Upregulation of the expression of Toll-like receptor 9 in basophils in patients with allergic rhinitis: An enhanced expression by allergens
Abstract
It was reported that the expression of Toll-like receptor (TLR) 9 may be related to Th2-type allergic inflammation including allergic rhinitis (AR). However, little is known about the expression of TLR9 in the basophils in AR. In the present study, the expression of TLR9 was examined by flow cytometry analysis, and the expression of TLR9 mRNA in KU812 was determined by quantitative real-time PCR. The results showed that the percentage of TLR9+CCR3+ cells in blood granulocytes increased by 46% in patients with AR, but not in peripheral blood mononuclear cells (PBMCs). Allergens namely Dermatophagoide allergen extract (DAE) and Platanus pollen allergen extract (PPAE) upregulated the expression of TLR9 in CCR3+ granulocytes by 76% and 84%, respectively. DAE and PPAE also enhanced the proportions of TLR9+CD123+HLA-DR− cells and TLR9+CCR3+CD123+HLA-DR− cells in granulocytes and PBMCs of patients with AR. In order to investigate the actions of allergens on basophils, KU812 cells were used. It was observed that all KU812 cells expressed TLR9, and the expression intensity of TLR9 in a single KU812 cell was elevated by CpG. IL-37, IL-31, IL-33, Artemisia sieversiana wild allergen extract (ASWAE), DAE, OVA and Der p 1 induced an increase in the expression of TLR9 mRNA and IL-6 production in KU812 cells. It was shown that the percentage of TLR9-expressing basophils increased in the blood of ovalbumin (OVA)-sensitized mice. In conclusion, an increased expression of TLR9 and the production of IL-6 in basophils implicate that the contribution of basophils to AR is likely via TLR9.
1 INTRODUCTION
Allergic rhinitis (AR) is a chronic, inflammatory disease characterized by inflammation of the nasal mucosa with hypersensitivity1 typically triggered by environmental allergens.2 With regard to the pathogenesis of AR, studies have shown that CD4+Th2 cells are not only the main source of Th2 cytokines, but also the main driving force of harmful immune response in the pathogenesis of AR.3 It is reported that innate immune stimulation via Toll-like receptors (TLRs) may also be involved in both the development and protection from AR.4
Toll-like receptor 9 is a member of the TLR family which is localized within the intracellular endolysosomal compartments.5 In peripheral blood, the expression of TLR9 was found in neutrophils, eosinophils, basophils, monocytes and lymphocytes.6 TLR9 recognizes the unmethylated CpG DNA of bacteria and viruses, as well as nucleic acids.7 It was reported that TLR9 expression by sinonasal epithelial cell in the AR group was significantly reduced compared with the normal controls.8 CpG dinucleotides, presumably acting through TLR9, serve as potent adjuvants for allergen desensitization therapy9 and induce interleukin (IL)-4 and IL-8 release from basophils.10 Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine appeared to offer long-term clinical efficacy in the treatment of ragweed AR.11 It has also been reported that a low expression of TLR9 in the nasal mucosa of patients with AR may be related to Th2-type allergic inflammation.12 However, there are few reports about the expression of TLR9 in basophils in patients with AR.
Basophils, as well as tissue mast cells, are the primary effector cells in IgE-mediated allergic reactions.13 AR patients develop specific IgE responses to allergens, and IgE may bind to high-affinity IgE receptors on mast cells and basophils.14 Activated basophils have the ability to rapidly elaborate Th2-type cytokines and support ongoing allergic immunity.15 It has been found that human basophils respond to TLR ligands in synergy with IgE-mediated activation, and the released cytokines can promote Th2 differentiation.10
The prevalence of allergic sensitization especially to airborne allergens has increased significantly over the last decades.16 The most common allergens that cause the disease are grass pollen, house dust mite (HDM),17 tree pollen and cat dander.18 A recent study shows that HDM major allergens can activate TLRs and upregulate proinflammatory cytokine expression,19 and the increased levels of IL-4, IL-31, IL-33 and thymic stromal lymphopoietin (TSLP) induced by dust mite and mugwort allergens may be involved in the pathogenesis of AR.20 However, altered expression of TLRs induced by allergens on basophils has not been examined.
The aim of the study was to investigate the expression of TLR9 in peripheral blood basophils of patients with AR, and the effect of allergens on TLR9 expression in basophils. We observed that the expression of TLR9 and the production of IL-6 in basophils increased, particularly upon allergen challenge.
