Allergens elicit upregulated IL-18 and IL-18Rα expression in blood monocytes of patients with allergic rhinitis
Abstract
Interleukin (IL)-18 is a potentially important molecule in allergic rhinitis (AR). However, expressions of IL-18, IL-18 binding protein isoform a (IL-18BPa) and IL-18 receptor alpha (IL-18Rα) in AR blood monocytes remain obscure. We, therefore, investigated IL-18, IL-18BPa and IL-18Rα expressions in monocytes using flow cytometry, murine AR model and quantitative real-time polymerase chain reaction (PCR). The results showed that the numbers of IL-18+ monocytes increased, whereas IL-18BPa+ monocytes decreased in the peripheral blood of AR patients. It was also observed that Platanus pollen extract provoked elevated expressions of IL-18 and IL-18Rα in the monocytes of AR patients. House dust mite extract, Artemisia sieversiana wild extract and Platanus pollen extract enhanced IL-18Rα protein and mRNA expression in the isolated primary monocytes from AR patients. Using ELISA kits, we observed that the levels of total IL-18 and free IL-18 in the plasma of perennial AR (pAR) and seasonal AR (sAR) patients were elevated, and the molar concentration ratio of free IL-18BPa/free IL-18 was 16.5 for healthy control subjects and 9.7 for patients with sAR, indicating that IL-18 likely plays a role in sAR. In the murine AR model, the number of IL-18Rα+ monocytes increased in the blood, and the number of IL-18Rα+ macrophages increased in the nasal lavage fluid of WT mice. In conclusion, IL-18 may serve as a causative factor for AR.
1 INTRODUCTION
Interleukin (IL)-18, initially known as IFN (interferon)γ-inducing factor, is constitutively expressed by monocytes and macrophages,1 airway epithelial cells2 and dendritic cells3 and plays regulatory roles in both innate and adaptive immunity.4 The inflammasome is responsible for the activation of caspases 1 and 5, leading to the processing and secretion of pro-inflammatory cytokines IL-1β and IL-18.5 A pathologic role for IL-18 in the induction of allergic diseases includes stimulating mast cell and basophil degranulation6 and activating basophils through pathways involving Syk and IκB kinases.7 It is reported that the polymorphism of the IL-18 gene was associated with allergic rhinitis (AR),8, 9 and elevated IL-18 levels have been found in both the nasal secretion and serum of AR patients10-12 and in the adenoid tissue of children with adenoid hypertrophy and coexistent AR.13 It is also observed that elevated serum IL-18 levels, caused by natural pollen exposure, are closely associated with bronchial hyperresponsiveness in seasonal AR (sAR) patients.14 These observations implicate that IL-18 likely contributes to the pathogenesis of AR.
IL-18 initially binds to IL-18 receptor alpha (IL-18Rα), initiates the MyD88-dependent signal pathway and exerts its immunomodulatory functions.15 Elevated IL-18Rα expression is observed on type 2 innate lymphoid cells (ILC2) of patients with AR, following IL-18 and house dust mite stimulation12 and on monocytes of patients with atopic asthma.16 IL-18 binding protein (IL-18BP) is an endogenous soluble antagonist of IL-18 because it binds to IL-18 with high affinity.17 Four human and two mouse isoforms of IL-18BP protein have been identified, of which human IL-18BP isoform a (IL-18BPa) and mouse IL-18BP isoform d (IL-18BPd) are structurally related, and exhibit the strongest affinity for IL-18.18 Decreased expression of IL-18BPa in the monocytes of patients with atopic asthma has previously been reported.16 Free serum IL-18BPa is physiologically present at a 20-fold higher level than free IL-18.19 It is suggested that a ratio of total serum IL-18BPa and total serum IL-18 lower than 12.8 may cause illness.16 These observations suggest that the imbalance between IL-18 and IL-18BPa expression may account for increased IL-18 activity in AR. Indeed, we demonstrated recently that the role of IL-18 in atopic asthma is determined by the balance of IL-18/IL-18BPa/IL-18Rα.16 Since the level of circulating blood IL-18BPa in AR has not been reported, we examined IL-18BPa levels in AR plasma in the present study.
