subjects had to be >16 years of age, reside in Norway and participate in the 91-km-long recreational mountain bike race (The North Sea Race). Subjects were excluded if they had known CV disease or CV medication, or if they had a resting ECG suggestive of underlying pathology at screening.9 Study subjects were assessed by electrocardiograms (ECG), clinical assessments, and blood samples at baseline and at 3- and 24 hours after the race. Study recruitment is outlined in Figure S1. In total, 61 subjects completed the NEEDED 2018 study protocol. Only participants without obstructive CAD at baseline CCTA were eligible to participate in NEEDED 2018. In NEEDED 2018, a cardiopulmonary exercise test was conducted prior to the cycling competition. All study participants in NEEDED 2018 were assessed by coronary computed tomography angiography (CCTA) within 1 month after the race. Progression of coronary artery calcium (CAC) score was defined as an increase above the 75th percentile of Δ CAC in the present cohort, corresponding with Δ CAC of ≥10 Agatston units. Linear regression was also performed, but due to skewed distribution of Δ CAC, logistic regression was preferred. Training volume was defined as self-reported number of hours per week of high-intensity exercise (>6 metabolic equivalents of task [MET] per minute) in the years between 2013 and 2018.
2.1 Cardiopulmonary exercise test
The cardiopulmonary exercise test (CPET) was performed 2-3 weeks prior to the North Sea Race in 2018, using the “Cyclus 2” and “Vyntus CPX” systems. This enabled subjects to perform the test on their own bikes. Individual ramp protocols, a face-covering mask, and a heart rate monitor were used to obtain data. Continuous gas analysis was done by using a mixing chamber gas analyzer. Subjects reached VO2max when a plateau in oxygen consumption occurred, as well as a respiratory exchange ratio above 1.05 and BORG score >18. VO2max was adjusted for age and gender by comparative data from a Norwegian cohort.10 One person did not achieve a plateau in oxygen consumption during testing. For this person, peak VO2 was recorded as the maximal VO2 at exhaustion.
2.2 Blood samples
Venous blood samples were drawn from the antecubital vein. Fresh serum samples were analyzed for creatinine, high-sensitive C-reactive protein (CRP), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) on Architect c16000TM (Abbott Diagnostics). High-sensitive cTnI was analyzed by a STAT assay from Abbott Diagnostics using Architect I2000SR. The cTnI assay had a limit of detection of 1.6 ng/L, and the overall 99th percentile was 26 ng/L.11 Hematology was analyzed using XE-5000 (Sysmex XN).
2.3 Coronary computed tomography angiography
CCTA was obtained using a Siemens Somatom Definition Flash Dual Source, with the same protocol as for the NEEDED 2013-2014 studies.7 Slice acquisition parameter was 0.6 × 128 mm. Those with heart rate more than 60 beats per minute were given atenolol or metoprolol tartrate prior to examination. All were given 0.8 mg nitroglycerine sublingual before the scan. A scout scan was performed from under the tracheal bifurcation to the diaphragm, followed by CT calcium score scans with gantry rotation 280 ms, 120 kV, and 80 mAs. A two-phase injection of Omnipaque 350 mg/mL at a rate of 6 ml/sec followed by 0.9% saline with high pitch or prospectively ECG triggered protocols was administered. CCTA was reconstructed with a slice thickness of 0.6 mm medium smooth tissue convolution and iterative reconstruction. Two independent radiologists with more than 5 years of experience in CCTA did the CAC analysis on SyngoVia cardiac work stations adhering to the guidelines on interpreting and reporting CCTA.12
2.4 Statistical analysis
Normally, distributed variables were reported as mean ± SD, while continuous variables with markedly skewed distributions were reported as median (25th-75th percentile). The Shapiro-Wilk test was used to test for normality. A two-tailed P-value of <0.05 was considered significant. The Mann-Whitney U test, Person chi-square test, or Fisher's exact test were used to assess differences between subjects with stable condition vs subjects with progression of CAC, as appropriate. For the multiple logistic regression analysis, the Hosmer-Lemeshow test was used to assess goodness of fit, with a P-value threshold of >0.05 for model acceptance. The following pre-specified variables were included in the multiple regression analysis: age, gender, baseline LDL, baseline BMI, baseline systolic blood pressure (SBP), and exercise volume per week during the follow-up period. A separate model (Model 2) was also calculated, which included the same variables as in Model 1, but also adjusted for the baseline CAC score. This was done in order to identify possible associations that could be obscured by including baseline CAC.13 No more variables were considered appropriate in the multiple regression analysis due to the moderate sample size (n = 61). A linear regression analysis with Δ CAC + 1 was also performed, using the same variables as in the presented logistic regression analysis. Residual analysis improved with Ln transformation of the dependent variable, but was still slightly skewed. For statistical analysis, the statistical software program SPSS version 24 was used.
