Oral treatment with Aloe polysaccharide ameliorates ovalbumin-induced atopic dermatitis by restoring tight junctions in skin
Abstract
Atopic dermatitis (AD) is a chronic inflammatory skin disease. A hallmark of AD is dry itchy skin that results from defects in the epidermal barrier function. Aloe vera is used widely to promote general health and is administered topically to treat skin conditions such as eczema, burns and wounds. However, effects of A vera on AD were not fully elucidated. In this study, we investigated the oral administration of processed A vera gel (PAG) containing low molecular weight Aloe polysaccharides to treat ovalbumin (OVA)-induced AD in mice. Oral administration of PAG suppressed total and OVA-specific IgE production in sera and decreased the epidermal thickness of skin. Numbers of Ki-67-positive cells were reduced by PAG treatment. Expression levels of tight junction genes, including those that encode ZO-1, Claudin-1 and Claudin-8, were decreased in AD skin lesions, whereas oral administration of PAG partially restored the expression levels of tight junction genes. In addition, IL-4 and IL-17A mRNA transcript levels were reduced in skin lesions after PAG treatment. Taken together, our findings suggest that oral administration of PAG ameliorated AD, normalized tight junction gene expression and suppressed inflammatory cytokines in AD skin.
1 INTRODUCTION
Atopic dermatitis (AD) is a chronic inflammatory skin disorder that results from defects in the epidermal barrier and immune dysfunction. AD affects up to 10% of adults and 30% of children in industrialized countries.1, 2 The complex pathophysiology of AD is known to include genetic risk factors, environmental triggers and dysregulation of innate and adaptive immunity. Patients with AD generally exhibit increased levels of Th2 type cytokines—IL-4, IL-5 and IL-13—during the acute phase of AD and increased levels of Th1 type cytokine IFN-γ during the chronic phase.3, 4 Current understanding of the aetiology of AD is that disruption of the epidermal barrier leads to increased permeability of the epidermis, pathological skin inflammation and percutaneous sensitization to allergens.5 One of the therapeutic approaches aims to maintain or improve epidermal barrier function to ameliorate AD. The skin is composed of two barrier structures, the stratum corneum (SC) and tight junctions (TJs) that provide a physical barrier against dehydration and environmental challenges.5, 6 Mutations in the gene encoding filaggrin and reduced expression of the gene encoding Claudin-1 (CLDN1) in the epidermis are strongly associated with barrier defects in AD.7-9 CLDN1 is a main component of TJs in the epidermis. Mutations or alterations in the CLDN1 gene result in human skin diseases and Cldn1 knockout mice die within one day of birth as a consequence of dehydration.9-12 Thus, most current treatment strategies seek to target specific aspects of skin barrier function or cutaneous inflammation, although successful therapeutic applications of this approach have been limited.
Aloe vera has been used both as a therapeutic and dietary health supplement in the treatment of various diseases.13-16 It is known to have immunoregulatory and skin moisturizing properties.17-19 Notably, A vera polysaccharides have been shown to modulate immunity in both humans and mice.13, 20 Topical application of A vera extract helped treat the AD.21, 22 It is not only used topically, but also has been administered orally via liquid formulations, tablets and capsules.13, 14 However, the effects of orally administered Aloe polysaccharides in AD-related diseases have not been fully elucidated.
The average molecular weight of native polysaccharides in A vera gel is 2000 kDa or higher. We used processed A vera gel (PAG), which has polysaccharides sized 5-400 kDa. PAG can enhance the immunomodulatory activity of the polysaccharide as compared with those isolated from native A vera gel.23 In this study, we evaluated the effects of oral administration of PAG in ovalbumin (OVA)-induced AD mouse model and investigated how these molecules affect skin barrier function, including the genes encoding the tight junction proteins TJP-1, claudin-1 and claudin-8, and cytokines that include IL-4 and IL-17A.
2 MATERIALS AND METHODS
2.1 Animals
7-week-old female Balb/c mice were used for all experiments (Orient). The mice were housed in a specific pathogen-free barrier facility at Inha University. Animal care and all experimental procedures were conducted in accordance with the Guide for Animal Experiments published by the Korean Academy of Medical Science. All animal experiments were approved by the Institutional Animal Care Use Committee (IACUC) at Inha University.