It has been reported that pollen allergen immunotherapy induced increased serum IL-18 level in AR patients,20 and pollen extract provoked IL-18 mRNA expression in peripheral blood mononuclear cells (PBMCs) of AR patients undergoing allergen immunotherapy,21-23 suggesting that allergens may contribute to AR through PBMC and IL-18-related mechanisms. Since monocytes are one of the major cell types of PBMCs, and little is known about the expressions of IL-18, IL-18BPa and IL-18Rα at protein and mRNA levels in the monocytes of AR patients, particularly upon allergen challenge, we investigated the levels of IL-18 and IL-18BPa in AR plasma, the expressions of IL-18, IL-18BPa and IL-18Rα in the monocytes of patients with AR and the influence of allergens on their expressions. We found that the numbers of IL-18+ monocytes increased, whereas IL-18BPa+ monocytes decreased in AR patient blood.
2 MATERIALS AND METHODS
2.1 Reagents
The following reagents were purchased from Biolegend: human RBC lysis buffer, Brilliant Violet 510™ (BV510)-conjugated donkey anti-rabbit IgG polyclonal antibody (pAb), PE/Cy7-conjugated mouse anti-human CD14 monoclonal antibody (mAb), BV510-conjugated rat anti-mouse Gr-1 mAb and its isotype control antibody BV510-conjugated Rat IgG2b κ, BV510-conjugated rat anti-mouse F4/80 mAb and its isotype control antibody BV510-conjugated Rat IgG2a κ, PerCP-conjugated rat anti-mouse CD11b mAb and its isotype control antibody PerCP-conjugated Rat IgG2b κ, human Fc receptor blocking solution, rat anti-mouse CD16/32 antibody, Brefeldin A, Zombie NIR™ and Zombie Green™ Fixable Viability Kit. Mouse IL-18BPd DuoSet ELISA kit, APC-conjugated mouse anti-human IL-18Rα mAb, PE-conjugated mouse anti-human IL-18 mAb, APC-conjugated rat anti-mouse IL-18Rα mAb and their respective isotype controls (catalogue #: IC002A, IC002P, IC005A) were supplied by R&D Systems. Rabbit anti-human IL-18BPa pAb and rabbit IgG isotype control antibody were obtained from Novus Biologicals. IFNγ was supplied by PeproTech. Trypan blue dye and Ovalbumin (OVA, Grade V) were purchased from Sigma-Aldrich. Human IL-18BPa and IL-18 ELISA kits were bought from ImmunoWay Biotechnology Company and ExCell Bio, respectively. Cytofix/Cytoperm™ Fixation/Permeabilization Solution Kit was purchased from BD Biosciences. Alhydrogel® adjuvant was purchased from InvivoGen. Lymphoprep™ solution was purchased from AXIS-SHIELD PoC AS. Foetal bovine serum (FBS, Hyclone), penicillin–streptomycin antibiotic mixture and RPMI 1640 medium were obtained from Gibco BRL. RBC Lysis Buffer (multi-species) for mouse study, mouse IL-18 ELISA kit and TRIzol reagent were purchased from Invitrogen. Anti-human CD14 MicroBeads and autoMACS Running Buffer–MACS Separation Buffer were obtained from Miltenyi Biotec. House dust mite extract (HDME) was bought from Greer Laboratories, Inc. Artemisia sieversiana wild extract (ASWE) and Platanus pollen extract (PPE) were purchased from Macro Union Pharmaceutical Co. Ltd. Allergens for skin prick tests were supplied by ALK-Abelló, Inc. Most of the general-purpose chemicals, such as salts and buffer components, were of analytical grade.
2.2 Subjects and animals
The general characteristics of 33 patients with perennial AR (pAR), 9 patients with sAR and 25 healthy control subjects (HC) recruited in this study were summarized in Table S1. The diagnosing criteria of pAR and sAR conformed to the Chinese Society of Allergy Guidelines for Diagnosis and Treatment of Allergic Rhinitis.24 The experiment was approved by the ethical committees of the First Affiliated Hospital of Jinzhou Medical University and the General Hospital of Northern Military Area. Approximately 5 mL of peripheral blood samples was drawn into a K2EDTA containing tube after obtaining written informed consent from each participant and centrifuged at 450 g for 10 min. The cells were kept for flow cytometric analysis, and the plasma was collected for ELISA analysis. For the CD14+ monocytes isolation study, approximately 180 mL of peripheral blood was taken from each donor.
