Increased expression of extracellular matrix metalloproteinase inducer (EMMPRIN) and MMP10, MMP23 in inflammatory bowel disease: Cross-sectional study
Gabriela Fonseca-Camarillo and Janette Furuzawa-Carballeda contributed equally to this manuscript.
Funding information
This work was supported by funds from the Inflammatory Bowel Disease Clinic at the National Institute of Medical Sciences and Nutrition.
Abstract
It has been reported that EMMPRIN is involved in the regulation of immune response and the induction of MMPs production by fibroblasts. The aim of this study was to describe the intestinal gene expression and protein production of EMMPRIN, MMP23 and MMP10 in patients with ulcerative colitis (UC) and Crohn’s disease (CD) and compared them with a control group. Gene expression of EMMPRIN, MMP10 and MMP23B was measured by RT-PCR. In order to determine EMMPRIN and MMP protein expression, colonic tissues were immunostained. The results of the study showed EMMPRIN gene expression was upregulated in rectal mucosa from active (a)UC versus aCD patients (P = .045), remission (r)CD group (P = .0009) and controls (P < .0001). We detected differences between rUC and aCD (P = .004), rCD (P < .0001) or control group (P < .0001). EMMPRIN showed a higher expression in mucosa (intraepithelial lymphocytes), submucosa and adventitia (endothelial cells) from aCD patients. MMP23 levels were increased in aUC and aCD compared to rUC and rCD and the control group (P = .0001). EMMPRIN+/MMP23+─expressing cells were localized mainly in mucosa, muscular and adventitia from active UC patients. MMP10 gene expression was increased in aUC versus CD patients and the control group (P = .0001). MMP10 gene expression is associated with inflammation in UC patients (P = .0001, r2 = .585). EMMPRIN+/MMP10+─producing cells were found mainly in all intestinal layers and perivascular inflammatory infiltrates from aUC patients. In conclusion, EMMPRIN, MMP23 and MMP10 were upregulated in patients with active UC versus remission UC , CD and control groups suggesting that, they are involved in the inflammatory process.
1 INTRODUCTION
Crohn's disease (CD) and Ulcerative Colitis (UC) are prototypical complex diseases characterized by chronic inflammation, induced by interacting environmental, genomic, microbial and immunological factors.1
Chronic inflammation and aberrant tissue remodeling with excessive accumulation or degradation of extracellular matrix components are hallmarks in pathogenesis of IBD.2
Matrix metalloproteinases (MMPs) can cleave and remodel extracellular matrix components in response to inflammatory stimuli and by their immunomodulating effects.3
EMMPRIN (extracellular matrix metalloproteinase inducer) or CD147 (cluster of differentiation 147) is a protein that in humans is encoded by the BSG gene,4 is a highly glycosylated immunoglobulin superfamily transmembrane protein.5
Functionally, EMMPRIN participates in the transport of nutrients, migration of inflammatory leukocytes and induction of MMPs5 and stimulates production of a collagenase MMP1 by fibroblasts in tumor cells.4-7
Previously, it has been reported that EMMPRIN is strongly expressed in activated lymphocytes4 and is the main inducer of MMPs production in the stromal fibroblast,8 for this EMMPRIN is involved in the regulation of immune responses.
MMPs including stromelysin (MMP10) and protease (MMP23) are transcriptionally upregulated in response to proinflammatory cytokines, cell-cell, or cell-extracellular matrix interactions and by the regulation of EMMPRIN.9
Petrey AC and de la Motte CA10 propose the functional and clinically significant interactions between immune as well as non-immune cells with the extracellular matrix components and MMPs in the pathogenesis of IBD.
There is limited knowledge about the expression of MMPs especially MMP23 in IBD patients and even less knowledge about gene expression and production of EMMPRIN. For this reason, to understand the pathophysiology, may help to figure out the disease and in turn to find therapeutic targets.
The aim of this study was to describe the gene expression and intestinal production of EMMPRIN, MMP23 and MMP10 in patients with active and remission UC, CD and compared them with control group, as well as to correlate them with clinical outcomes.
