The diagnostic and prognostic significance of flow cytometric bone marrow assessment in myelodysplastic syndromes according to the European LeukemiaNet recommendations in single-centre real-life experience
Abstract
Introduction
This analysis attempts to determine the diagnostic and prognostic value of bone marrow (BM) evaluation by multiparameter flow cytometry in patients with myelodysplastic syndrome (MDS).
Materials and methods
The study group consisted of patients who underwent diagnostic process in the years 2008-2017 due to cytopenia and finally were diagnosed with MDS (n = 71). The comparative group included patients with cytopenia diagnosed in the same period, whose definitive diagnosis was other than MDS (n = 39). Flow cytometric evaluation of BM was performed following the recommendations of the European LeukemiaNet (ELN) in all patients.
Results
The median number of immunophenotypic abnormalities found on granulocytes in the MDS group was significantly higher compared to the comparative group [2 (range 0-5) vs 0 (range 0-2); P < .0001]. Similarly, the median Ogata score was significantly higher in the MDS group [2 (range 0-4) vs 1 (range 0-3); P < .0001]. Since the disturbances of the CD11b/HLA-DR and CD11b/CD13 on granulocytes were significantly more common in MDS patients, the Ogata score was extended by these abnormalities, what resulted in its higher diagnostic sensitivity (82%) while preserving high specificity (87%). The positive correlation was found between risk score determined by the Revised International Prognostic Scoring System and the number of the BM immunophenotypic abnormalities (P = .017).
Conclusions
Our results indicate that the diagnostic usefulness of the Ogata score may be increased by including the abnormal expression of CD11b/HLA-DR and CD11b/CD13 on granulocytes. Moreover, our findings suggest the prognostic significance of the number of BM cytometric abnormalities in MDS.
1 INTRODUCTION
Myelodysplastic syndrome (MDS) is a heterogeneous group of clonal haematopoietic neoplastic diseases characterized by inefficient haematopoiesis with morphologically identifiable dysplastic lesions and an increased risk of transformation into acute myelogenous leukaemia. Despite the rapid development of new diagnostic methods, the gold standard for diagnosis remains the finding of dysplastic changes in hyperplastic bone marrow smear.1, 2 Proper diagnostic process should include histopathological, cytogenetic and molecular examination. Differential diagnosis should include cytopenias of different origin: deficiencies, infections or autoimmune processes, in which dysplastic changes in bone marrow may also occur.3, 4
The development of unified flow cytometric protocol for diagnosis of low-grade MDS with high sensitivity and specificity is now one of the greatest challenges. In 2007, National Comprehensive Cancer Network (NCCN), European LeukemiaNet (ELN) and International Working Group (IWG) experts, during the ‘Working Conference on MDS’, have presented guidelines for evaluation of the bone marrow immunophenotype in MDS.5 In 2012, ELN presented the bone marrow cytometry guidelines, which are valid until today.6 However, bone marrow assessment by multiparameter flow cytometry is not considered as obligatory according to WHO criteria of diagnosis.1
Although accurate immunophenotypic assessment of bone marrow when suspecting MDS is a valuable complementary examination, it requires the implementation of many parameters; therefore, the diagnostic process becomes time-consuming and expensive. For this reason, an important goal in flow cytometry development is to establish a simplified cytometric scoring system, which will allow MDS to be recognized with high sensitivity and specificity in a simple and cheap way. In 2009, Ogata et al presented a scale, which is characterized by 69% sensitivity and 92% specificity in MDS recognition.7 The study report assumes using only three monoclonal antibodies and the evaluation of four simple parameters, which are frequently aberrant in MDS. In 2012, the ELN experts simplified the Ogata score and proved that the assessment of similar parameters with the use of only two monoclonal antibodies can give comparable results (sensitivity 70%, specificity 93%).8 Thanks to the objectivity of all parameters, the result of the scoring system does not depend on the test conditions, type and settings of flow cytometer and the antibodies and conjugated fluorochromes used. In addition, it only slightly depends on the subjective assessment of the laboratory staff.