2 MATERIALS AND METHOD
2.1 Reagents
The following reagents were purchased from Biolegend: Human TruStain FcX™ (Fc Receptor Blocking Solution), Zombie Aqua™ Fixable Viability Kit, PE/Cy7-conjugated mouse anti-human CD123 and its isotype antibody PE/Cy7-conjugated mouse IgG1κ, PerCP/Cy5.5-conjugated mouse anti-human HLA-DR and its isotype antibody PerCP/Cy5.5-conjugated mouse IgG2aκ, APC/ Cy7-conjugated mouse anti-human CCR3 and its isotype antibody APC/ Cy7-conjugated mouse IgG2bκ, BV510 anti-mouse CD49b Antibody and PE/Cy7 anti-mouse FcεRIα Antibody. FITC-conjugated mouse anti-human TLR9 and its isotype antibody FITC-conjugated mouse IgG2bκ were obtained from Santa Cruz. Zombie NIR™ Fixable Viability Kit, APC Rat anti-Human TLR9 and its isotype antibody APC rat IgG2aκ were purchased from BD Biosciences Pharmingen. Mouse TLR9 Alexa Fluor® 647-conjugated was ordered from R&D Systems. Ovalbumin (OVA, grade V), DNase I and Trypan blue dye were purchased from Sigma-Aldrich. Human IL-33, human IL-37 were obtained from Peprotech. Human IL-4 and human IL-6 ELISA kits were bought from Cayman Chemical. IL-13 ELISA kit was bought from Invitrogen (Thermo Fisher), and the analytical sensitivity was 0.7 pg/mL. Oligonucleotide primers for qPCR were synthesized by Invitrogen Biotechnology Co. TRIzol reagent was obtained from Invitrogen. RNA was extracted by using a TaKaRa Mini BEST Universal RNA Extraction Kit. cDNA was synthesized using a PrimeScript RT reagent kit. Quantification of mRNA expression level was performed using a TAKARA SYBR Premix EX Tag Kit. A human chronic myelogenous leukaemia cell line, KU812 (ATCC® CRL2099™) cells and American Type Culture Collection (ATCC)-formulated RPMI-1640 medium were purchased from ATCC. Foetal bovine serum (FBS) and RPMI 1640 medium were purchased from PAN™ Seratech. Artemisia sieversiana wild allergen extract (ASWAE), Dermatophagoide allergen extract (DAE) and Platanus pollen allergen extract (PPAE) were purchased from Macro Union Pharmaceutical Co., Ltd. HDM allergen Dermatophagoide pteronyssinus (Der p)1 was synthesized by Beijing Protein Innovation Co., Ltd. Allergens for skin prick tests were supplied by ALK-Abelló, Inc. Most of the general-purpose chemicals, such as salts and buffer components, were of analytical grade.
2.2 Patients and samples
A total of 46 AR patients and 23 healthy control (HC) subjects were recruited for the study; their general characteristics are summarized in Table 1. The diagnostic criteria for AR in this study are in line with the 2015 AR Clinical Practice Guidelines issued by the American Academy of Otorhinolaryngology Head and Neck Surgery.21 The samples were obtained from the First Affiliated Hospital of Jinzhou Medical University, China. Informed consent was obtained from each volunteer according to the declaration of Helsinki and in agreement with the ethical committee of the First Affiliated Hospital of Jinzhou Medical University. Immediately after admission (acute exacerbation stage), blood from each patient with AR was extracted. Blood from HCs was collected in the outpatient clinic. Ten millilitre of peripheral blood was extracted from each individual and collected in an EDTA containing tube before centrifugation at 450 g for 10 minutes. The cells were used for flow cytometric analysis, and the plasma was collected and frozen at −80°C until use.
|
HC (n = 23) |
AR (n = 46) |
|
|---|---|---|
| Age (y) | 20-50 | 17-64 |
| Female/male | 19/4 | 22/24 |
| Median age at onset (y) | na | 35 (17-64) |
| Median disease duration (y) | na | 4 (1-40) |
| Number of positive skin prick for dust mite | 0 | 24 |
| Number of positive skin prick for artemisia | 0 | 28 |
| Number of positive skin prick for platanus pollen | 0 | 7 |
| Number of positive specific IgE for dust mite | 0 | 14 |
| Number of positive specific IgE for artemisia | 0 | 13 |
| Number of positive specific IgE for platanus pollen | 0 | 5 |
2.3 Animals
BALB/c male mice (18-22 g) were obtained from Vital River Laboratory Animal Technology Co. Ltd., CertificateNo.11400700056942-945. The animals were bred and reared under strict ethical conditions according to international recommendations. They were housed in the Animal Experimental Center of the First Affiliated Hospital of Jinzhou Medical University in a specific pathogen-free environment with free access to standard rodent chow and water at a constant temperature of 23-28°C and relative humidity of 60-75%. The animal experiment procedures were approved by the Animal Care Committee at the Shenyang Medical college.
