Interleukin 4 deficiency limits the development of a lupus-like disease in mice triggered by phospholipids in a non-bilayer arrangement
Abstract
Non-bilayer phospholipids arrangements (NPAs) are transient molecular associations different from lipid bilayers. When they become stable, they can trigger a disease in mice resembling human lupus, which is mainly characterized by the production of anti-NPA IgG antibodies. NPAs are stabilized on liposomes or cell bilayers by the drugs procainamide or chlorpromazine, which produce drug-induced lupus in humans. Here, we evaluated the participation of the TH2 response, through its hallmark cytokine IL-4, on the development of the lupus-like disease in mice. Wild-type or IL-4 knockout BALB/c mice received liposomes bearing drug-induced NPAs, the drugs alone, or an anti-NPA monoclonal antibody (H308) to induce the lupus-like disease (the last two procedures stabilize NPAs on mice cells). IL-4 KO mice showed minor disease manifestations, compared to wild-type mice, with decreased production of anti-NPA IgG antibodies, no anti-cardiolipin, anti-histones and anticoagulant antibodies, and no kidney or skin lesions. In these mice, H308 was the only inducer of anti-NPA IgG antibodies. These findings indicate that IL-4 has a central role in the development of the murine lupus-like disease induced by NPA stabilization.
1 INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic and multisystemic autoimmune disease characterized by deregulated innate and adaptive immune responses, which lead to the polyclonal activation of B cells and to a massive production of auto-antibodies that form immune complexes and cause tissue damage. SLE aetiology is not completely understood, but it involves genetic predisposition, a breakdown of immune tolerance, and environmental factors; furthermore, it is more frequent in women than in men.1-3
Mouse models have been used to study SLE pathogenesis.4 The best-characterized spontaneous lupus models include the F1 cross between New Zealand Black and New Zealand White (NZB/W F1) mice, the Murphy-Roths large/lymphoproliferative locus mice (MRL/lpr) and the cross between female C57BL/6 and male SB/Lc Y-linked autoimmunity accelerator (BXSB/Yaa) mice.5, 6 These three strains present hyperactive B and T cells, high titres of auto-antibodies against nuclear antigens, defective clearance of immune complexes and fatal antibody-induced glomerulonephritis.4
A lupus-like disease can be induced in mice by the deletion of genes that prevent excessive lymphocyte activity (like FcγRIIB, Lyn, Fyn, CD22, PD-1, CD45 E613R, p21 and Bcl2),5 by the injection of autoimmune sera,7 by the injection of pristane (an alkane isoprenoid from liver shark)8 or by immunization with peptides, like the decapeptide DWEYSVWLSN9 or with the peptides PPPGMRPP or PSQQVMTP10 from the Sm B/B' proteins. Anti-Sm autoantibodies are formed against these proteins in SLE patients. These induced lupus models have most of the features of human lupus.