2.2 Preparation of processed A vera gel
Processed A vera gel (PAG) was prepared as previously described and then characterized.23-25 Briefly, A vera gel was reacted with cellulase and then passed through a charcoal column to remove anthraquinones and other coloured substances. Finally, it was subjected to ultrafiltration in an Amicon Stirred Cell (MWCO, 5000 Da; Merck). The yield of PAG from A vera gel is ~0.5%. In order to eliminate the possibility of endotoxin contamination, we removed it using a previously reported method.23 It was confirmed that PAG contained <0.1 ng of endotoxin/mg PAG. In addition, we used a TSKgel G4000 PWxl column (7.8 mm × 30 cm; Tosoh Bioscience) to determine the molecular weight distribution of PAG. As described by Im et al,24 the molecular weight distribution of PAG polysaccharides was found to be much smaller than that of native A vera gel. The molecular weight of most polysaccharides contained in A vera gel was >1000 kDa. PAG used in this study consisted of more than 75% 10-500 kDa polysaccharides. PAG was dissolved in 1× phosphate-buffered saline (PBS) for in vivo experiments and in culture media for in vitro experiments.
2.3 AD induction
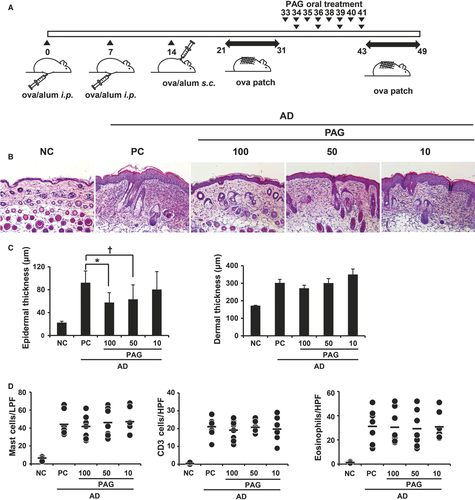
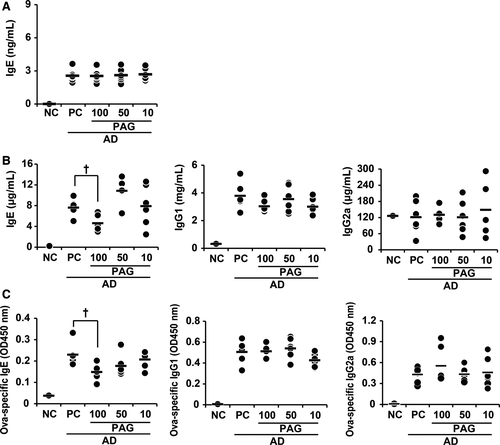
AD was induced as previously described, with some modifications.26, 27 Briefly, eight mice were used per group. The mice in the group except negative control (NC) were intraperitoneally immunized with 10 μg of chicken OVA (grade V; Sigma-Aldrich) mixed with 4 mg aluminium hydroxide (Imject®Alum; Pierce) in a volume of 150 µL on days 0 and 7. The third immunization was administered in the loose skin over the neck on day 14. On day 20, mice were anaesthetized with a mixture of 10 mg/kg zoletil (Virbac Laboratory) and 10 mg/kg xylazine (Bayer Korea Ltd.). During anaesthesia, dorsal skin was shaved with an electric clipper and hair removal creams. After 24 hours, the mice were epicutaneously sensitized with OVA patches. OVA (10 µg) prepared in 50 µL PBS was contained in a 1 × 1 cm patch of sterile gauze, which was placed on the shaved backs of mice and secured to the skin with a transparent dressing (Tegaderm™). The patch was changed daily, and the skin was kept in contact with OVA for 11 days (ie from days 21 to 31). Then, the indicated concentration of PAG (100 mg/kg for 4 times or 50 mg/kg and 10 mg/kg for 8 times) was administered orally to the mice. After PAG treatment, mouse dorsal skin was shaved again on day 42 and the skin was re-exposed to OVA for 7 days (ie from days 43 to 49). To adjust the AD severity in all mice groups, blood samples were harvested on day 32 before PAG treatment and total IgE levels were analysed using ELISA. Mice were grouped according to their IgE levels. IgE levels were similar in all groups (Figure 2A). On day 49, the mice were sacrificed by CO2 asphyxiation. After sacrifice, sera and skin biopsy specimens were obtained for ELISA and histological examination, respectively.