2 MATERIALS AND METHODS
2.1 Collection of rectal biopsies
This is a blind, cross-sectional study. We included a total of 90 rectal biopsies from 40 patients with UC, 30 patients with CD and 20 controls without colonic inflammation. The diagnosis of UC was based on clinical, radiologic, endoscopic and histologic criteria. Colonoscopy was performed in order to calculate the Mayo Score Activity Index and Riley Score for endoscopy and histology activity, respectively.11-15
All IBD patients were included during the period from January 2012 to July 2015, belonging to the Inflammatory Bowel Disease Clinic at the National Institute of Medical Science and Nutrition Salvador Zubirán.
Clinical and demographic information from enrolled patients were collected from interview and medical records. Variables included were age, gender, type of medical treatment (5-aminosalycilates, steroids and azathioprine), the presence of extra-intestinal manifestations such as articular affection, ankylosing spondylitis, sacroiliitis, sclerosing cholangitis, pyoderma gangrenosum, erythema nodosum or uveitis, disease endoscopic extension according to Montreal Classification and clinical course classified as: initially active and posteriorly remitted (first episode with activity and then long-term remission for more than 5 years), intermittent activity (>2 relapses per year) and chronic continual activity (persistent activity despite medical conventional therapy).11-15
The control group consisted of rectal biopsies without colonic inflammation (no documented inflammatory disease and use of 5-aminosalicylates and corticosteroids).
Rectal biopsies were taken with colonoscopy and were immediately placed in 0.5 ml of RNA later (Ambion) and stored at −80°C until processing.
2.2 Gene expression analysis
Such as other previous studies, we isolated total RNA using High Pure RNA Tissue (Roche Diagnostics), following the manufacturer's guidelines. Two hundred nanograms of total RNA were reverse-transcribed into cDNA with random hexamer primers (Roche Diagnostics).14-17
For gene expression analysis, we performed the methodology by Fonseca Camarillo et al14, 15 The PCR amplification of the above mentioned genes was carried out with 20 ng of cDNA, 200 nM forward and reverse primer, and Taqman Master Mix (Roche Diagnostics) in a final volume of 10 µL. PCR reactions were run in a Light Cycler 480 (Roche Diagnostics) for 45 cycles, each cycle consist in denaturation for 15 seconds at 95°, primer ligation for 15 seconds at 55°, and extension for 30 seconds at 72°C and cooling 30 seconds at 40°C.
The gene expression was measured by real-time polymerase chain reaction (RT-PCR). Reference gene GADPH transcripts were used for relative quantification and quality controls. GAPDH has been demonstrated as an appropriate control for mucosal tissues. There is no evidence of alterations in GAPDH gene expression regarding the age or gender of the donor. Moreover, for most tissues, the influence of delay in processing surgical and postmortem tissues is negligible. No differences in GAPDH mRNA expression has been determined between males and females. Furthermore, in gastrointestinal tract, including mucosal tissues GAPDH mRNA expression levels have been similar within groups of related tissues, such as: stomach antrum, body, and funds; left atria and left ventricle; kidney cortex, medulla, and pelvis; duodenum, jejunum, ileum, and colon. In mucosal tissues from patients with inflammation, GAPDH mRNA levels were equal when compared with samples without intestinal inflammation.18, 19 For q-PCR assays quality control, determination of linearity and reproducibility was evaluated (VC < 10%). The mRNA relative quantification of target genes was conducted using the LightCycler software 4.1, according to the 2-delta-delta Ct method. Table 1 shows the details of the primer's designs used for the RT-PCR.