Our research group developed a lupus model in BALB/c and NIH mice using phospholipids in a non-bilayer phospholipid arrangement (NPA); this murine lupus-like model recapitulates many characteristics of human lupus.7, 11 NPA is molecular associations of phospholipids different from lipid bilayers. NPA exists naturally in a transient form on cell membranes and participate in membrane fusion processes.12, 13 NPA can also be formed and stabilized on the lipid bilayer of liposomes or cell membranes in the presence of cationic inducers, such as manganese (Mn2+), or drugs like chlorpromazine (antipsychotic) and procainamide (antiarrhythmic), which produce as a side effect a drug-induced lupus erythematosus in humans.14, 15 NPA are formed by the interactions of membrane anionic phospholipids, such as phosphatidate, phosphatidylglycerol, phosphatidylserine and cardiolipin, with manganese or with the lupus-inducing drugs. The interactions between the anionic phospholipids and the inducers produce an inverted micelle, which is inserted into the lipid bilayer and leads to the formation of the NPA. A distortion occurs in the arrangement of the outer phospholipids of the NPA, so that their polar heads are spread and new antigens are exposed; these antigens induce the production of anti-NPA antibodies.11
Our lupus-like model can be developed in mice by the direct administration of NPAs or by administering inducers of NPA formation, which produce and stabilize NPAs on cell membranes. These NPA inducers include, in addition to manganese and the drugs chlorpromazine and procainamide, a NPA-specific monoclonal antibody (H308, which stabilizes pre-existing NPAs on mouse cell membranes), and sera from mice with the lupus-like disease. This model is characterized by the presence of anti-cardiolipin, anti-histones, anti-nuclear and anticoagulant auto-antibodies that appear one month after the anti-NPA IgG antibodies are detected.7, 11 These mice develop the following clinical features: piloerection, weight loss and symmetric facial lesions that in some cases resemble a ‘butterfly rash’. Skin from the facial lesions shows epidermal atrophy, widening of the hair bulb fibrous sheaths with disorganization of matrical cells, and the lupic band along the dermal-epidermal junction. Glomeruli show mesangial hyper cellularity and thickening of capillary walls.7, 11 Electron microscopy and immunofluorescence show deposits of immune complexes along the basement membranes of glomerular capillaries and in the mesangial matrix,7 suggesting the onset of kidney damage; however, proteinuria was not detected at the analysed times (4 months after the first injection of the lupus-like disease inductors).7 Mice have increased serum concentrations of proinflammatory cytokines and of the C3a and C5a complement components.16 B cells from these mice produce the anti-NPA IgG antibodies via germinal centres in the draining lymph nodes and the spleen17; anti-NPA antibodies are also found in the sera of patients with SLE.7
TH1- and TH2 cells participate in the development of human lupus. IFN-γ and IL-2, produced by TH1 cells, are necessary for antibody production by autoreactive B cells.18 IFN-γ activates macrophages, cytotoxic T cells and B cells, increasing the production of IgG antibodies. Patients and murine models of SLE show high levels of IFN-γ, and its decrease abolishes SLE development in mice.5 The TH2 cytokines IL-4 and IL-5 promote the recruitment of lymphocytes to the lymph nodes, and IL-4 induces immunoglobulin class-switching to IgG1 and IgE in mice.19, 20 In addition, the TH2 environment promotes the development of lupus nephritis.19 Also, it is important to highlight that IL-4, and IL-5 as well as the chemokine interferon gamma inducible protein 10 have been found in patients more than three years before they satisfied SLE classification criteria.21
In this study, we evaluated the participation of TH2 cells through the role of IL-4, its hallmark cytokine, in the development of the murine lupus-like disease, by inducing the disease in IL-4 gene knockout BALB/c (IL-4 KO BALB/c) mice and in wild-type BALB/c mice. We found an important role for IL-4 in the development of this disease, since the IL-4 KO BALB/c mice only showed minor disease manifestations.