The goal of this retrospective study was to evaluate the diagnostic and prognostic significance of flow cytometric evaluation of bone marrow according to ELN recommendation in patients with suspicion of MDS. In addition, we aimed to check if extension of the Ogata score by additional immunophenotypic aberrancies of maturing neutrophils and monocytes may increase its sensitivity and specificity.
2 MATERIALS AND METHODS
The retrospective study was approved by the local Bioethics Committee at the Poznan University of Medical Science.
2.1 Patients
The medical records of all patients who were suspected of having MDS and were diagnosed in the Department of Hematology and Bone Marrow Transplantation of the Poznan University of Medical Science between 2008 and 2017 year due to single- or multi-lineage cytopenia were included in this analysis. According to the final diagnosis, the patients were divided into the study group and the comparative group. All patients who were finally diagnosed with myelodysplastic syndrome in accordance to the WHO classification were included in the study group.1, 9 The diagnosis of MDS was established on the basis of bone marrow cytology, bone marrow biopsy and cytogenetic studies. An additional complementary study, routinely performed in patients with suspected MDS since 2008 in our Department, was the examination of the bone marrow using multiparameter flow cytometry (MFC). The comparative group consisted of patients who were suspected of having MDS due to single- or multi-lineage cytopenia over the same period of time, and underwent therefore a cytological, histopathological and flow cytometric examination of the bone marrow, but were eventually diagnosed with disorder other than MDS. The patients with cytopenia induced by chemotherapy or other anti-neoplastic treatment were excluded from the analysis.
2.2 Multiparameter flow cytometry
In brief, bone marrow samples were taken from the upper back hip spine and delivered to the laboratory within one hour from collection. To avoid the influence of EDTA on the expression of CD11b and CD16 on granulocytes, samples were immediately processed within 2 hours from collection.10 Monoclonal antibodies in the amount recommended by the manufacturer were added to 100 μL of the bone marrow. The antibodies panels used in the study are presented in Table 1. The panel was prepared to assess most of the disorders listed below in the period when only 6-colour cytometry was available. It was used in all patients with suspected MDS. Bone marrow samples were incubated with antibodies for 15 minutes at room temperature in a shaded area. Lysis of the erythrocyte red cell was then performed with the Becton Coulter IOTest3 lysis solution. The next step was rinsing twice with phosphate buffered saline (PBS) with 2000 rpm for 4 minutes. The samples were analysed immediately after preparation. All determinations were performed on a BD Calibur or Canto II flow cytometer, and images analysed using Diva 2.0 software.
| FITC | PE | PerCP | APC | PE-Cy-7 | APC-Cy7 | |
|---|---|---|---|---|---|---|
| Panel 1 |
CD45 2D1 |
CD13 L138 |
CD16 3G8 |
CD5 L17F12 |
CD19* J3-119 |
CD14 MφP9 |
| Panel 2 |
CD7 M-T701 |
CD 56 NCAM16.2 |
CD45 2D1 |
CD33* D3HL60.251 |
CD117 104D2 |
CD14 MφP9 |
| Panel 3 |
CD15 MMA |
CD11b D12 |
CD45 2D1 |
HLA-DR L243 |
CD34* 581 |
CD14 MφP9 |
| Panel 4 |
CD64** 10.1 |
CD11b D12 |
CD16 3G8 |
CD45 2D1 |
CD13 L138 |
CD14 MφP9 |
| Panel 5 |
CD65* 88HT |
CD2 S5.2 |
CD45 2D1 |
CD36 CB38 |
CD10 HI10a |
CD14 MφP9 |
Note
- Antibodies marked with (*) were purchased from Mab Beckman Coulter, marked with (**) from Mab Life technologies and other from Mab—Becton Dickinson.