2.4 Mouse sensitization and challenge
A total of 30 mice were randomly selected, 10 in OVA-sensitized-OVA-challenged group, 10 in OVA sensitized-normal saline (NS)-challenged group and 10 in NS control group. In the same day, 3 or 4 mice from each group were challenged, and these experiments were repeated three times. Mice were sensitized on days 0, 7, 14 and 21 with a subcutaneous multipoint injection of 50 μg of OVA and 1.5 mg of Al(OH)3 suspended into a total volume of 0.5 mL NS. Non-sensitized control animals received only an equal volume (0.5 mL) of NS on the same days. On day 28, the sensitized mice were challenged with nasal drops 20 µL of 1% OVA for 7 days, 10 µL on each side of the nasal cavity, and the control mice received 20 µL of NS. On the last day, 3 hours after nasal drip was administered, the animals were killed and the blood was collected for analysis.
2.5 KU812 cell culture and challenge
KU812 cells were cultured in ATCC-formulated RPMI 1640 medium supplemented with 10% heat-inactivated FBS and 100 units/mL penicillin/streptomycin in 75 cm2 tissue culture flasks (Falcon) at 37°C in a 5% (v/v) CO2, water-saturated atmosphere. The procedure for challenging KU812 cells was mainly adopted from a method previously described by Zhang et al for P815 cells.22 Briefly, cultured KU812 cells at a density of 1 × 106 cells/ml were incubated with various concentrations of Der p 1, OVA and CpG (ligand of TLR9), IL-31, IL-33, IL-37, TSLP, plasma from HC, DAE, ASWAE, plasma from AR allergic to dust mite alone, and plasma from AR allergic to Artemisia alone for 30 minutes, 2 hours and 16 hours at 37°C, before being centrifuged at 450 g for 10 minutes at 4°C. Cell pellets containing approximately 1 × 105 cells were resuspended in PBS for flowcytometric analysis, and 0.9 × 106 cells in TRIzol for qPCR, and the culture supernatant was collected and frozen at −80°C.
2.6 Flow cytometric analysis to detect the expression of TLR9 in peripheral blood basophils
To detect TLR9 expression in human blood basophils (CCR3+/ CD123+HLA-DR-/ CD123+HLA-DR-CCR3+ cells), blood cells were challenged with or without ASWAE, DAE23, 24 or PPAE25 (all at a concentration of 1.0 μg/mL) for 60 minutes at 37°C, respectively, and 2 μg/mL brefeldin A was also added into the tube. Cells were then incubated with the following antibodies: PE/Cy7-conjugated mouse anti-human CD123, PerCP/Cy5.5-conjugated mouse anti-human HLA-DR, and APC/Cy7-conjugated mouse anti-human CCR3, after which 100 μL of whole blood was added at room temperature for 15 minutes in the dark. Following ligation of red blood cells, white blood cells were fixed and permeabilized using a Cytofix/CytopermTM Fixation/Permeabilization Kit according to the manufacturer's instructions. FITC-conjugated mouse anti-human TLR9 was added to the test tube and FITC-conjugated mouse IgG2bκ was used as isotype control. Cells were incubated at 4°C for 30 minutes in the dark. After washing with BD washing buffer, the cell pellets were resuspended in fluorescence-activated cell sorting (FACS)-flow solution and analysed with FACS Verse flow cytometer (BD Biosciences). A total of 10 000 events were analysed per population for each sample.
To detect TLR9 expression in mouse blood basophils,26 PE/Cy7 anti-mouse FcεRIα and BV510 anti-mouse CD49b antibodies were added to test tubes after which 100 μL of whole blood was added at room temperature for 15 minutes in the dark. Following ligation of red blood cells, white blood cells were fixed and permeabilized using a Cytofix/Cytoperm kit according to the manufacturer's instructions. Mouse TLR9 Alexa Fluor® 647-conjugated were then added to each tube at 4°C and kept for 30 minutes. A total of 10 000 events were analysed for each sample.