2 MATERIALS AND METHODS
2.1 Ethics statement
This study was approved by the Bioethics Committee of our Institution, according to the “Guide for the Care and Use of Laboratory Animals” from the US National Institutes of Health.22
2.2 Lipids and mAb
Egg-yolk L-α-phosphatidic acid (PA), egg-yolk L-α-phosphatidylcholine (PC), chlorpromazine and procainamide were purchased from Sigma Aldrich (St. Louis, MO, USA). H308 is a murine IgM monoclonal antibody (mAb) that specifically binds to NPA and was prepared as described previously.7, 23
2.3 Preparation and characterization of smooth liposomes and liposomes bearing NPAs
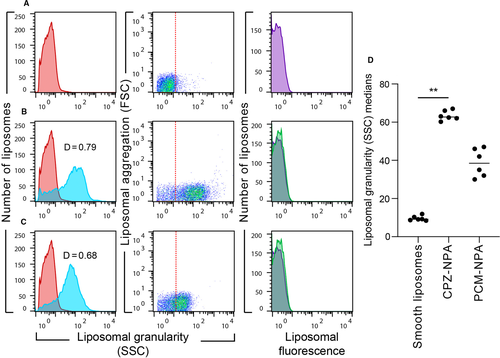
Smooth liposomes were prepared as previously described,7, 11 using PC and PA (2:1 molar ratio). Nine micromoles of the phospholipid mixture were dissolved in 1 mL of diethyl ether, 330 μL of TS (10 mmol/L Tris-HCl, 1 mmol/L NaCl, pH 7) was added and the mixture was sonicated three times in a Lab Supply G112SPI sonicator (Laboratory Supplies). The diethyl ether was subsequently removed, using a rotary evaporator, under a stream of oxygen-free dry nitrogen at reduced pressure and 37°C; the final volume was adjusted to 1 mL with TS. The smooth liposomes obtained were filtered through MF-Millipore membranes (Billerica, MA, USA) with 0.45 μm pores. To induce the formation of NPAs, smooth liposomes in TS were incubated 30 minutes at 37°C with the drugs chlorpromazine (3 mM) or procainamide (8 mM).11 The characterization of liposomes was made in a FACSCalibur flow cytometer (Becton Dickinson) using CellQuest software.7 Results from 10 000 events were analysed with FlowJo software (Tree Star) and reported as relative fluorescence histograms and relative FSC and SSC dot plots in logarithmic mode. FSC is proportional to liposome surface area, while SSC is proportional to the granularity of liposomal bilayers, and correlates with NPA content.7, 11, 16
2.4 Mouse strains
Two-month-old wild-type BALB/c and BALB/c-IL-4tm2Nnt/J (JAX 002496) (IL-4 KO BALB/c) female mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). All mice were maintained in a specific pathogen-free facility.
2.5 Development of the lupus-like disease in wild-type and IL-4 KO BALB/c mice
Wild-type and IL-4 KO BALB/c female mice were divided into six groups (I-VI), with six mice in each group. Mice from groups I and II received an intramuscular injection of 3 mg/kg chlorpromazine or 10 mg/kg procainamide, respectively, every third day for six months; the doses of these drugs were similar to those used for the treatment of psychotic dysfunctions (chlorpromazine) or cardiac arrhythmias (procainamide). Mice from groups III and IV were injected intrasplenically, on days 1 and 15, with PC:PA (2:1) liposomes (50 nmol PA/50 μL TS) that had been incubated with 3 mmol/L chlorpromazine or 8 mmol/L procainamide, respectively; on day 30 and every week for 6 months, these mice received the same doses of liposomes incubated with 3 mmol/L chlorpromazine or 8 mmol/L procainamide by intraperitoneal injection. Mice from group V received an intraperitoneal injection of 50 ng of H308 mAb every week for 6 months. Additionally, one group of ten male IL-4 KO BALB/c mice received the same doses of H308 mAb, in order to determine if male IL-4 KO BALB/c mice are less susceptible to develop the lupus-like disease than IL-4 KO female mice, as occurs in wild-type BALB/c mice.7 Mice from group VI were the negative controls and were injected with untreated liposomes, that is, liposomes that not were incubated with the drugs.
Blood was taken from the facial vein of mice before the lupus inducers and then each month after the first intraperitoneal injection, during 6 months. The development of the lupus-like disease was followed by the detection of anti-NPA, anti-cardiolipin, anti-histones and anticoagulant antibodies in the mice sera, and by histological evaluation of skin and kidneys.
2.6 Detection of anti-NPA antibodies by liposomal-ELISA
The liposomal-ELISA method was described previously.11 Goat peroxidase-conjugated anti-mouse IgM, IgG and IgA (total) antibodies, or goat peroxidase-conjugated anti-mouse IgG antibodies, were used as secondary antibodies. Results were reported as arbitrary units (AU) calculated by: (AsP – AsW)/(AsH – AsW) × 100, where AsP is the absorbance of sera from mice injected with the lupus inducers, AsH is the absorbance of sera from mice before the lupus inducers and AsW is the absorbance of the controls without sera.