In the period between 2008 and 2017, the ELN recommendations of antibodies panels for MDS diagnosis changed many times. For the purpose of this study, we selected only those parameters that were assessed in all patients included in the analysis. The parameters were used in the daily diagnostic practice according to the recommendations of the experts of the US National Comprehensive Cancer Network, the European LeukemiaNet and the International Working Group at the Working Conference on MDS, that were published between 2006 and 2012 years.5, 6, 11
- granulocytes granularity disorders;
- altered relationship of CD13 and CD16 expression on maturing neutrophils;
- altered relationship of CD33 and CD117 expression on maturing neutrophils;
- persistent CD34 expression on immature myeloid progenitors;
- altered relationship of CD11b and HLA-DR expression on maturing neutrophils;
- altered relationship of CD11b and CD13 expression on maturing neutrophils;
- altered relationship of CD15 and CD34 expression on maturing neutrophils;
- aberrant expression of CD56 on maturing neutrophils;
- aberrant CD14 expression on maturing neutrophils;
- aberrant expression of CD56 on maturing monocytes;
- aberrant expression of CD34 on maturing monocytes;
- absent expression of CD14 on maturing monocytes;
- absent expression of CD16 on maturing monocytes.
Occurrence of any of these aberrancies was weighted 1, and in their absence, the weight was 0. The total score of immunophenotypic disorders was the sum of weights of individual parameters.
In addition, a retrospective analysis of bone marrow cytometric images was performed to evaluate the aberrancies according to the Ogata score simplified and validated by ELN.8
2.3 Gating strategy
In 2009 and 2012, ELN experts standardized the conditions for the cytometric test and the gating standard in patients with suspected MDS. Since then, when assessing the bone marrow preparation for the presence of myelodysplastic features, we adopted the algorithm proposed by them. The gating strategy containing myeloid and monocytic compartment does not differ from that proposed in the ELN standards.5, 6
- exclusion of cell debris in FSC-H vs SSC-A plot
- exclusion of cell doublets
- granulocyte gating with high SSC-A signal and CD45 positive
The gating strategy of immature myeloid progenitors differed only in the last step in which events with low SSC-A signal and low CD45 expression were gated (Graph 2). Significant representation of other populations, that can be present in this gate, was further excluded by specific markers (CD10 or CD19- lymphoid progenitors, C117- basophils).
The gating strategy for maturing monocytes confined two first steps and selection of CD14+ cells and events with intermediate SSC-A signal and CD45 expression(Graph 3).
2.4 Statistics
Statistical hypotheses were verified at the significance level of α = 0.05. It was assumed that the differences between the studied groups are statistically significant, if P values calculated from statistical tests were lower than the assumed level of significance (P < α). For comparison between two groups of variables expressed in ordinal and interval scales, the Mann-Whitney U non-parametric test was used. For analysing the differences between more groups, the non-parametric Kruskal-Wallis test was used. The Pearson chi-square test or Fisher's exact test was used to show differences between groups in the distribution of qualitative and order variables. Analysis of the relationship between order and interval variables was performed using the Spearman rank correlation test. The sensitivity and specificity of individual flow cytometry scoring scales, as well as their positive predictive value (PPV) and negative predictive value (NPV), were determined. In order to determine the cut-off value for the number of cytometric aberrancies differentiating the compared groups, a receiver operating characteristic curve (ROC) analysis was used. Statistical analyses were performed using statistical packages SPSS v 13.0 and STATISTICA v 12.0.
3 RESULTS
3.1 Patients’ characteristics
The study group consisted of 71 patients (28 women and 43 men) at the median age of 66 years (range, 24-86) with single or multi-lineage cytopenia, who were diagnosed with myelodysplastic syndrome. The detailed patients’ characteristic is presented in Table 2. A subgroup of 40 patients, of which 50% (20/40) were women, was diagnosed with the low-grade MDS (MDS with single-line dysplasia, with multi-lineage dysplasia, with ringed sideroblasts, MDS unclassifiable, and MDS with del5q). The median age of the patients with the low-grade MDS was 62 years (range, 19-83 years). The median number of cytopenias in this subgroup was 1 (range, 1-3).