BALB/c mice, weighing 18-22 g, were purchased from Vital River Laboratory Animal Technology Co., Ltd. and maintained in the First Affiliated Hospital of Jinzhou Medical University under specific pathogen-free facilities with free access to standard rodent chow and water, at a constant temperature of 23-28°C and a relative humidity of 60%-75%. The animal experiment procedures were authorized by the Animal Care Committee at Jinzhou Medical University.
2.3 Isolation of CD14+ monocytes and allergen challenge
CD14+ cells were enriched by density gradient centrifugation and positive selection on magnetic cell sorting (MACS) according to instructions from the manufacturer. Purities of recovered CD14+ cells were evaluated by flow cytometry with PE/Cy7-conjugated anti-human CD14 mAb.
To further investigate the direct action of allergens on IL-18, IL-18BPa and IL-18Rα expressions in monocytes, isolated monocytes at a density of 1 × 106 per mL were cultured in RPMI 1640 medium containing 3% FBS and 100 U/mL penicillin/streptomycin in a 12-well cell culture plate (Nest) in the presence or absence of ASWE, PPE, HDME (all at a concentration of 1.0 μg/mL) or IFNγ (5 ng/mL, as positive control) for 10, 30 and 60 min,25-27 respectively, at 37°C in a 5% (v/v) CO2, water-saturated atmosphere. Brefeldin A at 2 μg/mL was added to the wells to detect the intracellular expression of IL-18 and IL-18BPa before stimulation. The cells were then harvested and centrifuged at 450 g for 10 min at 4°C. Cell pellets containing approximately 0.5 × 106 cells were resuspended in PBS for flow cytometric analysis, and 1 × 106 cells were resuspended in TRIzol reagent for real-time polymerase chain reaction (RT-PCR) assay.
2.4 Flow cytometric analysis of IL-18, IL-18BPa and IL-18Rα in human peripheral blood monocytes and isolated CD14+ monocytes
The procedures for detecting IL-18, IL-18BPa and IL-18Rα expression in human peripheral blood monocytes were mainly adopted from a previous study by Zhang et al.16 Briefly, whole blood cells were challenged with or without ASWE, PPE or HDME (all at a concentration of 1.0 μg/mL) for 1 h.
For cell surface molecules, whole blood cells were preincubated with human Fc receptor blocking solution and Zombie Green dye,28 then stained with PE/Cy7-conjugated anti-human CD14 and APC-conjugated anti-human IL-18Rα antibodies. Following erythrocyte lysis, leucocytes were analysed with FACSVerse flow cytometer (BD Biosciences). Fluorescence minus one controls for human IL-18Rα-APC, IL-18-PE, IL-18BPa and mouse IL-18Rα-APC and isotype controls (APC Mouse IgG1 κ Isotype Control catalogue #: IC002A, clone #: 11711, PE Mouse IgG1 κ Isotype Control catalogue #: IC002P, clone #: 11711, Rabbit IgG Isotype Control catalogue #: AB-105-C and APC Rat IgG1 Isotype Control catalogue #: IC005A, clone #: 43414) were applied.29 Dead cells and doublets were distinguished by SSC-A–live/dead cell dye and FSC-H–FSC-A gating strategies (Figure S1). The mean fluorescence intensity (MFI) of IL-18, IL-18BPa and IL-18Rα expressions in the monocytes was performed using FlowJo Software V10 (BD) (Figures S2 and S3).
For intracellular molecules, leucocytes were fixed and permeabilized according to instructions from the Cytofix/Cytoperm™ Fixation/Permeabilization Solution Kit, then stained with rabbit anti-human IL-18BPa and PE-conjugated anti-human IL-18 antibodies, which was followed by the addition of BV510-conjugated donkey anti-rabbit pAb.
As for isolated monocytes, the cells were stained with PE/Cy7-conjugated anti-human CD14 and APC-conjugated anti-human IL-18Rα antibodies. For intracellular molecules, the cells were stained with rabbit anti-human IL-18BPa and PE-conjugated anti-human IL-18 antibodies, which was followed by the addition of BV510-conjugated donkey anti-rabbit pAb.