2.7 Detection of anti-cardiolipin, anti-histones and anticoagulant antibodies
The ELISA described for the detection of anti-cardiolipin antibodies24 was used to measure these antibodies in mice sera. Results were also reported as AU, positive results had values ≥1.9. Anti-histone antibodies were detected by the ELISA described to measure these antibodies in mouse strains that spontaneously develop lupus25; results were reported as AU. Anticoagulant antibodies were measured using a modification of the kaolin-activated partial thromboplastin time.26
2.8 Cytometric analysis of anti-NPA antibodies
Liposomes with chlorpromazine-induced NPAs (0.1 μmol PA/100 μL TS) were incubated with 100 μL of mouse sera (1:50 dilution in TS buffer, previously heated at 56°C for 30 minutes to inactivate complement) at 37°C for 1 hour. Subsequently, 100 μL of FITC-conjugated goat anti-mouse IgG antibodies (1:2000 dilution in TS buffer) was added. After incubation at 37°C for 1 hour, liposomes were centrifuged at 200 000 × g at 18°C for 50 minutes and washed with TS buffer. Ten thousand liposomes were analysed in the FACSCalibur flow cytometer, as previously described.11 Binding of the H308 mAb to liposomes bearing chlorpromazine-induced NPAs (positive control) was detected with a FITC-conjugated goat anti-mouse IgM antibody.
2.9 Histological evaluation of mice
Mice were euthanized six months after the administration of H308 mAb and autopsies were performed. The skin and the kidneys were studied, since these are the most affected organs in wild-type BALB/c mice with lupus-like disease.7, 11 Tissue fragments from skin and kidneys were collected, fixed in 10% formaldehyde, embedded in paraffin, sectioned at 6 μm thickness and stained with haematoxylin and eosin for histological evaluation.
3 RESULTS
3.1 Characterization of smooth and NPA-bearing liposomes
The liposomes made from PC:PA (2:1) presented the characteristic granularity profiles of smooth unilamellar liposomes, in which phospholipids are in bilayer arrangements,11, 27 and showed basal fluorescence (Figure 1A). The liposomes with 3 mmol/L chlorpromazine (Figure 1B) or 8 mmol/L procainamide (Figure 1C) showed an increase in the granularity of their bilayers, produced by the formation of NPAs.11 When the granularity profile of smooth liposomes was compared with the Kolmogorov-Smirnov test to the granularity profile of liposomes bearing chlorpromazine- or procainamide-induced NPAs, D values of 0.79 and 0.68 were obtained, respectively (Figure 1B,C). D ≥ 0.5 with P < .001 denotes a statistically significant difference between the studied populations, and in this case, it indicates the presence of NPAs on liposomes.7, 11 NPAs did not modify the relative fluorescence of liposomes (Figure 1B,C), compared with the smooth liposomes (Figure 1A). Chlorpromazine was a better inducer of NPAs than procainamide, because the medians of the liposomal granularity of liposomes bearing chlorpromazine-induced NPAs were significantly higher than the medians of the smooth liposomes (Figure 1D), while the medians of the liposomal granularity of liposomes bearing procainamide-induced NPAs were not.
