Serum levels of epithelial-derived mediators and interleukin-4/interleukin-13 signaling after bronchial challenge with Dermatophagoides pteronyssinus in patients with allergic asthma
Abstract
Allergens are the main trigger that enhances airway type 2 inflammation, and the epithelium is the first line of defense that reacts to its exposure. Therefore, epithelial-derived mediators, such as interleukin (IL)-25, IL-33, thymic stromal lymphopoietin (TSLP) and ezrin, may play a role as alarmins in IL-4/IL-13 signaling in allergic asthma (AA). We investigated the serum levels of IL-25, IL-33, TSLP, ezrin, IL-4 and IL-13, after bronchial challenge with Dermatophagoides pteronyssinus in patients with AA. We examined 18 subjects: nine steroid-free stable patients with AA sensitized to D. pteronyssinus and nine non-atopic healthy subjects (HS). Bronchial allergen challenge was performed using inhaled D. pteronyssinus allergen. IL-4, IL-13, IL-25, IL-33, TSLP and ezrin levels in serum were measured by ELISA at two time points - before and 24 hours after bronchial allergen challenge. The serum levels of IL-25, TSLP and ezrin did not differ between AA and HS groups at baseline. However, after allergen exposure, significant increases in serum levels of IL-25, TSLP and ezrin were observed only in patients with AA. The serum level of IL-33 at baseline was significantly higher in the AA group compared with HS, but the allergen challenge did not provoke an increase of this cytokine in any group. IL-4 and IL-13 levels were significantly higher at baseline in the AA group compared with HS and, after allergen exposure, were significantly increased in the AA group, with no effect on HS. Thus, the epithelial-derived mediators IL-25, TSLP and ezrin, via IL4/IL13 signaling, enhance type 2 inflammation after bronchial challenge with D. pteronyssinus in AA.
1 INTRODUCTION
Asthma is a common life-long airway inflammatory disease, associated with a high social and economic burden. The most common phenotype of this disease is allergic asthma (AA),1 predominantly caused by urbanization, reduced activity, traffic or industry-related air pollution and increased exposure to allergens.2 An epidemic surge in asthma has been reported in the past 35 years, which is associated with the early uncontrolled asthmatic response to high doses of allergen.3
It is known that the primary molecular mechanism of AA pathophysiology is type 2 inflammation.4, 5 However, it has been observed that even in the case of the predominant type 2 inflammation, the clinical course of the disease is different. Two distinct inflammatory endotypes exist: Th2-low and Th2-high.6 The Th2-high endotype (type 2 asthma) is characterized by the presence of eosinophilic and Th2 airway inflammation, and high titres of antibodies, and correlates with the more complicated course of the disease.6 However, the predecessors initiating the entire immune cascade after contact with the inhaled allergen are not entirely clear.
Asthma is associated with epithelial cell dysfunction. The bronchial epithelium represents the first line of defense and protects the lung by acting as a physicochemical barrier of the submucosa.7 The bronchial epithelium has gained significant attention in recent years as a primary site of disease origin, perpetuation or resolution in lung diseases such as asthma.8 This tissue is also able to mount an inflammatory response releasing mediators following exposure to insulting agents including cytokines.7 Cytokines, such as interleukin (IL) 25, IL-33 and thymic stromal lymphopoietin (TSLP), are thought to be master epithelial-derived mediators of type 2 inflammation in asthma.5, 9 Bronchial epithelial cells release these cytokines, which may affect the epithelium itself, act on immune cells and affect airway remodeling processes and the production of cytokines such as IL-4 and IL-13.10 Additionally, ezrin, as a critical member of the ezrin/radixin/moesin family of proteins, acts at the cytoplasmic surface of cellular membranes by linking plasma membrane proteins to the cortical actin cytoskeleton.8 Therefore, ezrin plays an essential role in the development of bronchial obstruction: it is highly expressed in airway smooth muscle cells and modulates β2-adrenergic receptor signaling and muscle contraction in asthma.8, 11
IL-4 and IL-13 are called prototypical type 2 cytokines, and their signaling is crucial for the development of the allergic condition. IL-4 causes differentiation of naive T helper cells (Th0) into Th2 cells and together with IL-13 induces B cells to produce IgE. Increased IL-4 expression also intensifies eosinophilic inflammation. IL-13 is essential for the differentiation and hyperplasia stimulation of precursor cells, the activation of fibroblasts, an increase of bronchial hyperreactivity and hypersecretion of mucus, as well as being considered the central IgE synthesis regulator. IL-13 has a number of pro- and anti-inflammatory properties and correlates with peripheral blood eosinophil count.12
However, there are no data on epithelial-derived mediators, such as IL-25, IL-33 and TSLP, and ezrin release, and their role as alarmins in IL-4/IL-13 signaling after allergen expose in vivo. For this purpose, we have used bronchial challenge with Dermatophagoides pteronyssinus allergen mimicking an acute episode in AA patients.