2.5 Establishment of mouse AR model
Ovalbumin-induced mouse AR model was mainly adopted from a previous study by Mo JH et al.30 Briefly, mice were sensitized on days 0, 7 and 14 by intraperitoneal injection of 25 μg of OVA emulsified in 1 mg of alhydrogel. On days 21-27, the mice were challenged by intranasal instillation using 500 μg of OVA dissolved in PBS (10 μL/nostril) once daily. For control experiments, mice received normal saline only. At 24 h following the last OVA challenge, the mice were sacrificed by CO2 aspiration, and blood and nasal lavage fluid (NLF) samples were then collected. After centrifugation at 450 g for 10 min at 4°C, the total cell numbers were determined and the samples were used for flow cytometric analysis. Plasma and NLF supernatant were aliquoted and stored at −80°C.
To evaluate allergic symptoms, the numbers of sneezing and nasal-rubbing motions of the mice during the first 15 min after the allergen challenge were recorded and compared with healthy control mice (HM) by observers who were unaware of which mice they were studying. As presented in Figure S4A,B, the numbers of nasal rubbing and sneezing motions in AR mice (n = 7) were markedly higher than that in HM (n = 7) during a 7-day observation period.
2.6 Flow cytometric analysis of IL-18Rα expression in mouse blood monocytes and NLF macrophages
To detect IL-18Rα expression in mouse blood monocytes, whole blood cells were preincubated with anti-mouse CD16/32 antibody and Zombie NIR dye.31 Each labelled mAb including BV510-Gr-1, PerCP-CD11b and APC-IL-18Rα was added into the tubes before lysing the erythrocytes. The cells were then processed as in human blood samples and analysed using flow cytometry.
To detect IL-18Rα expression in NLF macrophages, the cells were incubated with anti-mouse CD16/32 antibodies and Zombie NIR dye,31 then subsequently incubated with BV510-conjugated anti-mouse F4/80 mAb32 and APC-conjugated anti-mouse IL-18Rα mAb and analysed as above.
2.7 Real-time quantitative PCR analysis for IL-18, IL-18BPa and IL-18Rα in isolated CD14+ monocytes
cDNA generated from total RNA of isolated human blood CD14+ cells was used as templates, and quantitative PCR (qPCR) assay was performed as described by Zhang et al.33 using specific primers of IL-18, IL-18BPa and IL-18Rα as listed in Table S2. Briefly, after synthesizing cDNA from total RNA using RT Master Mix Perfect Real Time, qPCR was performed using SYBR Premix Ex TaqII Kit on the Real-time Thermal Cycler (Thermo Fisher Scientific Oy). Each reaction contains 12.5 μL of 2 × SYBR green Master Mix, 300 nM oligonucleotide primers and 10 μL of the cDNA. Untreated controls were chosen as the reference samples, and the ΔCt for all experimental samples were subtracted by the ΔCt for the control samples (ΔΔCt). The magnitude change of test gene mRNA was expressed as 2−ΔΔCt. Each measurement of a sample was conducted in duplicate.
2.8 Measurement of cytokine levels in plasma and NLF supernatant, and calculation of molar concentration ratio of plasma IL-18BPa/IL-18
The levels of total IL-18 and total IL-18BPa or total IL-18BPd in plasma or NLF supernatant were determined using an ELISA kit under instructions from the manufacturer. The molar concentrations of human IL-18 and IL-18BPa and free IL-18 and free IL-18BPa were calculated as described by Novick D et al.34
2.9 Statistical analysis
Statistical analyses were performed using SPSS software (version 21.0, IBM Corporation). Data for the plasma levels of IL-18 and IL-18BPa were displayed as a Scatter plot, whereas the rest of the data were displayed as a boxplot, which indicates the median, interquartile range, the largest and smallest values for the indicated experiments. Where Kruskal–Wallis analysis indicated significant differences between groups, the paired Mann–Whitney U test was used for further analyses. Correlations were determined using Spearman rank correlation analysis. For all analyses, P < .05 was considered statistically significant.