3.2 Analysis of auto-antibody induction by chlorpromazine, procainamide or liposomes bearing drug-induced NPAs in IL-4 KO and wild-type BALB/c mice
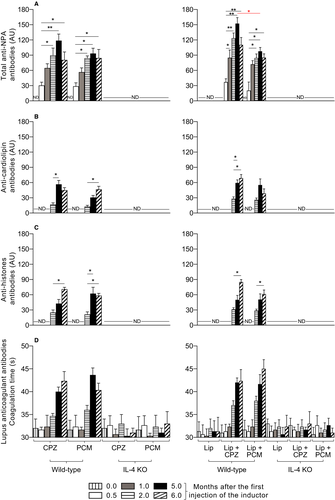
Female wild-type BALB/c mice that received chlorpromazine, procainamide or liposomes bearing drug-induced NPAs developed the lupus-like autoimmune disease characterized by the production of anti-NPA antibodies (Figure 2A). These antibodies were detected at 0.5 month, increased at 5 months and were conserved 6 months after the first administration of the lupus inducers (Figure 2A). The highest titre of anti-NPA antibodies was observed in mice that received liposomes bearing chlorpromazine-induced NPAs; the drugs alone and liposomes bearing procainamide-induced NPAs produced slightly lower titres of anti-NPA antibodies but were similar to each other (Figure 2A). In addition, anti-cardiolipin (Figure 2B), anti-histones (Figure 2C) and anticoagulant (Figure 2D) antibodies appeared 2 months after the first administration of the lupus inducers, 1.5 months after the anti-NPA antibodies, and increased 5 or 6 months after the first administration of the inducers. In female IL-4 KO BALB/c mice, the administration of the drugs alone, or of liposomes bearing drug-induced NPAs, did not induce the production of anti-NPA (Figure 2A), anti-cardiolipin (Figure 2B), anti-histones (Figure 2C) or anti-coagulant (Figure 2D) antibodies. In addition, in female wild-type BALB/c and female IL-4 KO BALB/c mice, which received untreated liposomes, there was not production of anti-NPA (Figure 2A), anti-cardiolipin (Figure 2B), anti-histones (Figure 2C) or anti-coagulant (Figure 2D) antibodies.
3.3 H308 mAb induces the production of IgG ANTI-NPA antibodies, but no histopathological lesions, in female IL-4 KO BALB/c mice
Because we previously demonstrated that the H308 mAb specifically binds to pre-existing NPAs on mice cell membranes and stabilizes them, leading to the production of anti-NPA antibodies in wild-type BALB/c mice,7 we investigated whether the administration of the H308 mAb induces the production of anti-NPA antibodies in the IL-4 KO BALB/c mice, compared to the wild-type BALB/c mice.
The H308 mAb is of the IgM class, so we only measured IgG antibodies in H308-administered mice. IgG anti-NPA antibodies were detected in female wild-type BALB/c mice 0.5 months after the initial administration of the mAb, and their titres increased until the sixth month (Figure 3A). In female IL-4 KO BALB/c mice, these antibodies were not detected until the fifth month, and their titres increased on the sixth month (Figure 3A). Six months after the initial administration of liposomes bearing chlorpromazine-induced NPAs, more than 80% of the total anti-NPA antibodies in female wild-type BALB/c mice were of the IgG class (data not shown), which is consistent with previous studies.17 Anti-cardiolipin (Figure 3B), anti-histones (Figure 3C) and anti-coagulant (Figure 3D) antibodies were detected in female wild-type BALB/c mice after 2 months, or 1.5 months after the IgG anti-NPA antibodies, and increased at 5 and 6 months after the first administration of the inducers. However, in female IL-4 KO BALB/c mice, no production of anti-cardiolipin (Figure 3B), anti-histones (Figure 3C) or anticoagulant (Figure 3D) antibodies were detected at the analysed times.