| Characteristics | The MDS group of patients | The comparative group of patients | P |
|---|---|---|---|
| n = 71 | n = 39 | ||
| Age | |||
| Median (range) | 66 (24-86) | 59 (21-84) | ns |
| Gender | |||
| Male | 43 (61%) | 20 (51%) | ns |
| Female | 28 (39%) | 19 (49%) | |
| The number of cytopenias | |||
| One | 23 (32%) | 19 (49%) | ns |
| Two | 19 (27%) | 10 (26%) | |
| Three | 29 (41%) | 10 (26%) | |
| The median number of cytopenias (range) | 2 (1-3) | 1 (1-3) | |
| Type of cytopenia: | |||
| Anaemia | 64 (90%) | 32 (82%) | |
| Leucopenia | 40 (56%) | 22 (56%) | |
| Thrombocytopenia | 41 (58%) | 15 (38%) | |
| MDS subtypes according to WHO 2016 classification | |||
| MDS-SLD | 18 (25%) | NA | |
| MDS-MLD | 15 (21%) | ||
| MDS-RS | 3 (4%) | ||
| MDS-U | 3 (4%) | ||
| MDS 5q- | 1 (1%) | ||
| MDS-EB1 | 13 (18%) | ||
| MDS-EB2 | 18 (25%) | ||
| The alternative diagnosis established for patients in comparative group | |||
| Drug-associated cytopenia | NA | 12 (31%) | |
| ACD | 8 (21%) | ||
| B12 deficiency | 7 (18%) | ||
| AA | 3 (8%) | ||
| AIHA | 2 (5%) | ||
| Iron-deficiency anaemia | 2 (5%) | ||
| Other | 5 (13%) | ||
| PNH | 1 (3%) | ||
| APS | 1 (3%) | ||
| PMF | 1 (3%) | ||
| SLE | 1 (3%) | ||
| Undetermined aetiology | 1 (3%) | ||
| R-IPSS | |||
| Very low | 7 (10%) | NA | |
| Low | 11 (15%) | ||
| Intermediate | 5 (7%) | ||
| High | 14 (20%) | ||
| Very high | 14 (20%) | ||
| Not available | 20 (28%) | ||
| Karyotype | |||
| Normal | 23 (45%) | NA | |
| Complex | 14 (27%) | ||
| del(-Y) 3 | 3 (6%) | ||
| 8 | 3 (6%) | ||
| Del 5q | 1 (2%) | ||
| inv (3;3) | 1 (2%) | ||
| Others (not characteristic for MDS) | 6 (12%) | ||
- Abbreviations: 5q-, with isolated 5q-; AA, aplastic anaemia; ACD, anaemia of chronic disease; AIHA, autoimmune haemolytic anaemia; APS, antiphospholipid syndrome; EB1, with excess of blasts 1; EB2, with excess of blasts 2; MDS, myelodysplastic syndrome; MLD, multi-lineage dysplasia; NA, not applicable; ns, not significant; PMF, primary myelofibrosis; PNH paroxysmal nocturnal haemoglobinuria; R-IPSS, Revised International Prognostic Scoring System; RS, with ringed sideroblasts; SLD, single lineage dysplasia; SLE, systemic lupus erythematosus; U, unclassifiable.
The comparative group consisted of 39 patients who were suspected of having MDS due to single- or multi-line cytopenia, but finally were diagnosed with other than MDS disorder. The detailed characteristics of patients who were included in the comparative group are shown in Table 2.
3.2 Immunophenotypic aberrancies assessed according to ELN recommendations
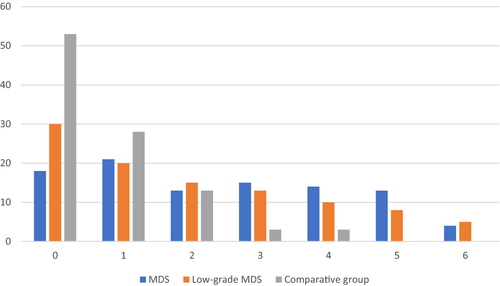
A significantly higher number of immunophenotypic aberrancies on maturing neutrophils (median = 2, range 0-6) were observed in the MDS compared to the comparative group (median = 0, range 0-4, P < .0001) (Figure 1). The number of monocytic maturation line disturbances did not differ between the groups (P = .89). The frequency of individual immunophenotypic aberrancies and the significance of differences in their occurrence (p) for the MDS group, low-grade MDS and the comparative group are given in Table 3.