3 RESULTS
3.1 Increased expression of IL-18 and IL-18Rα and decreased expression of IL-18BPa in monocytes of patients with pAR and sAR
Since the percentages of blood monocytes expressing IL-18, its specific receptor IL-18Rα and its natural neutralizer IL-18BPa in AR patients remain unknown, we investigated them by flow cytometry in the present study. The results showed that the proportion of IL-18+ monocytes increased by 4.2 and 9.1 folds (Figure 1A,B) and the proportion of IL-18BPa+ monocytes decreased by 77.5% and 56.0% in pAR and sAR patients, respectively, compared with HC (Figure 1C,D). PPE seemed to upregulate IL-18 and IL-18Rα expressions in the monocytes of pAR and sAR patients (Figure 1E,F).
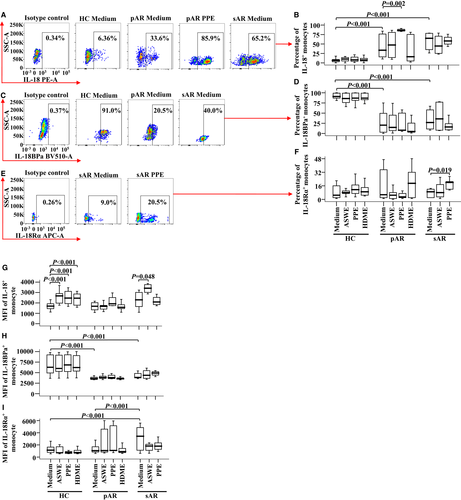
As for the expression intensity of a single positive cell, all allergens tested in this study including ASWE, PPE and HDME enhanced the MFI of IL-18 expression in the monocytes of HC, and ASWE increased the MFI of IL-18 expression in the monocytes of sAR patients (Figure 1G). However, pAR and sAR patients appeared to have lower MFI of IL-18BPa in monocytes than that of HC (Figure 1H), while sAR patients had a higher MFI of IL-18Rα on monocytes than that of HC and pAR patients (Figure 1I).
3.2 Allergens and IFNγ induced alteration of IL-18, IL-18BPa and IL-18Rα expressions in isolated monocytes
In order to evaluate the direct effects of allergens on the expressions of IL-18, IL-18BPa and IL-18Rα in purified monocytes (purity up to 99.7%), we then co-cultured HDME, ASWE, PPE or IFNγ with monocytes in a 12-well cell culture plate and examined their expressions using flow cytometry. The results showed that the proportion of IL-18BPa+ monocytes in AR patients was lower compared with HC (Figure 2B,E), and IL-18BPa expression appeared to be upregulated in HC following the stimulation of HDME for 10 min (Figure 2B,E). It was also shown that ASWE, PPE and IFNγ induced the elevated expression of IL-18Rα in the monocytes of AR patients at 10 min following incubation (Figure 2C,F). Moreover, HDME increased the expression of IL-18Rα in AR patients at 30 min following stimulation (Figure 2C,F). Additionally, the number of IL-18+ monocytes in AR patients was higher compared with that in HC (Figure 2A,D), which was consistent with the result seen in Figure 1A,B.
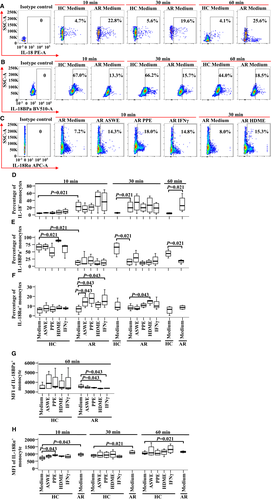
In terms of MFI, HDME and IFNγ seemed to downregulate MFI of IL-18BPa+ monocyte in AR patients at 60 min following incubation (Figure 2G). While enhanced MFI of IL-18Rα expression in the monocytes of AR patients was observed in comparison with HC (Figure 2H), PPE seemed to enhance the MFI of IL-18Rα expression in HC at 10 min following incubation (Figure 2H). However, allergens and IFNγ tested in the present study had little effect on IL-18 MFI expression in monocytes (data not shown).