2 MATERIALS AND METHODS
2.1 Subjects
The research protocol was approved by the Regional Biomedical Research Ethics Committee of the Lithuanian University of Health Sciences (BE-2-13). The study was registered in the US National Institutes of Health trial registry ClinicalTrials.gov with identifier NCT03388359.
The study included patients with newly diagnosed and steroid-free AA, and healthy subjects (HS) who formed the control group. The participants were men and women between the ages of 18 and 50 years who signed written informed consent. The patients were recruited from the Department of Pulmonology at the Hospital of the Lithuanian University of Health Sciences Kaunas Clinics.
The inclusion criteria for the AA group were newly established and untreated AA, approved with symptoms and medical history more than one year, positive skin prick test to the clinically relevant D. pteronyssinus allergen, and airway hyperresponsiveness to methacholine or a positive bronchial reversibility test. Exclusion criteria included: asthma exacerbation ≤1 month prior to study; clinically significant permanent allergy symptoms; and active airway infection 1 month prior the study.
The HS group was required to have been not atopic and without other chronic respiratory diseases.
The study included non-smokers only.
2.2 Pulmonary function testing
Lung function was evaluated for all study subjects by measuring baseline forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and FEV1/FVC ratio using a Ganshorn spirometer (Ganshorn Medizin Electronic, Germany), and compared with the predicted values matched for age, body height and sex according to the standard methodology. Each of these values was measured three times, and only the highest of three reproducible measurements was recorded.
2.3 Testing bronchial responsiveness
A bronchial responsiveness test was performed for AA patients and healthy individuals. For testing, we used inhaled methacholine via pressure dosimeter (ProvoX, Ganshorn Medizin Electronic, Germany). Aerolized methacholine was inhaled at 2 minutes intervals starting with a 0.0101 mg methacholine dose, increased by steps up to 0.121, 0.511, 1.31 mg of the total cumulative dose, or until there was a 20% decrease in FEV1 from the baseline. The bronchoconstricting effect of each dose of methacholine was expressed as a percentage of decrease in FEV1 from the baseline value. The provocative dose of methacholine causing a ≥20% fall in FEV1 (PD20M) was calculated from the log dose-response curve by linear interpolation of two adjacent data points.
2.4 Bronchial reversibility test
A bronchial reversibility test was performed for one subject from the AA group because testing bronchial responsiveness was not possible due to an observed bronchial obstruction at the screening visit. FEV1 and FVC were registered before and 20 minutes after administration of 400 mcg of salbutamol using a Ganshorn spirometer (Ganshorn Medizin Electronic, Germany). A positive response to a bronchodilator was defined as an increase of ≥12%, and ≥200 mL as an absolute value, compared with baseline in either FEV1 or FVC.
2.5 Bronchial allergen challenge test
The bronchial allergen challenge test was performed for all study subjects. Inhaled D pteronyssinus allergen (DIATER, Spain) was delivered via pressure dosimeter (ProvoX, Ganshorn Medizin Electronic, Germany). The starting point for the assessment of bronchoconstricting effect was 2 minutes after nebulized saline inhalation. Aerosolized allergen was inhaled at 10-min intervals starting with 0.1 histamine equivalent potency (HEP)/mL allergen concentration, increasing by steps up to 1.0, 10.0, 20.0, 40.0 and 60.0 HEP/mL, or a 20% decrease in FEV1 from the baseline was achieved. The bronchoconstricting effect of each dose of allergen was expressed as a percentage of decline in FEV1 from the baseline value. The provocative dose of allergen causing a ≥20% fall in FEV1 (PD20A) was calculated from the log dose-response curve by linear interpolation of two adjacent data points.