2.7 Flow cytometric analysis to detect the expression of TLR9 in KU812 cells following allergen challenge
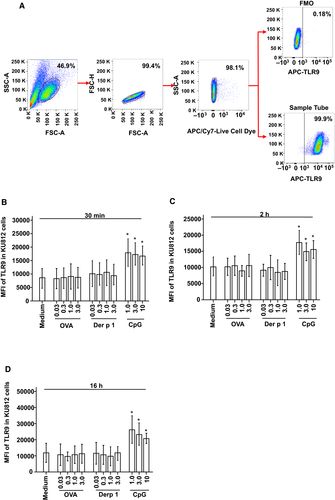
To detect the expression of TLR9 in KU812 cells, the cells were incubated in the presence and absence of various compounds or serum in 1 mL of 1% BSA/PBS for 30 minutes, 2 hours, 16 hours and pelleted by centrifugation. This was followed by the incubation of cells with Zombie NIR™ Fixable Viability Kit at room temperature for 15 minutes in the dark. This was followed by permeabilization using a Cytofix/Cytoperm kit according to the manufacturer's instructions. APC-conjugated rat anti-human TLR9 was added, and APC-conjugated rat IgG2aκ was used as isotype control. After washing, the cells were resuspended in fluorescence-activated cell sorting (FACS)-flow solution and analysed with FACS Verse flow cytometer and CellDevia software (BD Biosciences).
2.8 qPCR analysis of the expression of TLR9 in KU812 cells
Total RNA was extracted from the collected KU812 cells using a TaKaRa Mini BEST Universal RNA Extraction Kit. cDNA was synthesized using a PrimeScript RT Reagent Kit. The resulting cDNA was subjected to qPCR that was performed with a Light Cycler System using a SYBR Premix Ex Taq Kit. The amplified product was detected by the presence of a SYBR green fluorescent signal. Each reaction contains 10 μL of 2x SYBR green Master Mix, 300 nmol/L oligonucleotide primers, and 10 μL of the cDNA or plasmid DNA. β-actin cDNA was used as the internal control and the ΔCt of all experimental samples were subtracted by the ΔCt of the control samples (ΔΔCt). The magnitude change of test gene mRNA was expressed as 2−ΔΔCt. The primers of KU812 TLR9 were forward: cag cag ctc tgc agt acg tc and reverse: aag gcc agg taa ttg tca cg.
2.9 Determination of cytokine levels
Levels of IL-4, IL-6 and IL-13 were determined using ELISA kits according to the manufacturer's instruction.
2.10 Statistics
Statistical analyses were performed by using spss software (Version 13.0, IBM Corporation). Data for the expression of TLR9 in mouse blood basophils are displayed as box plots, which indicate the median, interquartile range and the largest and smallest values for the number of experiments indicated. Data for the expression of TLR9 in basophils of patients with AR are presented as scatter plots. The paired Mann-Whitney U test was employed in cases where the Kruskal-Wallis analysis indicated significant differences between groups, for the preplanned comparisons of interest. Data for the expression of TLR9 in KU812 cells and the expression of TLR9 mRNA were expressed as mean ± SEM. The Student's t test was applied in cases where the analysis of variance (ANOVA) indicated significant differences between groups with ANOVA. For all analyses, P < .05 was taken as significant.
3 RESULTS
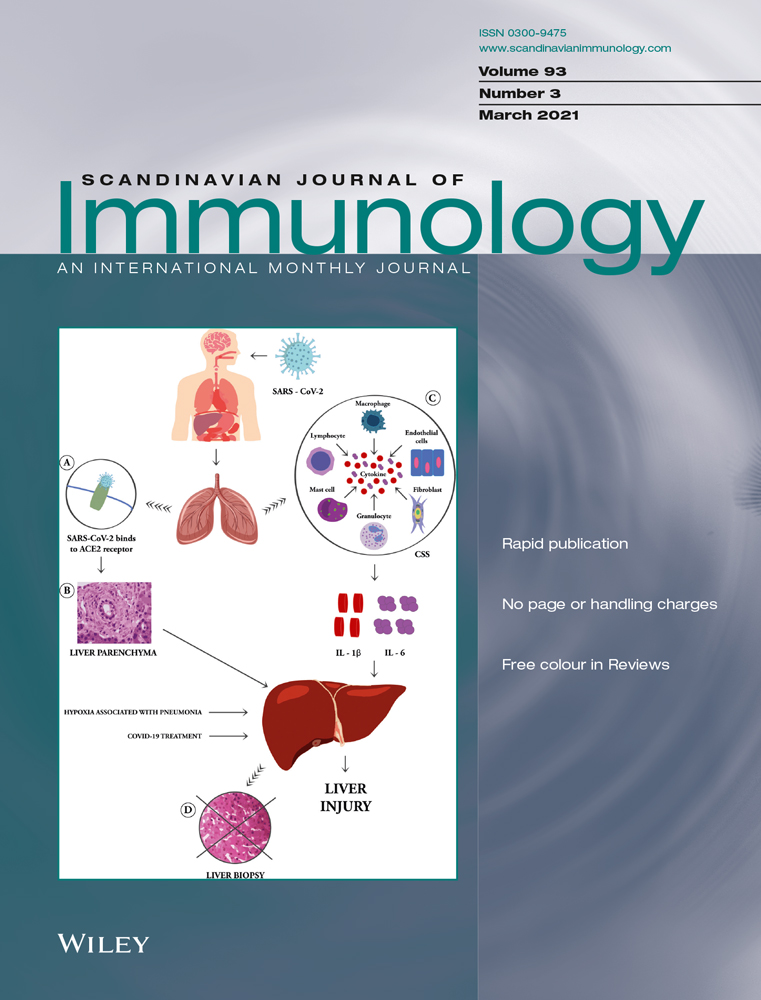
3.1 Altered proportions of TLR9-positive peripheral blood basophils in patients with AR