3.3 Allergen induced IL-18Rα mRNA expression in primary monocytes
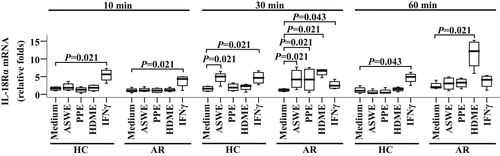
In order to further understand the effects of allergens on IL-18, IL-18BPa and IL-18Rα expressions in monocytes, we examined mRNA expressions of IL-18, IL-18BPa and IL-18Rα in primary monocytes using the qPCR technique. As seen in Figure 3, ASWE, PPE and HDME elicited an upregulated expression of IL-18Rα mRNA in the monocytes of AR patients by 2.5, 2.5 and 4.6 folds, respectively, at 30 min following challenge. At 60 min following challenge, only HDME-induced elevated expression of IL-18Rα mRNA in the monocytes of AR patients was observed. ASWE-provoked expression of IL-18Rα mRNA in monocytes was also found in HC at 30 min following challenge. The allergens tested had little effect on the expressions of IL-18 and IL-18BPa mRNAs in the monocytes of AR patients and HC following 10-, 30- and 60-min challenge periods (data not shown).
3.4 Elevated level of IL-18 in plasma of patients with pAR and sAR
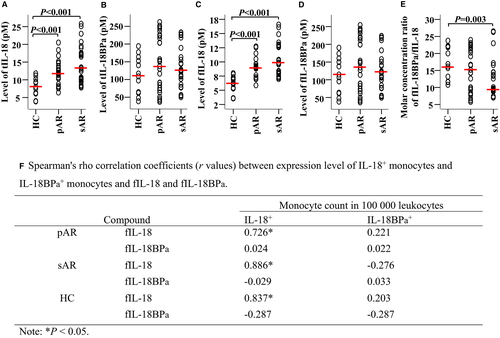
Using ELISA kits, we observed that levels of total IL-18 (Figure 4A) and free IL-18 (Figure 4C) in the plasma of pAR and sAR patients were elevated relative to HC. In contrast, there were no significant differences in plasma levels of total IL-18BPa (Figure 4B) and free IL-18BPa (Figure 4D) between patients with pAR or sAR and HC. It was also found that the molar concentration ratio of free IL-18BPa/free IL-18 for HC (16.5) was markedly greater than that for patients with sAR (9.7) (Figure 4E), suggesting that IL-18 may play a role in sAR. Meanwhile, significant correlations between free IL-18 and IL-18+ monocytes (Figure 4F) were found in patients with pAR, sAR and HC.
3.5 Upregulated IL-18Rα expression in blood monocytes and NLF macrophages of OVA-induced AR mice
To confirm the role of IL-18Rα in AR, we examined IL-18Rα expressions in both the blood monocytes and the NLF macrophages of AR mice. Compared with the HM group, the numbers of monocytes and IL-18Rα+ monocytes were increased by 25.6% (Figure 5A,B) and 1.4 folds (Figure 5A,C), respectively, in OVA-induced AR mice. The numbers of F4/80+ macrophages and IL-18Rα+ macrophages were increased by approximately 1.2 (Figure 6A,B) and 1.4 folds (Figure 6A,C), respectively, in AR mice. However, the MFI of IL-18Rα expression in the monocytes and the macrophages of AR mice had changed insignificantly (data not shown).
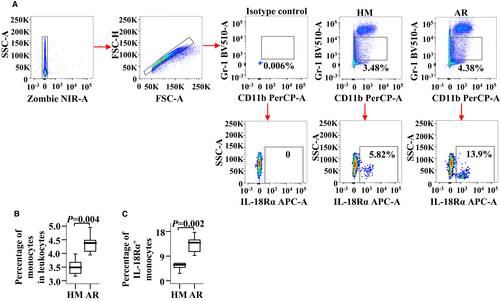
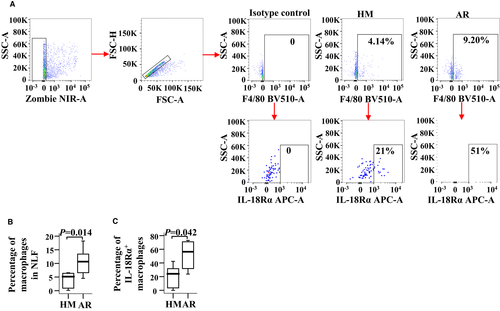
3.6 Increased levels of IL-18 in both plasma and NLF of AR mice
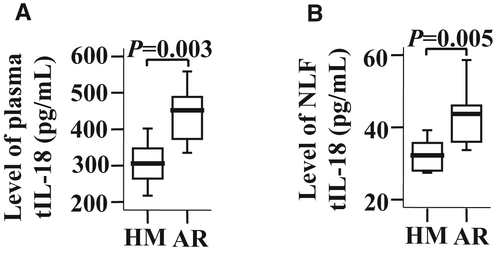
To further understand the role of IL-18 in AR, the effect of OVA challenge on IL-18 and IL-18BPd production in AR mice was examined. The results showed that the levels of IL-18 (Figure 7A,B), but not IL-18BPd (data not shown), were elevated in both the plasma and the NLF of AR mice.