2.4 Histology
Skin tissues from each mouse were filled with 10% neutral-buffered formalin and immersion fixed for 16 h at 4°C. Tissues were dehydrated, embedded in paraffin and sectioned at 5 μm. Mast cells, T cells and eosinophils in the skin were assessed using toluidine blue (Sigma–Aldrich), anti-CD3 antibodies (BD Bioscience), and haematoxylin and eosin (H & E) staining, respectively. Cells were counted from three randomly selected fields per slide using a microscope. Two slides per mouse were examined. The three fields were first identified by scanning the entire slide at ×100 magnification. Positively stained cells were counted in a high-powered field (HPF, ×400). Skin thickness analysis was performed on H & E staining photographs from individual mice skin at ×100 magnification. Dermal thickness was defined as the distance from the epidermal-dermal junction down to the adipose tissue of hypodermis. Epidermal thickness was defined as the distance from the epidermal-dermal junction up to the stratum corneum. Using Image J software (Image Processing and Analysis in Java, NIH), thickness measurements were obtained on light microscopy images by drawing a line to the average orientation. Four thickness measurements were taken per slide. The pixel-based thickness measurement was converted to micrometres. Antigens were detected with anti–Ki-67, claudin-1 (Abcam) and IL-4 (Abbiotec). Antigen-antibody complexes were detected with Alexa594-conjugated secondary antibodies (Molecular Probes). We performed control staining with secondary antibody alone (data not shown). Slides were counterstained with 1 μg/mL 4′,6-diamidino-2-phenylindole (DAPI). Images were acquired using a confocal microscopy (FluoView FV1000). For analysis of claudin-1, fluorescence area analysis was performed on immunofluorescence staining photographs from four mice skins at ×400 magnification. Epidermal area of the skin and positive fluorescence area were selected using the selection tool in ImageJ. Then, percentages of positive area per total epidermal area were calculated. The calculation is following; % of positive staining intensity = expression area/total epidermal area ×100. Ki-67-positive cells were counted at ×400 magnification of the immunofluorescence staining photographs in the basal layer using the selection tool in ImageJ.
2.5 Cytokine assay
To evaluate the effect of PAG on naïve T cells, lymphocytes were isolated from lymph nodes (inguinal, brachial, superficial cervical and axillary) in naïve Balb/c mice. To detect IL-4 cytokines, purified CD4+ T cells (1 × 106) were stimulated with 1 μg/mL of coated anti-CD3 (145-2C11) and soluble anti-CD28 (37.51) antibodies in the presence of PAG in a 24-well plate. To detect IFN-γ and IL-17A, total lymphocytes from lymph nodes were stimulated with 1 μg/mL soluble anti-CD3 and anti-CD28 antibodies. After 48 h of stimulation, cytokine levels were measured by ELISA according to the manufacturer's protocol (BD Bioscience). To evaluate the effect of PAG on antigen-specific T cell response, we first immunized Balb/c mice twice with OVA/alum at days 1 and 7. At day 15, after the 1st immunization, lymphocytes were isolated from the spleen and draining lymph nodes and then cultured with different concentrations of OVA and PAG. After 48 h, IL-4 levels were measured in culture media by ELISA.