It has been reported that a low expression of TLR9 in the nasal mucosa of patients with AR may be related to Th2-type allergic inflammation.12 However, little is known about the expression level of TLR9 in the basophils in AR. We therefore examined the expression level of TLR9 in peripheral blood basophils (Figure S1). Since several gating strategies have been proposed to determine the phenotype of basophils, and it is rather difficult to determine the best strategy, we therefore select 3 gating strategies, CCR3+,27 CD123+HLA-DR−28 and CD123+ HLA-DR-CCR3+29 cells (Figure 1A), to represent basophils in the present study. And the results showed that the percentages of TLR9+CCR3+ cells in blood granulocyte increased by 46% in patients with AR when compared with HC subjects, but not in peripheral blood mononuclear cell (PBMC). Allergens ASWAE, DAE or PPAE at a concentration of 1.0 µg/mL enhanced TLR9 expression in the CCR3+ granulocytes of HC subjects by 2.5-, 2.1- and 2.4-fold, whereas DAE and PPAE upregulated the expression of TLR9 in CCR3+ granulocytes by 76% and 84% respectively, in patients with AR (Figure 1B). Similarly, DAE increased the TLR9 expression in CCR3+ PBMC by 1.3-fold (Figure 1E).
As far as the other two cell populations are concerned, there was no significant difference in the proportions of TLR9+CD123+HLA-DR− cells and TLR9+CCR3+CD123+HLA-DR− cells of granulocytes (Figure 1C,D) and PBMC (Figure 1F,G) between AR patients and HC subjects. However, the allergens DAE and PPAE, both at a concentration of 1.0 μg/mL enhanced the proportions of TLR9+CD123+HLA-DR− cells and TLR9+CCR3+CD123+HLA-DR− cells by up to 10% and 12% in the granulocytes of patients with AR, but not the HC subjects (Figure 1C,D). DAE also enhanced the proportions of TLR9+CD123+HLA-DR− PBMC and TLR9+CCR3+CD123+HLA-DR− PBMC by 1.1- and 1.3-fold, respectively, in AR patients (Figure 1F,G), whereas allergen PPAE increased the percentage of TLR9+CCR3+CD123+HLA-DR− PBMC by 1.0-fold (Figure 1G).
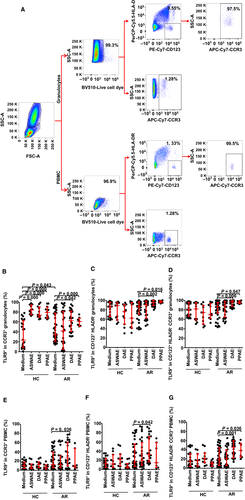
3.2 Altered TLR9 expression intensity in a single basophil in patients with AR
In order to further understand the expression of TLR9 in basophils, we investigated TLR9 expression intensity in a single basophil represented by mean fluorescent intensity (MFI). It was found that TLR9 MFI in CD123+HLA-DR− granulocyte (Figure 2B,E) and PBMC (Figure 2H,K) were enhanced by 63% and 38%, respectively, in patients with AR compared with HC subjects. Similarly, TLR9 MFI in CCR3+CD123+HLA-DR− granulocyte (Figure 2C,F) and PBMC (Figure 2I,L) increased by 76% and 30%, respectively, in patients with AR. The allergens DAE and PPAE at a concentration of 1.0 μg/mL increased the expression of TLR9 by 33% and 77%, respectively, in CD123+HLA-DR− granulocytes (Figure 2B,E) and by 33% and 64%, respectively, in CCR3+CD123+HLA-DR− granulocytes (Figure 2C,F) in patients with AR, but not the HC subjects.