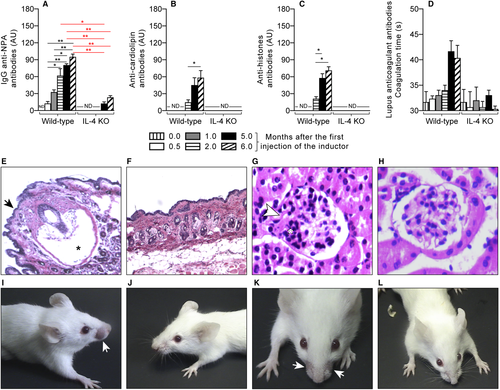
Six months after the initial administration of the H308 mAb, 55% of female wild-type mice showed symmetric facial lesions, 100% had kidney and 80% had skin histological abnormalities. Alopecic skin revealed epidermal atrophy, a decrease of terminal hair follicles and hair follicles with cystic degeneration, matrical cells disorganization and detachment (Figure 3E); the kidneys presented diffuse mesangial cell proliferation in the glomeruli and thickening of capillary walls (Figure 3G) in a similar way as was previously described.7, 11 However, female IL-4 KO mice did not develop histological abnormalities in their skin and glomeruli (Figure 3F,H), even though they produced anti-NPA antibodies after receiving the H308 mAb. In addition, representative photographs of the head of two female BALB/c wild-type mice (Figure 3I,K) and of two female IL-4 KO BALB/c mice (Figure 3J,L) after six months of the initial administration of H308 mAb, showed that the facial lesions were only observed in the female BALB/c wild-type mice that developed the lupus-like disease.
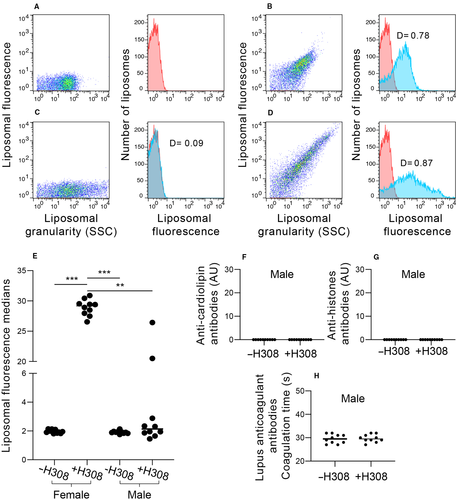
3.4 Female IL-4 KO BALB/c produce higher titres of IgG anti-NPA antibodies than male IL-4 KO BALB/c mice
We previously reported that the production of anti-NPA antibodies was more efficient in female than in male wild-type BALB/c mice.7 So, here we analysed one group of 10 male IL-4 KO BALB/c mice, compared with female IL-4 KO BALB/c, and measured the IgG anti-NPA antibodies by flow cytometry. Male IL-4 KO BALB/c mice were injected with H308 mAb to induce IgG anti-NPA antibodies, because only the administration of this monoclonal antibody, but not the administration of liposomes bearing drug-induced NPAs or the administration of the drugs alone, induced the production of anti-NPA antibodies in female IL-4 KO BALB/c (Figures 2A and 3A).
PC:PA (2:1) liposomes bearing chlorpromazine-induced NPAs that were incubated without or with the H308 mAb, were used as negative (Figure 4A) and positive (Figure 4B) controls for flow cytometry detection, respectively. IgG anti-NPA antibodies were analysed in the sera of IL-4 KO BALB/c mice before (Figure 4C) and six months after the injection of the H308 mAb; if the sera contain antibodies that bind to the liposomes bearing chlorpromazine-induced NPAs, an increase in the liposomal fluorescence is detected (Figure 4D). Female IL-4 KO mice were more susceptible to develop the lupus-like disease, since all the female IL-4 KO mice produced anti-NPA antibodies, but only 20% of the males produce these antibodies that trigger the lupus-like disease (Figure 4E). This is in accordance with the observation that SLE is more frequent in women than in men.1-3 These male IL-4 KO BALB/c mice did not produce anti-cardiolipin (Figure 4F), anti-histones (Figure 4G) or anticoagulant (Figure 4H) antibodies, in a similar form as was shown for the female IL-4 KO BALB/c mice (Figure 3B-D).