2.6 Isolation of RNA and RT-PCR analysis
To evaluate the mRNA levels of tight junction genes, we separated only the epidermis from AD skin lesions. To measure the mRNA levels of cytokines, skin lesions with epidermis and dermis were used. Total RNA was extracted from biopsied skin using easy-BLUETM (iNtRON), and cDNA was prepared using a QuantiTect Reverse Transcription Kit (Qiagen), according to the manufacturer's instructions. cDNA products were amplified using SYBR Green Quantitative PCR Master Mix (Applied Biosystems). The primers (QuantiTect Primer Assays) are all commercially available and validated by QIAGEN. Reactions were carried out in the StepOnePlus™ Real-Time PCR System (Applied Biosystems); the PCR running conditions were as follows: for activating the DNA polymerase, hot start was performed for 10 min at 95°C and then cycling at 95°C for 15 s and 60°C for 1 min, for a total of 40 cycles.
2.7 Statistical analysis
Statistical significance was determined by Student's unpaired t tests. Error bars represent mean ± SEM. Asterisks indicate statistical significance, where P < .05 was used as significance cut-off. Levels of statistical significance are indicated in the figure legends.
3 RESULTS
3.1 PAG ameliorates ovalbumin (OVA)-induced AD in mice
To investigate the effect of PAG on OVA-induced AD, mice were administered 10 μg OVA with aluminium hydroxide on days 0, 7 and 14 (Figure 1A). Epicutaneous sensitization was carried out using patches with 10 μg OVA in 50 μL PBS and comprised two 1-week exposures to OVA. PAG was administered orally between epicutaneous sensitizations. Mice received three different doses of PAG: 100 mg/kg every two days for 4 times or 50 or 10 mg/kg every day for 8 times. Two groups received 400 mg/kg PAG and one group received 80 mg/kg PAG in total. Mice were killed on day 49 after the second epicutaneous sensitization procedure (Figure 1A). Inflammation in skin lesions and immunoglobulin levels in sera were analysed. Histopathological examination revealed an amelioration of epidermal hypertrophy and hyperkeratosis in PAG-treated mice. H & E staining indicated decreased cell infiltration in the skin of PAG-treated mice compared with positive control (PC) mice (Figure 1B, C). Treatment with 100 or 50 mg/kg PAG resulted in decreased epidermal thickness, whereas treatment with 10 mg/kg PAG did not. Furthermore, there were no significant effects of PAG on dermal thickness or number of mast cells, T cells or eosinophils (Figure 1C, D). Serum immunoglobulin levels were measured by ELISA. Total and OVA-specific IgE levels significantly decreased only in the mice treated with 100 mg/kg PAG (Figure 2B, C). IgG1 and IgG2a levels were not affected by PAG treatment.
3.2 PAG inhibits keratinocyte proliferation and restores tight junction expression
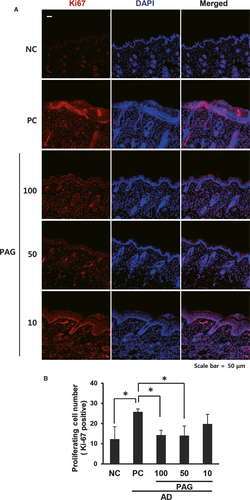
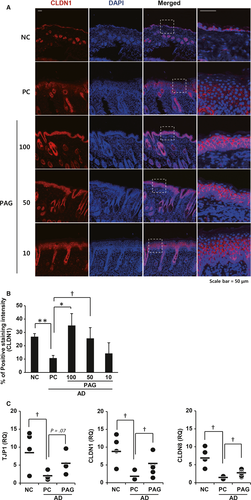
As we observed that treatment with PAG decreased epidermal thickness, we stained the skin tissues with Ki-67, a cell proliferation marker. Consistent with the H & E staining assessments, increased Ki-67 staining was inhibited by PAG in a dose-dependent manner. The least staining was observed in the 100 mg/kg PAG-treated skin, whereas the most staining was observed both in the PC and 10 mg/kg PAG-treated skin. There was more Ki-67 staining in the epidermal field than in the dermal field (Figure 3A). We quantified Ki-67-positive cells and observed decreased cell number in 100 and 50 mg/kg PAG-treated skin compared with the PC mice (Figure 3B).