| Frequency | P values | Frequency | P values | |||
|---|---|---|---|---|---|---|
|
MDS n=71 (%) |
Comparative Group n=39 (%) |
Low-grade MDS n=40 (%) |
Comparative Group n=39 (%) |
|||
| Aberrancies on maturing neutrophils | ||||||
| CD33/CD117 | 23 | 3 | .029 | 13 | 3 | .101 |
| CD34n | 15 | 3 | .114 | 13 | 3 | .166 |
| 11b/HLA-DR | 25 | 3 | .016 | 18 | 3 | .036 |
| CD13/CD11b | 27 | 3 | .004 | 28 | 3 | .001 |
| CD15/CD34 | 18 | 3 | .031 | 15 | 3 | .032 |
| CD56n | 15 | 0 | .033 | 10 | 0 | .077 |
| CD13/CD16 | 48 | 26 | .197 | 42 | 26 | .381 |
| CD14n | 6 | 3 | .680 | 8 | 3 | .427 |
| Aberrancies on maturing monocytes | ||||||
| CD56m | 38 | 18 | .158 | 35 | 18 | .295 |
| CD16 | 21 | 18 | .863 | 15 | 18 | .378 |
| CD34m | 10 | 10 | .681 | 10 | 10 | .731 |
| CD14m | 10 | 3 | .032 | 15 | 3 | .020 |
Note
- CD13/CD16, altered relationship of CD13 and CD16 expression on maturing neutrophils; CD33/CD117, altered relationship of CD33 and CD117 expression on maturing neutrophils; CD34n, persistent CD34 expression on immature myeloid progenitors; CD11b/HLA-DR, altered relationship of CD11b and HLA-DR expression on maturing neutrophils; D11b/CD13, altered relationship of CD11b and CD13 expression on maturing neutrophils; CD15/CD34, altered relationship of CD15 and CD34 expression on maturing neutrophils; CD56n, aberrant expression of CD56 on maturing neutrophils; CD14n, aberrant CD14 expression on maturing neutrophils; CD56m, aberrant expression of CD56 on maturing monocytes; CD34m, aberrant expression of CD34 on maturing monocytes; CD14m, absent expression of CD14 on maturing monocytes; and CD16, absent expression of CD16 on maturing monocytes. The shaded verses show the abberancies that statistically significantly differ MDS and the comparative group
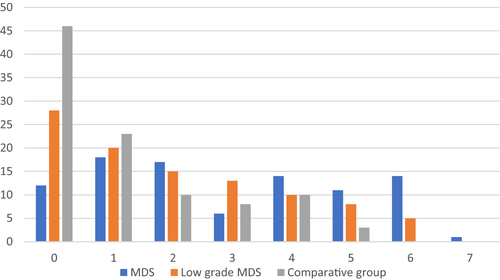
The total number of immunophenotypic aberrancies detected on maturing neutrophils and monocytes in the MDS group ranged from 0 to 7 (median 3), while in the comparative group from 0 to 4 (median 1) (Figure 2). The difference did not reach statistical significance (P = .07).
3.3 Evaluation of bone marrow cytometric abnormalities by the Ogata score
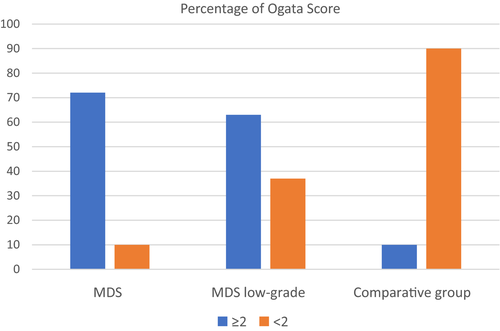
There was a significant difference in the summary number of the immunophenotypic disturbances of the bone marrow evaluated according to the Ogata score8 between the MDS and the comparative group (P < .0001). The numerical results in the study group were in the range of 0-4 (median 3) and in the comparative group of 0-3 (median 1) (Figure 3). For results in the range of 2-4, the sensitivity for MDS diagnosis was 73%, specificity 90%, with NPV and PPV of 0.64 and 0.93, respectively. The results are presented in Table 4.