3 RESULTS
Baseline characteristics of the total cohort (n = 61) are outlined in Table 1. In short, subjects were 46 ± 10 years old and 46 (74%) were male. The cohort had a low CV risk profile (Framingham Risk Score: 1 [0-5] %). Study subjects reported median 5 (4-6) hours/week of high-intensity exercise in the period between baseline and 2018, and 56 (33-106) MET hours per week (Figure 1).
| All (n = 61) | Stable condition (n = 46) | Progression of CAC (n = 15) | P-value | |
|---|---|---|---|---|
| Age, years | 45.9 ± 9.6 | 44.3 ± 8.9 | 52.9 ± 9.4 | .002 |
| Male sex, n (%) | 46 (74) | 33 (72) | 12 (80) | .74 |
| BMI, kg/m2 | 24.3 (23.5-26.6) | 24.5 (23.7-25.9) | 24.3 (24.1-26.7) | .56 |
| Waist circumference, cm | 83 (78-89) | 82 (78-89) | 84 (80-91) | .29 |
| SBP, mm Hg | 140 ± 18 | 138 ± 16 | 145 ± 26 | .33 |
| DBP, mm Hg | 81 ± 11 | 79 ± 12 | 84 ± 10 | .34 |
| Former smoker, n (%) | 28 (45) | 22 (48) | 6 (40) | .77 |
| Family history of CVD | 4 (7) | 2 (4) | 2 (13) | .26 |
| FRS, % | 1 (0-5) | 0 (0-2) | 4 (1-12) | .007 |
| Baseline blood samples | ||||
| LDL, mmol/L | 3.0 (2.5-4.1) | 2.9 (2.3-3.5) | 3.5 (2.9-4.3) | .03 |
| HDL, mmol/L | 1.6 (1.3-1.8) | 1.6 (1.3-1.8) | 1.6 (1.2-1.8) | .80 |
| CRP, mg/L | 0.5 (0.4-1.2) | 0.7 (0.3-1.3) | 0.4 (0.4-0.5) | .14 |
| eGFR, mL/min/1.73m2 | 91 (81-100) | 89 (81-100) | 96 (83-100) | .88 |
| Hemoglobin, g/dL | 14.3 ± 0.9 | 14.2 ± 0.9 | 14.5 ± 0.8 | .35 |
| Glucose, mmol/L | 5.3 (4.8-5.6) | 5.2 (4.8-5.5) | 5.6 (4.5-5.9) | .48 |
| cTnI, ng/L | 3.1 (1.7-7.0) | 2.3 (1.6-6.4) | 6.1 (2.7-10.1) | .14 |
| Training experience | ||||
| Years of endurance training | 10 (6-35) | 5 (3-8) | 9 (3-30) | .06 |
| Number of prior competitions | 9 (3-15) | 7 (3-12) | 10 (3-21) | .41 |
| MET h/wk | 56 (33-106) | 50 (34-82) | 68 (33-122) | .16 |
| Race duration, h | 3.7 (3.4-4.0) | 3.6 (3.4-4.0) | 3.9 (3.2-4.1) | .56 |
- Abbreviations: BMI, body mass index; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; FRS, Framingham Risk Score; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MET, metabolic equivalents of task (obtained by the IPAQ-SF questionnaire).