| Gene | NM Gene Bank | Primer left | Primer right | UPL |
|---|---|---|---|---|
| MMP23 | NM_006983.1 | ggaccacttcaacctcacct | ggacacgtcgctccacat | 12 |
| MMP10 | NM_002425.1 | caaaagaggaggactccaaca | ttcacatccttttcgaggttg | 61 |
|
EMMPRIN (CD147)* |
NM_198591.1, NM_001728.2 | gggagagtactcctgcgtctt | acttcacagccttcactctgg | 42 |
| GAPDH | NM_002046.3 | agccacatcgctcagacac | gcccaatacgaccaaatcc | 60 |
- *Oligonucleotides were designed considering all the alternative splicing variations
2.3 Double-staining procedure
In order to determine the EMMPRIN, MMP-10 and MMP-23 positive cells, we followed the methodology of double staining procedure by Furuzawa- Carballeda J. et al.17, 20 Briefly, 5 µm-thick sections of formalin-fixed and paraffin-embedded tissue were placed on positively charged slides. Sections were deparaffinized and rehydrated through a series of xylene and graded alcohols. Enzyme antigen retrieval was carried out during 2 min (Enzo Life Sciences, Inc) and endogenous peroxidase of the tissue was blocked with 3% H2O2. Then non-specific background staining was avoided with the immunohistochemistry serum-free background blocking solution (Enzo Life Sciences). EMMPRIN and MMP23-producing cells were detected by a Multiview (mouse-HRP/rabbit-AP) immunohistochemistry kit and EMMPRIN and MMP-10-producing cells were detected by a Multiview (mouse-HRP/mouse-AP) immunohistochemistry kit (Enzo Life Sciences, Inc., Farmingdale, NY, USA). This procedure is a sequential double staining method in which the cocktail of mouse monoclonal IgG1 EMMPRIN (8D6) antibody (SC21746; Santa Cruz Biotechnology, CA, USA) and C-Terminal Polyclonal Rabbit IgG1 anti-MMP23, antibody (M6184; SIGMA) or the cocktail of mouse monoclonal IgG1 EMMPRIN (8D6) antibody and mouse polyclonal MMP10 to 50 µg (ABCAM) was incubated during 40 min at room temperature.
Slides were washed and then incubated with PolyView IHCh reagent (anti-mouse-HRP polymer)/PolyView IHCh reagent (anti-rabbit-AP polymer) or with PolyView IHCh reagent (anti-mouse-HRP polymer)/PolyView IHCh reagent (anti-mouse-AP polymer) for 20 min. Finally, EMMPRIN antigens were visualized using horseradish peroxidase (HRP)/3,3′-diaminobenzidine (DAB) (in brown) and the MMP10 and MMP23 antigens with alkaline phosphatase (AP)/Permanent Red (in pink). Tissues were counterstained with Mayer's hematoxylin and mounted in aqueous mounting medium. Negative control staining was performed with the universal negative control reagent specifically designed to work with rabbit, mouse, and goat antibodies (IHCh universal negative control reagent, Enzo Life Sciences, Inc) The blank was incubated with phosphate buffered saline-egg albumin (SIGMA-Aldrich) instead of the primary antibody. Both controls satisfactorily excluded nonspecific staining and endogenous enzymatic activities.
Double staining positive cells were counted in at least three optical fields from each slide in 320× high power magnifications. The average values per slide were used for statistical analysis.
2.4 Statistical analysis
The statistical analysis for gene expression analysis was performed using SPSS version 20 and Prism GraphPad version 6 using Kruskall Wallis non-parametric test, Spearman`s correlation, Fisher's exact test and Odds Ratio (OR) to determine the strength of association. A P value < .05 was considered statistically significant.
Immunohistochemistry statistical analysis was done using the Sigma Stat 11.2 program (Aspire Software International). Results were expressed as the mean ± standard error of the mean (SEM) of cells quantified by the program Image Pro-Plus version 5.1.1 For the analysis of percentage of immunoreactive cells we employed One Way Analysis of Variance on Ranks by Holm-Sidak Method. The P values smaller than or equal to .05 were considered as significant.
3 RESULTS
For this transversal and descriptive study, we included a total of 90 rectal biopsies that were divided in 5 groups: (a) Active UC (n = 20); (b) Remission UC (n = 20); (c) Active CD (n = 15); (d) Remission CD (n = 15) and control group of non-inflamed donors (n = 20). The demographic and clinical characteristics of the IBD patients and controls are shown in Table 2.