CLDN1, a major component of TJs in the epidermis, which plays a key role in barrier function, is decreased in the skin of patients with AD.9, 28, 29 Therefore, we stained AD skin with CLDN1 antibodies. Consistent with the results of previous studies, we observed decreased CLDN1 expression in AD skin lesions. CLDN1 expression increased in 100 and 50 mg/kg PAG-treated skin lesions, whereas it decreased in the outer layer compared to the inner layer of 10 mg/kg PAG-treated skin lesions (Figure 4A). We quantified the percent of claudin-1 positive area per total epidermal area. As shown in Figure 4B, treatment of 100 and 50 mg/kg PAG can restore the claudin-1 expression. In addition, we also measured other tight junction gene mRNA levels. Expression of the tight junction components ZO-1 (TJP1), claudin-1 (CLDN1) and claudin-8 (CLDN8) was decreased in AD epidermis compared with normal epidermis. These decreases were partially rescued by treatment with PAG (Figure 4C), suggesting that PAG ameliorates AD through restoration of tight junction gene expression.
3.3 PAG suppresses the production of pro-inflammatory cytokines in AD skin lesions
Inflammatory cytokines are known to be involved in AD. We measured the expression of IFN-γ, IL-4 and IL-17A in AD skin lesions. The mRNA expression of IFN-γ does not significantly change in any group. Consistent with AD symptoms, mRNA levels of IL-4 and IL-17A were increased in AD, and PAG treatment decreased these cytokine levels (Figure 5A). We could also detect lower number of IL-4-positive cells in skin lesions of PAG-treated mice compared with PC mice in a dose-dependent manner (Figure 5B, C). In addition, we investigated whether PAG treatment affected the OVA-specific response. We immunized mice twice with OVA/alum at days 1 and 7. At day 15 after the 1st immunization, lymphocytes were isolated from the spleen and draining lymph nodes and then cultured with different concentration of OVA and PAG. After 48 h, IL-4 levels in the culture media were measured by ELISA. As shown in Figure 5D, we could observe decreased IL-4 expression by PAG treatment in a dose-dependent manner.
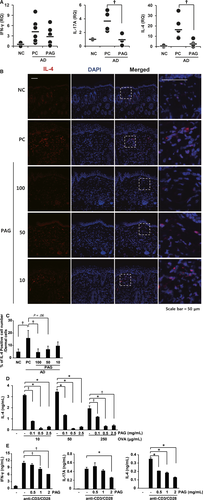
Furthermore, we isolated lymph nodes from naïve mice and total lymphocytes were stimulated with anti-CD3 and anti-CD28 antibodies in the presence or absence of different concentrations of PAG. Cytokines in the culture media were measured by ELISA. IL-4 and IFN-γ levels were significantly suppressed in a dose-dependent manner (Figure 5E). IL-17A significantly decreased after treatment with 2 mg/mL PAG. These results indicated that PAG suppresses the production of AD-related pro-inflammatory cytokines, not only in total T cells but also in ova-specific T cells.