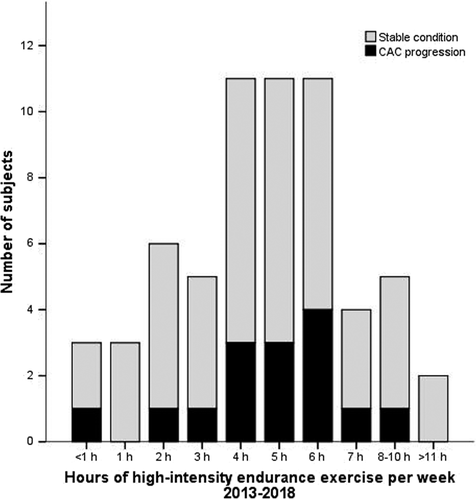
At baseline, the median CAC score was 0 (0-6) Agatston units. Forty subjects (66%) had normal coronary arteries without CAC or non-calcified plaques. In subjects with CAC, 13 (62%) had calcified plaques only (Table 2). On follow-up CCTA in 2018, 15 subjects had an increased CAC score (≥10 Agatston units) as compared to the baseline CCTA examination.
| Inclusion (2013/14) | Follow-up (2018) | |||||
|---|---|---|---|---|---|---|
| Stable condition (n = 46) | Progression of CAC (n = 15) | P-value | Stable condition (n = 46) | Progression of CAC (n = 15) | P-value | |
| Any CAD | 6 (13) | 15 (100) | <.001 | 11 (27) | 15 (100) | <.001 |
| Calcified plaques | 5 (83) | 8 (53) | 9 (82) | 11 (73) | ||
| Non-calcified plaques | 1 (17) | 1 (7) | 0 (0) | 0 (0) | ||
| Mixed morphology | 0 (0) | 6 (40) | .18 | 2 (18) | 4 (27) | 1.00 |
| Number of affected arteries | ||||||
| Normal arteries | 40 (87) | 0 (0) | 35 (76) | 0 (0) | ||
| 1 artery affected | 6 (13) | 8 (53) | 10 (22) | 4 (27) | ||
| ≥2 affected arteries | 0 (0) | 7 (47) | <.001 | 1 (2) | 11 (73) | <.001 |
| CAC, Agatston units | 0 (0-0) | 22 (3-58) | <.001 | 0 (0-0.13) | 40 (21-142) | <.001 |
The overall median Δ CAC from baseline to the 2018 CCTA was 0 (25th-75th percentile: 0-10) Agatston units (mean 15 ± 40 Agatston units, Figure 2). In the group with stable condition, the median Δ CAC score was 0 (0-0.1), while in the subjects with progressive CAC, median Δ CAC was 28 (12-105) Agatston units (Table 3).