| Extension of the Ogata Score | Cut-offs | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| Ogata score | >1 pt | 73% | 90% | 0.64 | 0.93 |
|
Ogata score (0-4 points) plus four immunophenotypic aberrancies on granulocytes CD11b/HLA-DR (1 pt) CD11b/CD13 (1 pt) CD15/CD34 (1 pt) plus lower expression of CD14 on monocytes (1 pt) |
>1 pt | 83% | 82% | 0.89 | 0.73 |
|
Ogata score (0-4 points) plus two immunophenotypic aberrancies on granulocytes CD11b/HLA-DR (1 pt) CD11b/CD13 (1 pt) |
>1 pt | 82% | 87% | 0.92 | 0.72 |
3.4 Extension of the Ogata score with selected immunophenotypic aberrancies
Table 4 presents a comparison of the specificity, sensitivity and predictive values of the Ogata score8 extended by adding the aberrancies which were more frequent in patients with MDS than in patients from the comparative group, that is abnormal expression pattern of CD13/CD11b, CD11b/HLA-DR, CD34/CD15 on maturing neutrophils and CD14 on monocytes. The highest sensitivity (83%) in the MDS diagnosis was achieved by the Ogata score extended by all four immunophenotype parameters. However, the Ogata score extended by only two immunophenotypic parameters (disruption of CD13 to CD11b and CD11b to HLA-DR, as presented in Graph 4) provided the highest specificity (87%), while only slightly lower sensitivity (82%). The Ogata score extended by these two immunophenotypic aberrancies was also characterized by high positive predictive values; therefore, it was selected for correlation analyses with R-IPSS.
3.5 Cytometric scales in patient with low-grade MDS
In the subgroup of patients diagnosed with low-grade MDS, a median of 2 (range, 0-6) immunophenotypic aberrancies was found. The number was significantly higher than in the comparative group (P = .002). Similarly as in the whole study group, the total number of immunophenotypic aberrancies on maturing neutrophils was higher than in the comparative group (P < .0001). The total number of immunophenotypic aberrancies was also significantly higher compared to the control group (P < .0001). Such difference was not found for monocytes (P = .261). The comparison of the sensitivity, specificity, PPV and NPV in the diagnosis of low-grade MDS is presented in Table 5.
| Extensions of the Ogata Score | Cut-offs | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| The total number of immunophenotypic aberrancies assessed according to ELN recommendations | >1 pt | 58% | 69% | 0.66 | 0.61 |
| Ogata score | >1 pt | 63% | 90% | 0.86 | 0.7 |
|
Ogata score (0-4 points) plus immunophenotypic aberrancies on maturing neutrophils CD11b/HLA-DR (1 pt) CD11b/CD13 (1 pt) CD15/CD34 (1 pt) plus lower expression of CD14 on monocytes (1 pt) |
>1 pt | 73% | 82% | 0.73 | 0.82 |
|
Ogata score (0-4 points) plus immunophenotypic aberrancies on maturing neutrophils CD11b/HLA-DR (1 pt) CD11b/CD13 (1 pt) |
>1 pt | 70% | 87% | 0.85 | 0.74 |
|
Ogata score (0-4 points) plus immunophenotypic aberrancies on maturing neutrophils CD11b/HLA-DR (1 pt) plus lower expression of CD14 on monocytes (1 pt) |
>1 pt | 68% | 87% | 0.84 | 0.72 |
3.6 The R-IPSS classification
R-IPSS values were determined on the basis of medical records in 72% (51/71) of patients. The median R-IPSS was 4.8 (range: 0.3-9). In the remaining 16/71 (23%), R-IPSS was not determined due to the lack of a cytogenetic result.
3.7 The association between parametric scores and cytogenetic aberrations
Attempts were also made to find the association between cytometric scores and the results of cytogenetics. In summary, no differences were found between patients categorized according to cytogenetic abnormalities and cytogenetic risk, probably to the small number of patients in cytogenetic subgroups.