2.6 FeNO measurement
All study subjects underwent fractional exhaled nitric oxide (FeNO) analysis with an online method using a single breath exhalation and an electrochemical assay (NIOX VERO®, Circassia, UK), according to guidelines.13 Patients made an inspiration of exhaled nitric oxide-free air via a mouthpiece immediately followed by full exhalation at a constant rate (50 mL/s) for at least 10 seconds. The mean of three readings at the end of the expiration (plateau phase) was taken as the representative value for each measurement. Readings of 25 ppb or more were considered elevated FeNO values.
2.7 Skin prick test
All study subjects were tested for allergies by the skin prick test using standardized allergen extracts (Stallergenes SA, France) for suspected and/or most common allergy-causing substances (D. pteronyssinus, D. farinae, cat and dog dandruff, 5 mixed grass pollen, birch pollen, mugwort allergen). Negative control was performed with temoin, positive control—with histamine hydrochloride (10 mg/mL). Results of the test were evaluated 15 minutes after application. The skin prick test was considered appropriate when the mean wheal diameter was ≥3 mm. Only AA patients with a positive skin prick test to D. pteronyssinus allergen were included in the study.
2.8 Detection of protein level in blood serum
Protein (IL-4, IL-8, IL-13, IL-25, IL-33, ezrin and TSLP) levels in blood serum samples were measured by the enzyme-linked immunosorbent assay (ELISA). ELISA kits used for experiments with a lower limit of detection (LLD) of: IL-4 (R&D Systems, USA)—31.3 pg/mL; IL-8 (R&D Systems, USA)—31.3 pg/mL; IL-13 (R&D Systems, USA)—93.8 pg/mL; IL-25 (R&D Systems, USA)—11.7 pg/mL; IL-33 (R&D Systems, USA)—23.4 pg/mL; TSLP (R&D Systems, USA)—7.8 pg/mL; ezrin (Abbexa Ltd, UK)—0.312 ng/mL. Serum samples of 100 µl were used for experiments. Blood was collected into BD Vacutainer® SSTTM II Advance blood collection tubes and allowed to clot for 30 minutes. After, tubes were centrifuged at 1300 × g for 10 minutes at room temperature to separate serum from clotted blood. Serum was immediately collected and divided into 1-mL cryogenic tubes that were frozen at −80°C for further protein level analysis. ELISA measurements were performed after the sufficient number of samples was collected. The results were expressed as protein concentration per 1 mL of serum. The assay used 96-well plates precoated with primary antibodies against investigated proteins. After a sufficient period of incubation with blood serum or sample standards and appropriate washing off of the excess and unbound materials, the bound analyte was allowed to associate with biotinylated detection antibodies. The wells are washed again, and a streptavidin-HRP (SPP) conjugate was added to the antibody-antigen-antibody complex. After another washing process, a chromogenic substrate 3,3′,5,5′-tetramethylbenzidine (TMB) was used to produce a blue-coloured product with an intensity related to the amount of analyte in the sample. A sulphuric acid solution was added to stop the enzymatic reaction (changing the colour to yellow), and optical density OD was read at 450 nm wavelength (540 nm for wavelength correction) by using a microplate spectrophotometer (Biotek Epoch™, Vermont, USA).
2.9 Statistical analysis
Statistical analysis was performed by using the GraphPad Prism 6 for Windows (ver. 6.05, 2014; GraphPad Software Inc, San Diego, CA). Protein concentration data represented as mean [range]. Significant differences between two independent groups were determined using the Mann-Whitney U test. The Wilcoxon matched-pairs signed rank test was used for dependent groups. P < .05 was considered as statistically significant.