4 DISCUSSION
Mice models are key for human disease research and treatment development.28 The murine lupus-like model induced by phospholipids in NPAs provides an opportunity to understand the immune mechanisms underlying lupus onset, as well as the genetic, endocrine and metabolic influences in this multifactorial illness. In this study, we analysed the role of IL-4, the hallmark TH2 cytokine, in the development of a lupus-like disease by comparing the response of female IL-4 KO BALB/c mice with the response of female wild-type BALB/c mice to NPAs. We used three different procedures to induce the lupus-like disease: (1) administration of NPA-stabilizing drugs (chlorpromazine or procainamide), (2) administration of liposomes bearing drug-induced NPAs, or (3) administration of the NPA-stabilizing H308 mAb. Anti-NPA antibodies are the hallmark of this lupus-like disease, because their presence is indicative of the development of the disease in mice.17 We observed that, in female BALB/c mice that received the drugs, or liposomes bearing drug-induced NPAs, or the H308 mAb, anti-NPA antibodies appeared at 0.5 months, increased after 5 months and remained 6 months after the first administration of the NPA-inducer. At 6 months, 80% of the total anti-NPA antibodies were of the IgG class, which is consistent with our previous reports.17 Anti-cardiolipin, anti-histones and anticoagulant antibodies appeared in sera at 2 months, or 1.5 months after the anti-NPA antibodies; 55% of the sick mice showed symmetric facial lesions, 100% had kidney and 80% had skin histological abnormalities. The facial lesions disclose atrophy in the epidermis and large histopathological changes in the hair follicles; the glomeruli show diffuse mesangial cell proliferation and thickening of capillary walls.
Because our previous studies, using electron microscopy and immunofluorescence, showed deposits of immune complexes along the basement membranes of glomerular capillaries and in the mesangial matrix,7 it is possible that the glomerular damage observed here could be attributed to the deposit of autoantibodies as immune complexes in the glomeruli, and constitute the onset of an immune-complex glomerulonephritis, as is described in other spontaneous and induced murine models of lupus.29
It is important to point out that, among all the different mouse models of lupus (spontaneous, induced, transgenic and knockout models), facial lesions, which are similar to the rash that is observed in SLE patients, have only been described in the MRL/lpr mice4, 5, 29 and in the induced model studied here.
In contrast, in female IL-4 KO BALB/c mice, only the administration of the H308 mAb, but not the administration of liposomes bearing drug-induced NPAs, or the administration of NPA-stabilizing drugs, induced the production of anti-NPA antibodies. We previously reported that the anti-NPA mAb H308 induces the most pronounced features of the lupus-like disease (including very low fertility and foetal absorption in female wild-type BALB/c mice), compared to other lupus inducers,7 which is in accordance with the results reported here. In addition, the induction of anti-NPA antibodies by H308 mAb was delayed in female IL-4 KO mice compared to female wild-type mice, since it started at 5 months and increased at 6 months after the first administration of the H308 mAb. H308-administered female IL-4 KO BALB/c mice did not produce anti-cardiolipin, anti-histones or anticoagulant antibodies, and they did not show facial lesions, or histological abnormalities in skin and glomeruli. Only 20% of male IL-4 KO BALB/c mice produced anti-NPA antibodies, without producing the other three auto-antibodies, or developing facial lesions or histological abnormalities. The lower proportion in the development of the lupus-like disease in male IL-4 KO BALB/c mice could be caused by sex hormones, sex chromosomes or both, as has been described for many autoimmune diseases, which are characterized by a female predominance, such as SLE.2, 30
IL-4 is a pleiotropic cytokine that is produced by TH2 cells, basophils and mast cells.31-33 It drives the suppression of IFN-γ-producing TH1 cells, and it is required for the differentiation of antigen-activated CD4+ T cells into TH2 cells, since it activates STAT6 and induces the expression of GATA-3, which in turn increases the expression of IL-4, IL-5, IL-9 and IL-13. IL-4 is an autocrine growth factor for TH2 cells, and it is also a B-cell growth factor that induces B-cell proliferation and immunoglobulin class switching to IgG1 and IgE in mice and to IgG1, IgG4 and IgE in humans. It inhibits switching to IgG2a, IgG2b, IgG3 and IgG4 in mice, and to IgG3 in humans. Additionally, IL-4 induces alternative macrophage activation.32, 34