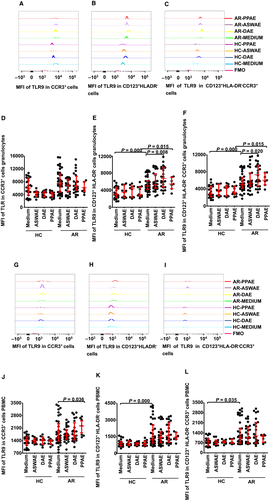
3.3 Induction of enhanced TLR9 MFI in KU812 cells by CpG
In order to understand the influence of allergens and CpG on basophils, a basophilic cell line of KU812 cells was employed, which has been previously shown to be a suitable model for studying the activation and degranulation of human basophils30 (Figure 3A). The results showed that all KU812 cells expressed TLR9 (data not shown). However, the expression intensity of TLR9 in a given KU812 cell is variable. Thus, CpG at 1.0, 3.0 and 10 µg/mL provoked up to 1.1-, 0.73- and 1.2-fold increase in the expression of TLR9 MFI in KU812 cells, respectively, at 30 minutes (Figure 3B), 2 hours (Figure 3C) and 16 hours (Figure 3D) following incubation. On the other hand, OVA and Der p 1 at the concentrations tested did not provoke enhanced expression of TLR9 MFI in KU812 cells (Figure 3B-D). IL-31, IL-33, IL-37, TSLP, as well as the allergens DAE and ASWAE with or without plasma from perennial or seasonal AR failed to alter TLR9 expression in KU812 cells (data not shown).
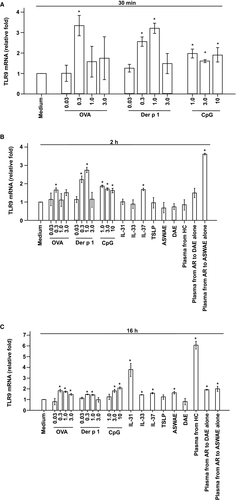
3.4 Enhanced TLR9 mRNA expression in KU812 cells
In addition to examining the protein level expression of TLR9 in KU812 cells, the expression of TLR9 mRNA in KU812 cells was also examined. The results showed that OVA at 0.3 μg/mL and Der p 1 at 1.0 μg/mL induced up to 2.3- and 2.2-fold increase in the expression of TLR9 mRNA, respectively, at 30 minutes (Figure 4A) and 2 hours following challenge (Figure 4B). CpG at the concentrations tested induced up to 0.9-fold increase in TLR9 mRNA expression in KU812 cells (Figure 4A,B). On the other hand, IL-37 and plasma from seasonal AR allergic to Artemisia alone provoke enhanced expression of TLR9 mRNA in KU812 cells following 2 hours incubation. IL-31, IL-33, TSLP, plasma from HCs, as well as DAE and ASWAE with or without plasma from perennial or seasonal AR did not induce a significant increase in the expression of TLR9 mRNA in KU812 cells following 2 hours incubation periods (Figure 4B). At 16 hours following incubation, OVA up to 3.0 μg/mL, Der p 1 up to 1.0 μg/mL, CpG up to 10 μg/mL, IL-31, IL-33, ASWAE, plasma from HC, plasma from AR to DAE alone and plasma from AR to ASWAE alone significantly upregulated TLR9 mRNA expression in KU812 cells (Figure 4C).