4 DISCUSSION
Increased plasma levels of IL-18 and upregulated expressions of IL-18 and IL-18Ra in the monocytes of patients with AR imply that monocytes are likely involved in the pathogenesis of AR via IL-18-related mechanisms. More precisely, the upregulated expression of IL-18 in monocytes suggests that IL-18 may originate from monocytes, and the upregulated expression of IL-18Ra in monocytes implies that the action of IL-18 on monocytes may have been conducted by IL-18Ra, which implicates a paracrine mechanism that monocytes secrete IL-18 and IL-18 acts on adjacent monocytes via IL-18Rα. The previous reports that IL-18 is constitutively expressed by monocytes and macrophages4 and is closely related to the pathophysiology of allergic respiratory disorders,6 in addition to the findings that elevated IL-18 level is found in the serum of AR patients11, 12 and that elevated serum IL-18 during natural pollen exposure is closely associated with bronchial hyperresponsiveness in sAR patients,14 emphasize further the potential of IL-18 as a causative factor in AR.
However, the contribution of IL-18 to AR is not only determined by the plasma concentration of IL-18 but also depends on the free IL-18 activity in plasma. Generally, the plasma concentration of IL-18 (total IL-18) consists of free IL-18 and bound IL-18, and the pathological plasma level of free IL-18 in HC is lower than 40 pg/mL.19 Free serum IL-18BPa is physiologically present at a 20-fold higher level than free IL-1819 and exhibits the highest affinity for IL-18.18 It is suggested that a ratio of total serum IL-18BPa and total IL-18 lower than 12.8 may cause illness16 and that excessive free IL-18 can cause inflammation.35, 36 A molar excess of 10 of IL-18BPa over IL-18 is required to reduce the pathological level of 400 pg/mL of IL-18 to the level for HC (40 pg/mL)19 as IL-18BPa has a neutralizing capacity toward IL-18.17 In the present study, we found that the molar concentration ratio of free IL-18BPa/free IL-18 for HC is 16.5, whereas the ratio for patients with sAR is 9.7, suggesting that IL-18 may play a role in sAR. Since plasma levels of total IL-18BPa and free IL-18BPa in patients with pAR or sAR did not decrease, the reduced molar concentration ratio of free IL-18BPa/free IL-18 for sAR should result from overproduction of IL-18. Obviously, the normal plasma levels of total IL-18BPa and free IL-18BPa in patients with pAR or sAR contrast with the fact that IL-18BPa+ monocytes decrease by 77.5% and 56.0% in pAR and sAR patients. Since there are weak correlations between the plasma levels of free IL-18BPa and IL-18BPa+ monocytes observed in pAR and sAR patients in the present study, and decreased expression of IL-18BPa in monocytes of patients with atopic asthma has previously been reported,16 we anticipate that besides IL-18BPa+ monocytes, there must be other sources of production of IL-18BPa in the body.
Enhanced MFI of IL-18Rα expression in the monocytes of AR patients is observed, implicating that the density of IL-18Rα expression in given monocyte is increased and subsequently has a better chance of being activated by IL-18. The reports that elevated IL-18Rα expression is observed on ILC2 cells of patients with AR, following IL-18 and house dust mite stimulation,12 and on monocytes of patients with atopic asthma16 may support our earlier observation.