| Variable | Non-inflamed controls (n = 20) | Remission UC patients | Active UC patients (n = 20) | Remission CD patients | Active CD patients (n = 15) |
|---|---|---|---|---|---|
| Age, y | |||||
| Mean | 50.4 | 40.8 | 40.32 | 49.3 | 25 |
| Sex, female/male | 13/7 | 10/10 | 8/12 | 4/11 | 8/7 |
| Treatment | |||||
| Mesalazine | ND | 13/20 | 14/20 | 8/15 | 1/15 |
| Azathioprine | ND | 4/20 | 1/20 | 5/15 | 10/15 |
| Prednisone | ND | 3/20 | 5/20 | 2/15 | 5/15 |
| Mercaptopurine | ND | 0/20 | 0/20 | 0/15 | 0/15 |
| Disease extension | |||||
| Distal colitis | ND | 10/20 | 12/20 | 8/15 | 0/15 |
| Pancolitis | ND | 10/20 | 8/20 | 7/15 | 15/15 |
| Extra-intestinal manifestations | |||||
| Absent | ND | 8/20 | 19/20 | 8/15 | 0/15 |
| Present | ND | 12/20 | 1/20 | 7/15 | 15/15 |
- Abbreviations: CD, Crohn's Disease patient group; ND, not determined; UC, Ulcerative colitis patient group.
The most typical endoscopic features are continuous, confluent, and concentric colonic involvement proximal to the anal verge. Endoscopic severity may be best reflected by the presence of mucosal friability, spontaneous bleeding, and deep ulcerations. Typical pathologic findings are composed of widespread crypt architectural distortion (cryptitis, crypt abscess, and crypt atrophy), diffuse lamina propria cell infiltration, and basal plasmacytosis.
3.1 Relative mRNA gene expression of EMMPRIN, MMP23 and MMP10 in patients with IBD and controls
The EMMPRIN (CD147) mRNA expression was detectable and quantifiable by RT-qPCR in colonic biopsies from UC and CD patients and controls. EMMPRIN was up regulated in rectal mucosa from patients with active UC compared to active CD patients (P = .045), remission CD group (P = .0009) and controls (P < .0001). Also, we detected differences between UC in remission and active CD (P = .004), remission CD (P < .0001) and control group (P < .0001; No significant differences were found between active CD patients in compared to the control group shown in Figure 1A).
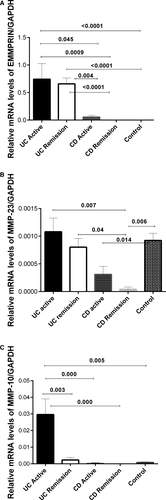
 , differences among groups were assessed by Kruskall Wallis test, and p values are presented in the figure
, differences among groups were assessed by Kruskall Wallis test, and p values are presented in the figureGene expression of MMP23b was increased in the rectal biopsies from patients with active UC compared to remission CD group (P = .007). Additional significant difference was found between remission UC and remission CD (P = .04) as shown in Figure 1B. The MMP23b levels were decreased in active and remission CD compared with controls (P = 0.014 and P = 0.006; Figure 1B).
The results showed that patients with active UC had significantly higher MMP10 gene expression in mucosa compared to remission UC patients and controls (P = .003 and P = .005 respectively). Also, high levels of relative gene expression of MMP10 was associated with the severe histological activity (P = .000) in patients with UC. Significant differences were found between active UC and active CD (P = .000; Figure 1C).
3.2 Intestinal production of EMMPRIN, MMP23 and MMP10 in patients with IBD
A total of 20 IBD patients were included (10 patients with active UC and 10 with active CD) and 10 patients without intestinal inflammation were included in the study.
Patients with active UC and CD had mucosal lesions consist of epithelial alterations and a cellular inflammatory response. Abundant inflammatory infiltrates of mononuclear cells, which extend from the serous layer to the mucosa, being more abundant at the level of the epithelium and the serosa were observed in active UC and CD tissues. In the control group, the histopathological characteristics of the colon resemble normal tissue.