3 RESULTS
3.1 Characteristics of study population
We examined 18 non-smoking adults (6 men and 12 women): 9 steroid-free AA patients and 9 HS. AA group differed by the increased peripheral blood eosinophil count and serum total IgE level, and higher FeNO concentration (Table 1). The atopy component had only AA group patients. Following the allergen exposure, only in AA group was a significant increase in peripheral blood eosinophil count and FeNO concentration.
| AA group patients | Healthy subjects | |||
|---|---|---|---|---|
| Number, n | 9 | 9 | ||
| Sex, M/F | 5/4 | 1/8 | ||
| Age, y | 25 ± 2.1 | 30 ± 2.9 | ||
| Body mass index, kg/m2 | 25.6 ± 2.4 | 22.1 ± 1.3 | ||
| PD20M, mga | 0.09 (0.03-0.26) | NR | ||
| PD20A, HEP/mL | 0.39 (0.15-3.04) | NR | ||
| FEV1, l | 4.0 ± 0.3 | 3.9 ± 0.2 | ||
| FEV1, % of predicted | 96.0 ± 6.7 | 108.8 ± 2.8 | ||
| Maximum fall in FEV1after bronchial allergen challenge, mean % [min-max] | -44.4 [−70.0 – −23.5] | -4.2 [−9.6 - 0.0] | ||
| Total serum IgE, IU/mL | 132.0 ± 27.4b | 35.4 ± 9.3 | ||
| Before allergen challenge | 24 h after allergen challenge | Before allergen challenge | 24 h after allergen challenge | |
| Blood eosinophil count, x109/l | 0.37 ± 0.07b | 0.46 ± 0.05c | 0.16 ± 0.03 | 0.14 ± 0.03 |
| FeNO, ppb | 56.7 ± 14.0b | 79.8 ± 15.0c | 14.7 ± 1.7 | 14.4 ± 1.8 |
Note
- Data presented as mean ± standard error of the mean, except PD20M and PD20A provided as geometric mean (range).
- Abbreviations: AA, allergic asthma; M, male; Ffemale; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1s; HEP, histamine equivalent potency; IgEimmunoglobulin E; NA, not applicable; NR, not responded; PD20M, the provocation dose of methacholine causing a 20% decrease in FEV1; PD20A, the provocation dose of D pteronyssinus allergen causing a 20% decrease in forced expiratory volume in 1 s.
- a Bronchial responsiveness was tested for 8 AA subjects; 1 subject had positive bronchial reversibility test.
- b P < .05 comparing with HS group before allergen challenge.
- c P < .05 comparing with AA group before allergen challenge.
3.2 Serum levels of IL-25, IL-33, TSLP and ezrin
We investigated serum levels of IL-25, IL-33 and TSLP from subjects from AA and HS groups before and 24 hours after bronchial allergen challenge. The level of IL-25 did not differ between the groups at baseline: 37.4 [32.4-46.4] pg/mL in AA vs 37.4 [17.5-55.8] pg/mL in HS. Allergen exposure significantly increased serum IL-25 levels to 63.5 [38.5-139.2] pg/mL only in the AA group (P < .05), whereas there was a slight and not significant difference in the HS group (to 44.6 (18.3-79.2)) (Figure 1A). Serum IL-33 level at baseline in the AA group was significantly higher by almost twofold, 65.7 (28.6-119.3) pg/mL compared with 37.8 (13.0-119.9) pg/mL in HS (P < .05), but the bronchial allergen challenge test did not provoke an increase of IL-33 level in any group: 63.4 (37.4-97.4) pg/mL in AA patients and 38.0 (9.88-95.5) pg/mL in HS (Figure 1B). At the baseline, TSLP level did not differ between the AA and HS groups (311.7 (216.9-581.7) vs 319.1 (179.7-435.4) pg/mL); however, a significant difference was observed after allergen exposure in the AA group: the TSLP level increased to 383.5 (261.0-773.3) pg/mL (P < .05) without changes in HS (Figure 1C).
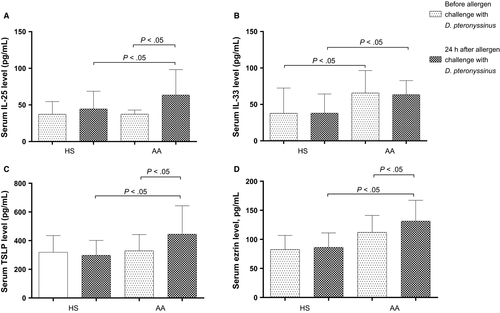
The serum ezrin level did not differ between AA and HS groups at baseline—112.1 (77.0-167.7) vs 82.7 (61.2-126.3) pg/mL—but after allergen exposure significantly increased to 131.3 (95.6-185.8) pg/mL only in the AA group (P < .05), while significant changes were not observed in HS (86.2 (47.0-116.4) pg/mL) (Figure 1D).