The findings that PPE upregulates IL-18 and IL-18Rα expression in the monocytes of pAR and sAR patients and ASWE enhances the MFI of IL-18+ monocyte in sAR patients suggest that aeroallergens can directly affect IL-18 and IL-18Rα expression in monocytes, even though direct contact of allergens with blood monocytes seldom occurs in the body. Unexpectedly, the MFI of IL-18+ monocytes in HC can be enhanced by all allergens tested in this study including ASWE, PPE and HDME. Since grass pollen extracts induce secretion of IL-18 from human nasal epithelial cells,37 and monocytes are one of the major sources of IL-18,38 the enhanced expression of IL-18 in monocytes may imply that this cytokine may facilitate the sensitization of HC to aeroallergens. The finding that synergy of IL-5 and IL-18 in eosinophils mediated the pathogenesis of allergic diseases39 may also support the notion that IL-18 promotes allergy.
Using primary human monocytes, the elevated IL-18Rα expression in the monocytes of AR patients, induced by allergens ASWE, PPE and HDME, is confirmed at both protein and mRNA levels in the current study. This result suggests that allergen-induced upregulation of IL-18Rα expression is most likely a direct event. Although the proportion of IL-18BPa+ monocytes in AR patients is lower compared with HC and HDME seems to downregulate the MFI of IL-18BPa+ monocytes in AR patients, the allergens tested in the present study show little effect on IL-18BPa mRNA expression in the monocytes of AR and HC. These findings imply that reduced IL-18BPa expression in monocytes most likely occurs during protein synthesis such as during the elongation, transporting or modification stages.
Our observation that IL-18 levels are elevated in both the plasma and the NLF of AR mice, following OVA challenge, agrees with the finding that IL-18 level is increased in AR patients. Meanwhile, the numbers of blood monocytes, IL-18Rα+ monocytes, NLF macrophages and IL-18Rα+ macrophages are found to be elevated in AR mice. Since tissue-resident macrophages originate from blood monocytes,40 the upregulated expression of IL-18Rα may implicate that not only monocytes but also the monocyte–macrophage system participate in IL-18 mediated promotion of AR.
In conclusion, we demonstrated for the first time that enhanced free IL-18 is found in AR plasma. Furthermore, upregulated expressions of IL-18 and IL-18Rα have been observed in the monocytes of AR patients. These findings strongly implicate that IL-18 may serve as a causative factor for AR. The regulation of the expressions of IL-18, IL-18BPa and IL-18Rα in monocytes by specific allergens suggests that allergens contribute to the pathogenesis of AR by directly modifying monocyte behaviour. Further investigation of the relationships between allergens, IL-18 and IL-18Ra will be critical for understanding the pathogenesis of AR.
AUTHOR CONTRIBUTIONS
JL Wang and FQ Gu performed most of the experiments and drafted the original manuscript. MM Zhan, L Wang and RM Yang participated in magnetic cell sorting and flow cytometry. HY Zhang conducted the ELISA and Bio-plex experiments and generated the figures. D Chen, H Xie and RN Chai recruited volunteers and collected their general information. N Zhao conducted the PCR experiment and data interpretation. Z Li contributed to animal study and literature review. SH He designed and supervised the study, analysed the data and wrote the final version of the manuscript. All authors commented on previous drafts of the manuscript, read and approved the final manuscript.
FUNDING INFORMATION
This project was sponsored by the grants from the 2021 Youth Science and Technology Talents Support Plan from Boze Project of Jinzhou Medical University (JYBZQT2110); Natural Science Foundation of Liaoning Province (2019-ZD-0801; LJKZ0796; 201601354); Key Laboratory of Research on Pathogenesis of Allergen Provoked Allergic Disease of Liaoning Province (2018-30); Allergic Disease Clinical Medicine Research Center of Liaoning Province (2017-37); Climbing Scholar Project for Institution of Higher Education in Liaoning Province (2018-38), Science and Technology Project of Liaoning Province (2019JH8/10300053); Shenyang Science and Technology Plan Project (19-109-4-17); Innovation and Entrepreneurship Project for High-Level Talent in Shenyang (2017CXCY-C-01).
CONFLICT OF INTEREST
The authors declare they have no competing interests regarding the publication of this article.
Open Research
DATA AVAILABILITY STATEMENT
Raw data support the findings of this study will be available from the corresponding author upon reasonable request.