| Stable condition (n = 46) |
Progression of CAC (n = 15) |
P-value | |
|---|---|---|---|
| Δ BMI, kg/m2 | 0.3 (−0.6-1.1) | 0.4 (−0.1-1.4) | .38 |
| Δ Waist circumference, cm | 4 (−0.5-7.2) | 6 (2-11) | .18 |
| Δ SBP, mm Hg | -4 ± 12 | -4 ± 22 | .60 |
| Δ DBP, mm Hg | 2 ± 10 | 0 ± 11 | .44 |
| On statin therapy in 2018, n (% of subjects with CAD at baseline) | 1 (17) | 5 (33) | .62 |
| Blood sample changes | |||
| Δ LDL, mmol/L | 0.2 (−0.2-0.6) | -0.2 (−1.7-0.2) | .02 |
| Δ LDL (non-statin users only), mmol/L | 0.2 (−0.1-0.7) | 0.1 (−0.2-0.5) | .26 |
| Δ HDL, mmol/L | -0.1 (−0.2- 0.0) | -0.1 (−0.2-0) | .41 |
| Δ CRP, mg/L | 0.2 (0-0.7) | 0.2 (0-1.1) | .50 |
| Δ eGFR, mL/min/1.73m2 | 3.6 (−0.4-13.1) | 3.8 (−1.8-7.1) | .65 |
| Δ Glucose, mmol/L | 0.3 (−0.5-1.1) | 0.4 (−0.9-1.2) | .91 |
| Δ Resting cTnI, ng/L | -0.1 (−0.7-1.8) | 1.1 (0.3-2.9) | .07 |
| Exercise parameters | |||
| Δ MET h/wk | 10 (−5-25) | -11 (−24-27) | .35 |
| Δ Race duration, h | 0.4 (0.2-0.7) | 0.5 (0.2-0.5) | .99 |
| CCTA indices 2018 | |||
| Δ CAC, Agatston units | 0 (0-0.1) | 28 (12-105) | <.001 |
Note
- Data are presented as mean ± SD or median (25th-75th) percentile, whenever appropriate.
New CAC in 2018 occurred in six subjects with no CAC at baseline (Table 2). The median Δ CAC in these subjects was 5 (3-12) Agatston units. The only subject with a CAC progression exceeding 10 Agatston units (CAC of 20 Agatston units in 2018) was a 37-year-old male with a non-calcified plaque on the baseline CCTA examination in 2014.
3.1 Differences between subjects with stable condition vs progressive CAC score
Compared with subjects without CAC progression, subjects with CAC progression (n = 15) were older (53 ± 9 vs 44 ± 9 years old, P = .002) and had a higher FRS (4 [1-12] vs 0 [0-2] %, P = .007) and higher levels of low-density lipoprotein (LDL) (3.5 [2.9-4.3] vs 2.9 [2.3-3.5] mmol/L, P = .031). None of the included subjects were current smokers at inclusion, and a similar number of subjects in both groups had a history of tobacco use (P = .78). All of the 15 subjects with CAC progression had CAD at baseline, with 7 (47%) having CAD in two or more of the epicardial coronary arteries (Table 2).
During follow-up, both groups had similar changes in body composition, blood pressure, CRP level, and kidney function. Borderline significant differences in resting cTnI level were found, with subjects with progression of CAC showing a slight increase (P = .07). LDL decreased in the group with progression of CAC, but when subjects with current statin-use were excluded (n = 8), similar cholesterol levels were evident in both groups (Table 3).
In multiple regression analysis, age and baseline CAC score were associated with an increased risk of progression of CAC (Table 4, Table S1).
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Endurance training 2013-18, h/wk | 1.02 (0.81-1.29) | .85 | 0.97 (0.62-1.50) | .87 |
| Age, y | 1.14 (1.03-1.25) | .009 | 1.03 (0.90-1.18) | .67 |
| Male gender | 1.16 (0.20-6.84) | .87 | 1.23 (0.12-13.0) | .86 |
| LDL at baseline, mmol/L | 1.32 (0.58-3.00) | .51 | 0.77 (0.22-2.55) | .67 |
| BMI at baseline, kg/m2 | 0.98 (0.73-1.33) | .92 | 1.02 (0.66-1.56) | .94 |
| SBP at baseline, mm Hg | 0.99 (0.95-1.03) | .61 | 1.00 (0.96-1.05) | .96 |
| Baseline CAC, Agatston units | 82.1 (6.0-1122.2) | .001 | ||
Note
- Baseline CAC only included in Model 2.