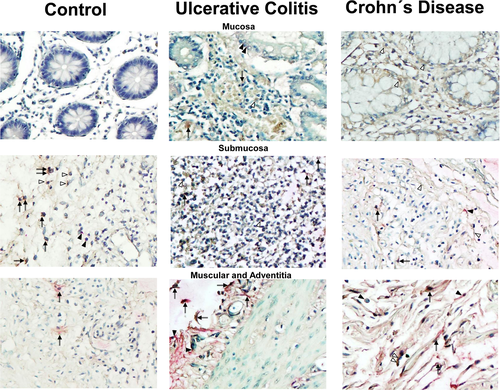
EMMPRIN showed important expression levels in submucosa, and cells that presumably could be lymphocytes and macrophages, remarking an important degree of immunopositivity in the case of intraepithelial lymphocytes and endothelial cells in serosa.
3.3 Co-localization of EMMPRIN+/MMP23+ in colonic tissue of patients with IBD
Double-staining procedure of EMMPRIN+/MMP23+ (stained in burgundy) showed an increased expression of EMMPRIN (stained in brown) and MMP23 (stained in pink) in submucosa and muscular layer of colonic tissue from patients with UC and CD compared with controls, as shown in Figure 2 and 3. EMMPRIN+/MMP23+ cell percentage was lower in CD compared with UC in all intestinal layers (Figure 3).
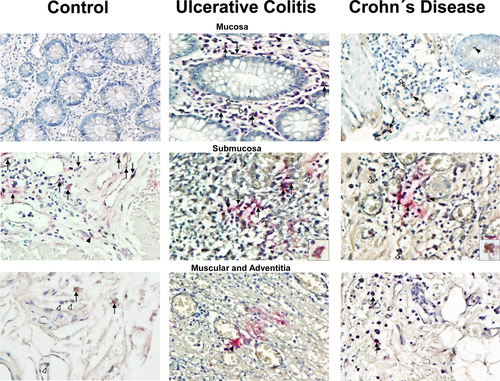
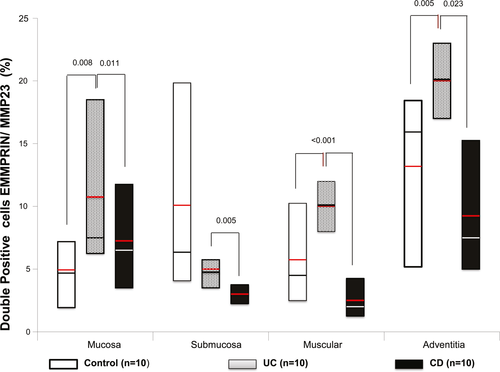
Higher double positive cells for EMMPRIN+/MMP23+—producing cell percentage (stained in burgundy) was localized mainly in mucosa, submucosa muscular and adventitia layers of patients with active UC compared with active CD (P = 0.011, P = 0.005, P ≤ 0.001 and P = 0.023, respectively; Figure 3).MMP23 was potentially produced by inflammatory infiltrate cells and parenchyma cells.
Also increased double positive cells were detected in active UC compared with controls in mucosa, muscular and adventitia (P = 0.008, P = <0.001 and P = 0.005).
3.4 Co-localization of EMMPRIN+/MMP10 + in colonic tissue of patients with IBD
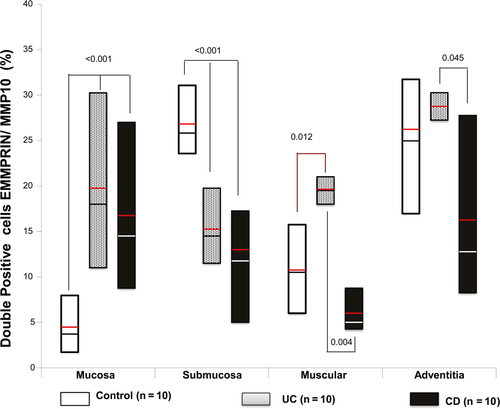
Co-localization of EMMPRIN+/MMP10+ (stained in burgundy) showed an increased expression of EMMPRIN (stained in brown) and MMP10 (stained in pink) in all layers (mucosa, submucosa and muscular) of colonic tissue from patients with UC and CD compared with controls, as shown in Figure 4 and 5.
Higher double positive cells for EMMPRIN/MMP10/—producing cell percentage (stained in burgundy) was localized mainly in muscular and adventitia layers of patients with active UC compared with active CD (P = 0.004, P = ≤0.045 respectively; Figure 5).