3.8 Correlation between parametric scores and R-IPSS
There was a statistically significant positive correlation of the R-IPSS value with the total number of immunophenotypic aberrancies (P = .017), and the severity of cytometric abnormalities quantified on the Ogata scale (P = .027). In addition, a statistically significant positive correlation between R-IPSS and the Ogata score extended by selected immunophenotypic aberrancies (the relation of expression of CD11b/HLA-DR and CD11b/ CD13) was also observed (P < .001).
4 DISCUSSION
4.1 Immunophenotype disorders characterizing myelodysplastic syndromes
A great challenge nowadays is to determine the algorithm of cytometric examination in the diagnosis of the low-grade MDS (MDS-SLD, MDS-MLD, MDS-U, MDS RS-SLD, RS- MLD, MDS del 5q). In 2012, the ELN working group published a report on the standardization of the phenotypic and immunophenotypic examination of the bone marrow by multiparameter flow cytometry in patients with suspected MDS.6 In the above study, it was shown that among the disorders mentioned in ELN recommendations, the altered relationship CD11b/CD13 and CD15/CD34 expression on maturing neutrophils and the aberrancies of CD14 expression on monocytes have the highest diagnostic value. Although aberrant expression of CD11b/HLA-DR on maturing granulocytes is not listed in the ELN recommendations, the results of our study suggest its value in MDS diagnosis.
Disordered relationship of expression of CD13 and CD16 is often described as occurring in MDS.12, 13 In our study, it was shown that this aberrancy is the most common disorder in MDS, but does not significantly differentiate inefficient haematopoiesis in the course of MDS from other causes of cytopenias, since its incidence rate was the same in MDS patients and the patients with cytopenia of the other origin.
Frequently found immunophenotypic disturbance in MDS is an altered relationship of CD33 and CD117 expression.14 The altered relationship of CD33/CD117 expression was significantly more frequent in MDS patients than in the comparison group. However, this difference was not found if the subtypes groups with excess blasts were excluded from MDS group. Hence, we are not able confirm that this disorder as a single aberrancy differentiates early MDS from other cytopenia.
4.2 Cytometric scales in the diagnosis of myelodysplastic syndromes
To date, many different cytometric scoring systems have been created that quantitatively describe the phenotypic and immunophenotypic phenomena found in MDS. Most of them assume the evaluation of a large number of parameters, which reduces their repeatability and the possibility of widespread use in practice. Relatively easily applicable, objective and repeatable scoring system proposed by Ogata et al in 2008,7 and the simplified modification of this scale presented by ELN experts in 20128 was proved to have high diagnostic value in the diagnosis of MDS. One of the aims of our study was to confirm the diagnostic value of the simplified Ogata score in daily practice. We have shown, as in original work of Della Porta et al, that the Ogata score differentiates the MDS and comparative group with high specificity (90%) and high PPV (0.93). The sensitivity (73%) and NPV (0.64) were slightly lower, what is also in line with the results published by Della Porta et al.8 From practical point of view, it is extremely important that the parameters included in the scale are expressed as relative values, independent of the subjective assessment. Therefore, many other study groups have achieved similar diagnostic values.15, 16
Since the time when the Ogata score was published, numerous related scales have been created for the diagnosis of MDS, many of which assume the assessment of similar parameters. Karai et al extended this scale to include erythrocyte maturation cell lines by adding CD71 and mast cell percentage, achieving higher sensitivity (84%) for low-grade MDS.17 Gupta et al have presented cytometric scale which assumes evaluation of parameters included in Ogata score extended by evaluation of CD13/CD11b, CD13/CD16 and CD11b/CD16.18
An important element of our work was an attempt to extend the Ogata score with immunophenotypic disorders in order to increase its diagnostic value. The highest sensitivity (83%) was achieved by adding to the Ogata score the evaluation of all 4 immunophenotypic parameters which were statistically more frequent in low-grade MDS compared to cytopenias of a different aetiology. Unfortunately, such modification of the Ogata score led to a significant reduction in its sensitivity and PPV. The extension of the Ogata score by only two immunophenotypic parameters, the altered pattern of CD11b/CD13 and CD11b/HLA-DR expression, has allowed to increase its sensitivity (82%) and NPV (0.72), while maintaining high specificity (87%) and PPV (0.92). However, the subjectivity of the assessment of expression of CD11b/ CD13 and CD11b/HLA-DR remains the problem. Still, the decisive advantage of this scale is the ability to perform all tests in one test tube which allows to shorten the test time and reduce its costs.