3.2 Endurance exercise and progression of CAC score
Both groups reported similar number of prior endurance competitions and similar MET hours per week at baseline (Table 1). Both groups had similar race performance at baseline (3.9 [3.2-4.1] vs 3.6 [3.4-4.0] hours, P = .56). No significant differences in reported MET hours or race performance between the two groups were evident in 2018 (Table 2). In addition, there was no difference in maximal VO2 level in 2018 between those with progression of CAC vs those with stable condition (38.8 ± 7.0 vs 42.4 ± 8.9 mL/kg/min, P = .23, as percent of expected, adjusted for age and sex: 97.3 ± 16 vs 100 ± 20%, P = .57). Reported MET hours/week at inclusion and hours of endurance training per week during the follow-up period correlated well (rho = 0.60, P < .001). MET hours/week reported in 2018 and hours of endurance training had a moderate correlation (rho = 0.39, P = .002).
During the follow-up period, both groups reported 5 (4-6) hours of high-intensity endurance exercise training per week. There was no association with high volumes of high-intensity endurance exercise training and progression of CAC in either unadjusted (OR = 0.99 [0.82-1.19], P = .88) or in adjusted models (Table 4, Figure 2, Figure S2). No association with exercise volume was found in a sensitivity analysis conducted with Δ CAC + 1 as the dependent variable (Table S1).
3.3 Statin therapy
Subjects were excluded if they were taking statins prior to inclusion. However, following the baseline CCTA, the 21 subjects with CAD at baseline were advised to start statin therapy. In 2018, only 8 subjects reported that they were currently taking a statin, two of whom had started statin therapy during the follow-up period independently of findings on CCTA at baseline (both had normal coronary arteries). As such, 15 subjects had discontinued the drug during follow-up. There was no significant difference in current statin therapy between the subjects with stable condition and those who had progression of CAC (Table 2).
4 DISCUSSION
This is the first longitudinal coronary computed tomography angiography (CCTA) study to assess the progression of coronary artery calcification (CAC) in recreational athletes. There are several important findings: First, the volume of endurance exercise training was not associated with progression of CAC. Second, the prevalence of CAC was less in the present cohort of highly active middle-aged subjects compared with previously published studies on well-trained subjects. Third, the progression of CAC was associated with age and with baseline coronary artery disease (CAD).
4.1 Clinical consequences of coronary artery calcium (CAC)
The presence of CAC assessed by CCTA is associated with increased risk of clinical events.1, 2 It has therefore raised concern when previous studies have demonstrated a higher prevalence of CAC among recreational athletes than in age- and gender-matched sedentary populations.3, 5, 6 Although CAC is a marker of the burden of coronary artery atherosclerosis, assessment of CAC does not identify vulnerable plaques, that is, plaques with large necrotic cores, and macrophage infiltration in the fibrous cap. Subjects with purely calcified plaques may have less clinical events than subjects with non-calcified or mixed plaques.14 Calcified coronary plaques may consequently be considered more stable than non-calcified and mixed plaques.14, 15 Statin therapy for CAD decreases all-cause and cardiovascular mortality, but concomitantly, statin therapy is associated with increased CAC.13, 16-19 It has been suggested that the increased prevalence of CAC among recreational athletes might be indicative of a similar plaque stabilizing effect of exercise.3, 5
Exercise has been hypothesized to increase CAC due to shear stress forces and a hyperdynamic coronary circulation causing non-laminar flow.3, 5 Exercise-induced hypertension, systemic inflammation with repeated bouts of exercise, and exercise-induced parathyroid hormone increases may also increase CAC formation.5, 20 In the present study, we found no relationship between exercise training volume and the progression of CAC in either logistic regression analysis (Table 4) or in linear models (Table S1). Similarly, there was no relationship between progression of CAC and the other exercise variables explored. In line with previous publications, age and baseline CAC were major predictors of progression of CAC.13, 21, 22 Our findings suggest that the progression of CAC rather reflects underlying CV risk factors and CAD than the volume of exercise training.