MMP10—producing cells were found mainly in mucosa muscularis, adventitia and perivascular inflammatory infiltrates in UC and CD patients. MMP10 was synthesized by presumably epithelial cells, inflammatory infiltrate cells and parenchyma cells.
The protein production of MMP10 was detected in colonic tissue from patients with UC, CD and controls. In the muscular layer of patients with active CD we detected an increased number of positive cells of MMP10 versus active UC group (P = .004) and control group (P = .012; Figure 5).
4 DISCUSSION
The main findings of this study showed a significant increased gene and protein expression of EMMPRIN in colonic tissue of patients with active ulcerative colitis compared with active Crohn's disease. This expression was differential between diseases regarding co-localization with MMP10 and MMP23.
Specifically, our study demonstrates that EMMPRIN is up regulated in patients with active UC and the protein is mainly produced by mononuclear cell infiltrates and endothelial cells.
Thus overall, our findings indicate that EMMPRIN-mediated cellular interactions may act in a pro-inflammatory manner in the colonic tissue.
EMMPRIN have been implicated in modulation of the epithelial barrier function through an MMP-mediated occluding cleavage.21 Moreover, EMMPRIN is up-regulated on activated T cells and also shown recently to serve as a marker for active Tregs (CD25+/EMMPRIN+) versus resting Treg or naive Treg (CD25+/EMMPRIN-) in the human system. Furthermore, the labeling with FoxP3, a well-known intracellular Treg marker, and EMMPRIN enables the identification of 3 distinct populations: 1) EMMPRINlowFoxP3+ cells representing resting Tregs; 2) EMMPRINmediumFoxP3+ cells, representing Tregs that can produce cytokines, including IL-2, IFN-γ, TNF-α, and IL-17; and 3) EMMPRINhighFoxP3+ cells, representing active Tregs that do not secrete high levels of cytokines, demonstrate highly suppressive activity, and exhibit demethylation in the Treg-specific demethylated region of the FOXP3 gene, a region whose demethylation status correlates to stabilized FoxP3 expression and suppressive activity.22
It is unknown if EMMPRIN plays a role in immune homeostasis, but it may serve as a useful marker for Treg subsets when evaluating disease progression.In the intestine, is unclear the mechanism of EMMPRIN in the modulation of the epithelial barrier regulated by MMPs.
MMP23 is a member of the matrix metalloprotease family of zinc- and calcium-dependent endopeptidases, which are involved in a wide variety of cellular functions including tissue remodeling, cell proliferation, cell migration, differentiation, angiogenesis, apoptosis and the immune response.22 There is evidence that MMP23B functions through the TNF signaling pathway. MMP23B directly interacts with TNF and mediates its release (shedding) from the cell membrane. On the other hand, MMP23 may regulate the surface expression of Kv1.3 channels in primary and metastatic colorectal cancer cells and thereby regulate cellular function. Colon cancer cells secreting processed active MMP23 enzyme containing the Kv1.3 channel-blocking toxin domain may block Kv1.3 channels on infiltrating anti-tumor T cells, and suppress them as a means of immune evasion.23
Velasco et al, provided evidence that this proteinase could play some specific role in any of the matrix-remodeling processes in a large type of tissues.24
Regarding the implication of EMMPRIN in epithelial barrier function and MMP23, we decided to explore the colonic production of EMMPRIN+/MMP23-producing cells in patients with IBD.
Interestingly, we found an increased synthesis of EMMPRIN+/MMP23 in colonic tissue of patients with active UC, predominantly in the areas of the intestinal mucosa, muscular, adventitia and perivascular inflammatory infiltrates in compared with active CD and controls.
Remarkably, our results show a decreased gene expression of MMP23 in CD patients, it is different from MMP10 and EMM. This should be explained by the source of MMP23. Specifically, MMP23 was produced by immune cells such as monocytes/macrophages and endothelial cells in lamina propria from patients with CD.
MMP23 protein was scarcely produced by epithelial cells from the colonic mucosa from IBD patients.
To our knowledge, this is the first evidence of EMMPRIN and MMP23 secretion in intestinal tissue and immune cells, suggesting a role of this poorly characterized metalloproteinase in patients with IBD.