Xu et al published the results of the extension of the Ogata scoring system by the assessment of myeloblast immunophenotype. The authors added to the Ogata scale the assessment of CD15, CD11b, CD4 and CD56 expression on myeloblasts. It is particularly interesting that in a report by Xu et al a significant difference in the expression of CD11b on CD34+ cells between low-grade MDS and non-clonal cytopenia was demonstrated.19 In our experience, the aberrancies of CD11b expression on maturing neutrophils may be an important parameter that distinguishes low-grade MDS from other diseases involving cytopenia.
4.3 Prognostic significance of multiparametric flow cytometry in myelodysplastic syndromes
The first attempts to use cytometry as a prognostic tool mainly concerned the evaluation of single antigens or their combinations on maturing granulocytes. There is some evidence in the literature that important factor affecting the prognosis in MDS is the expression of antigens on granulocytes and monocytes that are specific for other cell lines, as well as an increased percentage of CD34+ cells. Ogata et al have demonstrated that patients with a numerical score of 2 and higher have higher IPSS and WPSS values, and thus a worse prognosis.8 In the publication of Xu et al, a positive correlation of the cytometric score was confirmed with the R-IPSS score (P < .001).19 In line with the results of the above-mentioned studies, we showed a statistically significant positive correlation between the Ogata score extended by selected immunophenotype disorders and the score of R-IPSS. The strongest correlation was demonstrated when the Ogata score was extended by the assessment of the expression pattern of CD11b/HLA-DR and CD11b/CD13 on maturing neutrophils (P < .001).
Improvement of diagnostic results can also be obtained by additional assessment of red cell parameters. The use of two monoclonal antibodies anti-CD36 and anti-CD71 allows the assessment of four red cell parameters. If you add the result of this determination to the parameters tested in the Ogata score, we get the result in the RED-scale. It allows to recognize MDS with high sensitivity (87.9%) and specificity (88%). Unfortunately, the use of this scale requires the performance of non-lysed whole bone marrow sample, which requires much more work during the analysis.20
4.4 New generation sequencing in MDS diagnosis
Next-generation sequencing is now playing an increasing role in the diagnosis of MDS.
In the population of patients with myelodysplastic syndrome, up to 80%-90% have mutations that may be associated with the disease. Unfortunately, the diagnosis of MDS based on the presence of molecular disorders is hampered by the fact that the same abnormalities are present in the population of people without cytopenia, especially in the elderly. This phenomenon is known as ‘clonal haematopoiesis of indeterminate potential’ (CHIP). In the population of people aged 70-79, CHIP was found in 9.5%.21 It seems worth developing various diagnostic techniques and using these tests as complementary.22 Multiparameter flow cytometry may be especially important as a diagnostic tool in patients without molecular abnormalities or with CHIP, because this relatively cheap and broadly available technique may provide the additional data confirming abnormal maturation of granulocytes and monocytes in the bone marrow.
5 CONCLUSIONS
In our experience, the Ogata score as original presented by ELN, or extended by selected immunophenotypic aberrancies of maturing neutrophils, that is expression of CD11b/HLA-DR and CD11b/CD13, is characterized by high specificity and high PPV in the diagnosis of MDS.8 Flow cytometric quantitative assessment of immunophenotype disorders in MDS due to high reproducibility and relatively easily application in experienced laboratories may be very helpful in daily haematological practice. The results of our study also suggest prognostic value of flow cytometric scoring systems, what should be explored in future prospective trials in patients treated with new agents and targeted therapies.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