4 DISCUSSION
Aloe polysaccharide, a component of A vera, is reported to have various therapeutic effects, which include antitumor, antiviral and antioxidant activities.23, 30-33 Previous studies have reported the immunomodulatory functions of aloe polysaccharide, which exhibit mitogenic activity by causing the maturation of immature dendritic cells,34 increased macrophage viability against C albicans 35 and enhanced generation of cytotoxic T cells in vitro.36 Aloe extracts also inhibited inflammation in several autoimmune diseases such as streptozotocin-induced diabetes, myelin peptide-induced experimental autoimmune encephalomyelitis and adjuvant-induced arthritis.37-39
The PAG used in this study is characterized by a significantly smaller molecular size (<500 kDa) of polysaccharides than those from native A vera gel (more than 2000 kDa).24 Recently, PAG has been reported to exhibit robust therapeutic or prophylactic effects in various diseases. For example, PAG inhibited colon cancer carcinogenesis by inhibiting chronic inflammation and cell cycle progression.40 PAG also reduced the growth of C albicans in streptozotocin-induced diabetic mice and ameliorated cyclophosphamide-induced immunotoxicity.24, 41 Notably, PAG was effective in the treatment of food allergy.15 Moreover, A vera is known to have skin protection, hydration and wound healing activities. AD is an allergic skin disease. Two key studies have used animal models to study A. vera in AD 21, 22; however, these studies obtained contrasting results. One study that applied A vera gel topically in 2,4 dinitrofluorobenzene-induced AD mice for 10 days reported significant reduction in serum IgE levels and decreased epidermis thickness compared to in the control animals.21 However, a study that evaluated the effects of oral administration of A vera gel extracts on spontaneous AD-like NC/Nga mice 22 showed that six weeks of treatment with 0.8 mg/kg A vera gel extracts resulted in decreased serum IL-5 and IL-10 concentrations, but increased IgE levels compared with those in the control group.22 Oral administration of A vera extracts does not cause an improvement in AD symptoms under the study conditions. Here, we used an OVA-induced AD mouse model in which we orally administered PAG between topical ovalbumin challenges. Thus, we could evaluate sustained treatment effect of PAG. In contrast to the NC/Nga mouse model, we observed a decrease in total and antigen-specific IgE levels only in the sera from 100 mg/kg PAG-treated mice and decreased epidermis thickness both in the 100 and 50 mg/kg PAG-treated mice. This finding indicated that the immunomodulatory functions of A vera varied depending on the dose of PAG or on the mouse models used. We also observed decreased keratinocyte proliferation and IL-4 and IL-17A mRNA transcript production in the skin of PAG-treated mice. It has been reported that hyper-proliferation of keratinocytes is driven by pro-inflammatory cytokines from T cells, such as Th1, Th17 and Th22 cells.42 Particularly in psoriasis, IL-17 plays a role in keratinocyte proliferation and epidermal thickening with inflammatory responses.42 Therefore, we speculated that the decreased epidermal thickness and keratinocyte proliferation were likely a consequence of decreased IL-17-producing cell infiltration into AD skin lesions after PAG treatment. Consistently, we observed decreased IFN-γ, IL-4 and IL-17A production in lymphocytes stimulated with anti-CD3 and anti-CD28 antibodies in vitro. Furthermore, PAG could inhibit OVA-specific IL-4 expression. Therefore, PAG can suppress ongoing immune responses in certain conditions.
In addition, we focused on tight junction genes, which were recently identified to play important roles in skin barrier function in AD. Several studies showed that expression of the genes encoding filaggrin, claudin-1 and claudin-4 decreased and the number of IL-17-producing cells increased in skin specimens from patients with AD.9, 28, 43 One of the major components of barrier dysfunction in AD is CLDN-1. Studies have shown enhanced transepidermal water loss and an AD-like phenotype in CLDN-1-deficient mice.29, 44 Topical application of A vera gel can be used for the healing of dry and sun-damaged skin. However, the effect of A vera gel on TJs has not yet been reported. Here, we showed for the first time that expression levels of the tight junction genes, TJP-1, CLDN1 and CLDN8 were restored after oral treatment with PAG, and CLDN1 protein expression was increased in the 100 and 50 mg/kg PAG-treated AD mice compared with those in the PC mice. Therefore, oral administration of PAG suppressed inflammatory cytokine production and restored the expression of tight junction genes in AD skin lesions.
5 CONCLUSIONS
Our study of the in vivo response to PAG indicated that oral administration of A vera ameliorates AD through the suppression of inflammatory cytokines such as IFN-γ, IL-4 and IL-17A and enhancement of TJs to protect skin barrier function.
ACKNOWLEDGMENT
This study was supported by the National Research Foundation of the Korean government (NRF-2017M3C7A1031108 and 2018R1A2B6005861) and a CAP research grant funded by Univera Inc
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR’S CONTRIBUTIONS
Kwangmin Na participated in the design of the study, laboratory work, data analysis and statistical analysis. Eunju Shin and Chin-Kil Lee contributed to the design of the study and data analysis. Enkhmaa Lkhagva-Yondon, Minha Kim and Yu-Ree Lim participated in the laboratory work. Myung-Shin Jeon participated in the design of the study, analysed the data and wrote the manuscript.