Further studies are required to elucidate the mechanism of action of this MMP in the inflammatory response in IBD.
In the absence of any mechanistic studies, increased expression of EMMPRIN in disease state suggest a pathophysiologic role.
In this context, the role of MMPs in inflammatory process of IBD depends on environment and the degree of severity of the ulcers and they are likely to be significantly different from normal healing after a surgical procedure in terms of cytokine expression, bacterial balance, and medication (eg heavy anti-inflammatory steroid medication).25
Saarialho-Kere et al23 have been shown in vivo, in intestinal ulcers associated with IBD, that stromelysin-2 (MMP10), and matrilysin-1 (MMP7) are expressed by epithelial cells bordering ulcerations, indicating their role in either epithelial closure or basement membrane (BM) remodeling during intestinal wound healing.26
MMP10 is a rational target for investigation in IBD because its expression has been described at healing ulcer edges in human specimens of UC, suggesting a possible role in disease resolution rather than disease progression.27
Koller FL et al., have been shown28 that MMP10 is produced predominantly by infiltrating myeloid cells in both murine and human colitis and this proteinase is required for resolution of DSS-induced colonic damage, and in its absence, chronic inflammation and ultimately dysplasia occurs.
Interestingly, we found an increased gene expression of MMP10 in patients with active CD compared to UC patients and the control group (P = .0001). MMP10 gene expression was associated with the state of inflammation in patients with UC (P = .000, r2 = .585).
Even though, this is a descriptive study, the findings are of interest, as far as we know, it is the first depiction of the association of MMP10 gene expression with histological activity in UC.
According, with our results Dobre et al, showed an upregulation of MMP10 trend in both non-inflamed and inflamed mucosa compared to controls.29
Furthermore, we detected double positive cells of EMMPRIN/ MMP10—producing cells in by epithelial cells, fibroblasts, endothelial cells and lymphocytes in muscularis, adventitia and perivascular inflammatory tissue of patients with active UC compared with active CD and controls.
Additional studies should be performed to illustrate the mechanism of regulation these proteins by inflammation, the regulation of EMMPRIN, MMP10 and MMP23 in the gut mucosal inflammatory process can begin to support the protective role of EMMPRIN in the possible induction of production of MMP10 and MMP23 in the intestinal inflammation and wound healing in IBD.
Moreover, the role EMMPRIN and MMPs by neutrophils in the pathogenesis of IBD remains to be elucidated. It may differ between CD and UC. In UC, unrestricted neutrophil activation may cause significant tissue damage that further leads to chronic pathology, whereas in CD, defective neutrophils and chemotaxis may not be able to limit invasion by microorganisms, leading to subsequent uncontrolled inflammatory reaction.30
Translational studies like this in IBD are important, and there is some evidence that EMMPRIN could play a role in the pathogenesis of murine models of intestinal inflammation, as well as human IBD.
EMMPRIN, MMP10 and MMP23 were up-regulated in patients with active UC as compared to UC remission, CD active or remission and control groups suggesting that these proteins may participate in the repair of the intestinal mucosa.
ACKNOWLEDGEMENTS
We would like to thank all participants to the study without whom this work would have not been possible.
CONFLICT OF INTEREST
The authors declared that they have no competing interests.
AUTHORS' CONTRIBUTIONS
GFC participate during the sample processing, performed RT-qPCR analysis for MMPs transcript level quantification data analysis and prepared the manuscript, critical reviewing and bibliographic analysis. JFC participate during the sample processing, data analysis and prepared the manuscript, critical reviewing and bibliographic analysis. BMB and RBZ participate during the sample processing. JKYF designed and provided the research idea, directed, reassessed both clinical and histological diagnostic, and coordinate the manuscript editing.
ETHICAL APPROVAL
The study was approved by the Ethical and Medical Committee from National Institute of Medical Science and Nutrition Salvador Zubirán. Informed consent was obtained from all subjects. This study was performed according to the principles expressed in the Declaration of Helsinki.
Open Research
DATA AVAILABILITY STATEMENT
Data availability will be provided upon request.