Some studies have found that TH2 cells promote the development of lupus nephritis in mice lacking the Src family protein tyrosine kinase Lyn (Lyn(-/-) mice),19 and low IL-4 appears to predispose lupus in BXSB mice.20 However, in studies with other mice strains, IL-4 deficiency is associated with reduced disease. In BALB/c mice that receive a lupus-inducing decapeptide (DWEYSVWLSN), IL-4 deficiency does not inhibit the production of IgM and IgG3 anti-DNA antibodies, but it strongly inhibits the deposition of antibodies in glomeruli.35 In lupus-prone NZM2410 mice, which develop glomerulosclerosis and renal disease and overexpress IL-4, treatment with an anti-IL-4 antibody decreases TH2 responses, improves glomerulosclerosis and delays or even prevents the renal disease, despite the presence of high levels of IgG anti-DNA antibodies.36 Finally, in the lupus-prone MRL/Mp-lpr/lpr strain, IL-4 deficiency leads to reduced serum levels of IgG1 and IgE, and to significantly reduced lymphadenopathy and an end-organ disease.37 Our findings are in accordance with those models where IL-4 deficiency improves or even prevents kidney damage in mice,35-37 and support the notion that the role of IL-4 in the pathogenesis of lupus differs in mice strains with different genetic backgrounds.
The reduced manifestations of the lupus-like disease in IL-4 KO BALB/c mice, compared to wild-type BALB/c mice, could be attributed to the fact that the IgG anti-NPA antibodies were detected in lower titres in the IL-4 KO mice, and only until the fifth month, compared to wild-type mice, in which these antibodies were detected 2 weeks after the first administration of the lupus inducers. IgG antibodies have a longer half-life and a higher antigen affinity than other antibody classes, and their diffusion to extravascular sites is efficient.32 IgG1, which is depleted in IL-4 KO BALB/c mice,38 is the most abundant of the IgG subclasses and probably accounts for the reduced levels of anti-NPA IgG antibodies that we observed in these mice. IgG1 binds to FcγRIII and activates NK cells,39 and some IgG1 antibodies activate the complement system,40, 41 so the loss of IgG1 would lead to a significant reduction of IgG-dependent complement activation and NK cell-mediated killing. NK cells can kill auto-antibody-coated cells and cause tissue damage.42 Similarly, auto-antibodies can activate the complement system, which causes cytotoxicity 43 and is associated with renal damage in SLE.6 We propose that anti-NPA antibodies of the IgG class activate these two mechanisms (we previously reported that wild-type BALB/c mice with the lupus-like disease have increased concentrations of C3a and C5a16), leading to a disruption of cellular membranes that exposes intracellular molecules, which now become auto-antigens. This explains the delayed appearance of antibodies against intracellular antigens (anti-cardiolipin, anti-histones and anticoagulant antibodies), which can be detected in serum six weeks after the appearance of anti-NPA antibodies. Therefore, an important decrease in IgG anti-NPA antibodies, particularly of the IgG1 subclass, could lead to the milder disease observed in IL-4 KO mice.
5 CONCLUSIONS
IL-4 has a central role in the development of the murine lupus-like disease induced by the stabilization of NPAs, since IL-4 KO mice had only minor manifestations of the lupus-like disease and a reduced production of anti-NPA IgG antibodies, no antibodies against intracellular antigens and no histological alterations in the skin and kidneys. Therefore, IL-4 inhibition may cause a clinical improvement in human lupus.
CONFLICT OF INTEREST
The authors declare no commercial or financial conflict of interest. The funding source was not involved in any part of this study.
AUTHOR CONTRIBUTIONS
ARM, CWB, AEG, RHP, IWB and IB conceived and designed the experiments. INL, CLS, EMG and RHP performed the experiments and the acquisition of data. All authors analysed the data, interpretation, and discussion, wrote and revised the paper, involved in final approval of the version to be published. Also, the authors declare that there is no conflict of interest regarding the publication of this paper.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.