4.2 The prevalence and progression of coronary artery calcium (CAC) in recreational cyclists
In the present cohort, the prevalence of CAC at baseline was 34%, with a median CAC of 0 (0-6) Agatston units. For subjects with CAC at baseline, the median CAC was 12 (3-37) Agatston units. Thus, the baseline CAC score in the present cohort was comparable with the CAC scores in similar age groups from other population-based cohorts,23, 24 but lower than previously reported in highly active subjects.3-6, 25 During the 4.1 ± 0.3 years of follow-up, only 15 subjects (25%) had progression of CAC ≥ 10 Agatston units. The mean Δ CAC was 15 ± 40 Agatston units. This rate of progression of CAC was modest compared with other studies of the progression of CAC on CCTA.13, 21, 22, 26-28 In the Heinz Nixdorf Recall Study (HNRS), subjects 45-50 years of age had a mean Δ CAC progression of 38 Agatston units over a 5-year period.21 In HNRS, authors reported that once the CAC process had begun, the progression of CAC followed an exponential trajectory.21
In previous studies, age, gender, and baseline CAC score explained 80% of the variance of CAC progression in men and 72% of the variance of CAC progression in women. Additional adjustment for baseline risk factors only increased the explained variance by 1%-3%.21 In the present study, age was a significant predictor of CAC progression in univariable and the simplest multiple regression models, but was no longer a significant predictor factor in multiple regression models including baseline CAC. Furthermore, all subjects with CAC progression during follow-up had some degree of CAC or CAD at baseline. Subjects with CAC progression had a decrease in LDL levels during follow-up; however, this finding was abolished when statin users were excluded (Table 3). Consequently, this finding underscores the importance of established CAC on the progression of coronary artery calcification.
4.3 Limitations
The main strength of this study is a low CV risk profile at baseline, with no smokers or diabetic subjects during follow-up. This allows an analysis of the effects of exercise on CAC without the influence of pre-existing CV conditions. However, when interpreting the results, in particular with regard to the multiple regression models, it should be noted that the present study was limited to only 61 subjects, with a follow-up of > 4 years. Also, the present study is an observational study without a sedentary control group.
The present study used self-reported retrospective data to explore the effects of training volume on CAC. Each athlete was asked to report the average amount of exercise training per week for the total follow-up period. This was done to assure a measure of training volume that would cover the whole follow-up period, including seasonal fluctuations in training volume and periods of interrupted training. However, this data may be subject to recall bias. Interestingly, the amount of endurance training hours/week during the follow-up period was well correlated with the reported MET hours/week at inclusion in 2013/14 (rho = 0.60, P < .001) and moderately at follow-up in 2018 (rho = 0.39, P = .002). The MET hours/week were obtained by the use of the International Physical Activity Questionnaire. This questionnaire assesses physical activity during the last 7 days prior to data registration, thereby reflecting the training during the last week prior to the North Sea Race. Since the last 7 days prior to a competition are adapted to improve competitive performance, the exercise pattern during this period may not be representative for the general exercise volume. We therefore believe that our global measure of exercise training is better suited to assess the impact of high-intensity exercise on CAC. Future studies examining the effect of exercise on progression of CAC should include an exercise log to minimize the risk of over- and underestimation of exercise.
Lastly, only 25% of the included subjects had exercise volumes exceeding 6 hours per week. The relationship between CAC progression and exercise in middle-aged recreational athletes with very high training volumes should be further evaluated before concluding with certainty on the effect of exercise volume on CAC progression.
5 PERSPECTIVE
In this longitudinal follow-up study of highly active middle-aged recreational athletes with a low CV risk, the progression of coronary artery calcification was modest. The volume of high-intensity exercise was not significantly associated with progression of CAC. In fact, all subjects with progression of CAC ≥ 10 Agatston units during follow-up had some CAC or CAD at baseline. These findings challenge the belief of a causal effect of exercise on coronary artery calcification in masters athletes.
ACKNOWLEDGEMENTS
We thank the participants and the medical staff at Stavanger University Hospital who contributed to the data acquisition, in particular T. Svihus and J. Havnen for organizing and evaluating the CCTA examinations.
CONFLICT OF INTEREST
None.