3.5 Induction of elevated IL-6 and IL-13 release from KU812 cells
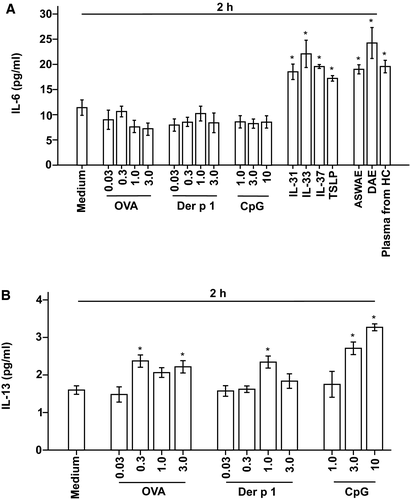
To further understand the influence of allergens, cytokines and TLR9 ligand on KU812 cells, we investigated their effects on IL-4, IL-6 and IL-13 release from KU812 cells. The results showed that IL-31, IL-33, IL-37 and TSLP all at 3 ng/mL stimulated 62%, 94%, 71% and 51% increase in IL-6 release from KU812 cells respectively, whereas DAE and ASWAE both at 3 μg/mL and plasma from HC provoked 67%, 113% and 72% elevation of IL-6 release from KU812 cells respectively, after incubation for 2 hours (Figure 5A), but not after 30 minutes and 16 hours incubation periods (data not shown). OVA, Der p 1 and CpG at the concentrations examined had little effect on IL-6 release (Figure 5A). On the other hand, OVA at 0.3 and 3.0 μg/mL, Der p 1 at 1.0 μg/mL and CpG at 3.0 and 10 μg/mL provoked up to 48%, 47% and 104% increase in IL-13 release from KU812 cells, respectively, at 2 hours following incubation (Figure 5B). All reagents tested failed to induce detectable IL-4 release from KU812 cells following 30 minutes, 2 hours and 16 hours incubation periods (data not shown).
3.6 Increased proportion of TLR9+ basophils in OVA-sensitized mice
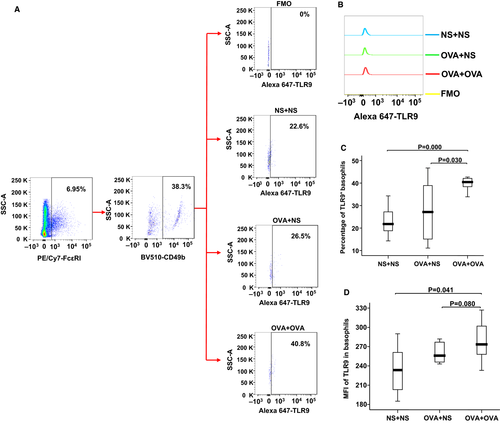
Mechanisms associated with allergic sensitization are important in the induction of TLR9 expression, we therefore investigated the expression of TLR9 in the basophils of AR mice using flow cytometry analysis. The results showed that the percentage of TLR9 expressing basophils was increased in the blood of OVA-sensitized-OVA-challenged mice compared with OVA sensitized-NS-challenged mice and NS control mice (Figure 6A,C). The MFI of TLR9 expressing basophils was also elevated in the blood of OVA-sensitized-OVA-challenged mice compared with NS control mice (Figure 6B,D).
4 DISCUSSION
It was found that the expression of TLR9+CCR3+ cells increased in the granulocytes of patients with AR and that the allergen extracts DAE and PPAE can enhance the expression of TLR9+CCR3+ cells, TLR9+CD123+HLA-DR− cells and TLR9+CCR3+CD123+HLA-DR− cells in the granulocyte and PBMC of patients with AR, suggesting that basophils may contribute to the pathogenesis of AR via TLR9, and the allergens may participate in AR through the induction of increased expression of TLR9 in basophils. Since basophils are primary effector cells for allergic inflammation31 including AR, our results may provide some valuable information on understanding mechanisms of AR.
Among the 3 gating strategies for peripheral blood basophils, CD123+HLA-DR-CCR3+ cells are basophils.29 However, CCR3+ cells cannot exclusively represent basophils as eosinophils in granulocytes32 and a small proportion of lymphocytes in PBMC do express CCR3.33 CD123+HLA-DR− cells in PBMC are solely basophils,34 but they are possibly myeloid-derived suppressor cells (MDSCs) in granulocytes.35 Our results indicate that the percentages of TLR9+CCR3+ cells in blood granulocyte increased by 46% in patients with AR, but not in PBMC, and that the proportions of TLR9+CD123+HLA-DR− cells and TLR9+CCR3+CD123+HLA-DR− cells in granulocytes and PBMC between AR patients and HC subjects had no significant difference, suggesting that the 46% increase in TLR9+CCR3+ cells may have resulted from elevated TLR9 in eosinophils, and not basophils. However, the allergens appear to enhance TLR9 expression in the CCR3+ cells of both granulocytes and PBMC, suggesting that the upregulated expression of TLR9 provoked by allergens is likely in basophils. The results stating that allergens enhanced the proportions of TLR9+CD123+HLA-DR− cells and TLR9+CCR3+CD123+HLA-DR− cells in the granulocytes and PBMC of patients with AR also indicate that the upregulated expression of TLR9 provoked by allergens is in basophils.
The proportions of basophils increased, along with an increase in the TLR9 expression intensity in CCR3+, CD123+HLA-DR− and CCR3+CD123+HLA-DR− granulocytes or PBMC cells in patients with AR. This strongly suggests that TLR9 in basophils may be involved in the pathogenesis of AR.
It is rather difficult to obtain purified basophils as 1-2 million of highly isolated basophils require at least 100 mL of fresh peripheral blood, and ethical permission. Therefore, basophilic cell line KU812 cells were employed for the present study. Since all KU812 cells naturally express TLR9, it is impossible to examine the change in the proportion of TLR9+ KU812 cells. Unlike allergens tested in the present study, which had little effect on TLR9 MFI in KU812 cells, CpG, a TLR9 ligand, provoked an increase in the expression of TLR9 MFI in KU812 cells. Since CpG can act through TLR9 to induce an antimicrobial response while suppressing allergic Th2 responses,36 our findings may implicate that CpG upregulates TLR9 expression, therefore desensitizing basophils. Meanwhile, CpG as well as the allergens OVA and Der p 1 induce an enhanced expression of TLR9 mRNA in KU812 cells. Induction of upregulated TLR9 mRNA expression in KU812 cells by CpG, IL-37, IL-31, IL-33, ASWAE and plasma from AR sensitive to DAE alone and ASWAE alone suggest that TLR9 ligand, cytokines and allergens can regulate TLR9 mRNA expression in basophils. Obviously, more detailed work is required to further address this issue.
Induction of IL-6 release from KU812 cells by IL-31, IL-33, IL-37, TSLP, DAE and ASWAE, but not by OVA, Der p 1 and CpG, and provocation of IL-13 release from KU812 cells by OVA, Der p 1 and CpG seem confusing, but it certainly suggests that the induced IL-6 and IL-13 release from KU812 cells is most likely via different cell signalling pathways. The ability of certain cytokines such as IL-31 and IL-37 can provoke IL-6 release, and an upregulated TLR9 expression implicates that these cytokines may induce IL-6 release through activated TLR9. Our observation that cytokines and allergens provoke IL-6 and IL-13 release from KU812 cells may suggest that basophil-derived cytokines may contribute to blood level of these cytokines in allergy.
The mouse AR model is an important tool to study of mechanism of AR in vivo.37 We clearly observed that the proportion of TLR9 expressing basophils in the blood of AR mice are greater than that of the healthy control mice. The result supports our finding that allergens can enhance TLR9 expression in basophils of granulocytes and PBMC. Since JNK-TLR9 signal pathway can mediate OVA-induced allergic airway inflammation,38 and basophils play an important role in AR, we believe that an increased TLR9 expression in basophils is likely to contribute to the pathogenesis of AR.
5 CONCLUSIONS
In conclusion, TLR9 expression intensity in basophil is enhanced in the blood of the patients with AR, and allergens increase the expression of TLR9 in basophils, indicating that the contribution of basophils to AR is likely via TLR9. Induction of IL-6 and IL-13 release from KU812 cells by cytokines and allergens once again confirms that basophils are involved in the pathogenesis of AR.
ACKNOWLEDGMENT
The study was conducted by the First Affiliated Hospital of Jinzhou Medical University in close collaboration with the School of Shenyang Medical College. We gratefully acknowledge the contributions of Dr Fangqiu Gu and Miss Lihong Wang for their assistance with flow cytometry and sensitization of mice. The authors would also like to thank Miss Wei Liu for her help with the ELISA test.
CONFLICT OF INTEREST
The authors declare that there is no competing interest regarding the publication of this article.
AUTHOR CONTRIBUTIONS
Qing Zhong carried out most of the experiments, generated the majority of the data, and wrote a large part of the first draft of the manuscript. Mengmeng Zhan and Junling Wang performed flow cytometry, cell culture and challenge test and wrote a part of the first draft of the manuscript. Dong Chen carried out the clinical study and participated in data analysis. Nan Zhao and Ling Wang performed ELISA and real-time quantitative PCR and wrote a part of the first draft of the manuscript. Wei Wang, Xiaowen Zhang and Yixia Huang participated in animal experiment and challenge test. Shaoheng He and Huiyun Zhang designed and conducted the study, analysed the data, and wrote the second and final drafts of the paper. All authors read and approved the final paper.
ETHICAL STATEMENT
The study has been approved by the Medical Ethical Committee of Shenyang Medical College and the Medical Ethical Committee of the First Affiliated Hospital of Jinzhou Medical University (Trial registry: Chinese clinical trial; registration number: ChiCTR-BOC-16010279). Written informed consent was obtained from volunteers.
Open Research
DATA AVAILABILITY STATEMENT
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.