3.3 Serum levels of IL-4 and IL-13
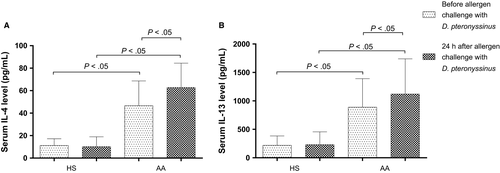
IL-4 levels were significantly higher at baseline in the AA group compared with HS—46.7 (12.7-92.7) vs 11.3 (0.4-16.4) pg/mL—and after the bronchial allergen challenge, the AA group significantly increased to 62.8 (35.2-104.6) pg/mL (P < .05), with no differences in the HS group (10.2 (3.6-28.7) pg/mL) (Figure 2A). IL-13 distinguished at its highest level and reached 889.1 (276.0-1624.0) pg/mL at the baseline visit and 1121.0 (413.5-2141.0) pg/mL after exposure of allergen (P < .05) without significant changes in the HS group (218.5 (11.0-491.0) pg/mL and 230.6 (11.0-553.5) pg/mL, respectively) (Figure 2B).
4 DISCUSSION
In this study, we investigated changes in the serum levels of IL-25, IL-33, TSLP and ezrin—considered epithelial-derived biological active substances—and IL-4 and IL-13—named central type 2 inflammatory cytokines—after bronchial challenge with D. pteronyssinus in patients with AA. A significant increase in IL-25, TSLP and ezrin serum levels was found after allergen exposure, which is associated with early bronchial epithelial damage. IL-33 did not respond to the allergen challenge, but its serum level at baseline in the AA group was significantly higher by almost twofold compared with HS. IL4/IL13 signaling—higher serum levels of IL-4 and IL-13—was enhanced in AA subjects, especially following allergen challenge.
AA is a heterogeneous airway disease. Although the basis of pathogenesis is airway type 2 inflammation, there are multiple pathogenetic features.14 The growing need for a better understanding of the disease pathogenesis leads to in-depth studies of different stages of the ongoing immune process. The bronchial epithelium has continuous contact with environmental agents—in the case of AA, with allergens—so, for some time, attention has been focused on the analysis of its immunomodulatory functions, especially on initiating and maintaining airway type 2 inflammation. Despite emerging evidence for the key role for the epithelial-derived mediators in the pathogenesis of asthma,15 there is a paucity of studies on them in the context of allergen exposure. The advantage of this study is that an acute AA episode was provoked by allergen expose in vivo, and analysis of a number of cytokines related with type 2 inflammation gives a better understanding of AA pathogenesis.
IL-25, IL-33, TSLP and ezrin are increasingly being recognized to play crucial role in the pathophysiology of allergic diseases. 8-10, 16-20 All the bioactive substances in AA cases are mainly produced by epithelial cells and are increasingly called alarmins.20-22 Existing evidence suggests associations between these cytokines and allergic diseases, but the mechanisms by which these cytokines influence the allergic immune responses and airway type 2 inflammation have only recently begun to be revealed. It was observed an increase in IL-25, IL-33 and TSLP immunoreactivity in the bronchial epithelium and submucosa followed allergen exposure and a correlation with the level of late-phase bronchial obstruction was found. This affirms these molecules as potential targets for the inhibition of allergen-induced airway inflammation.23
IL-25 plays a crucial role in promoting the recruitment and proinflammatory function of eosinophils in AA,22 mucus over-secretion and remodeling in the airway.24 Recent studies with AA subjects also demonstrated an increased serum level of IL-25 as a response to inhaled allergen.22, 25, 26 Our data re-emphasize and complement these results. We also found a significantly increased serum IL-25 level 24 hours after allergen challenge. In contrast to the studies that highlighted the importance of IL-25 to boost the ongoing internal immune cascade by enhancing eosinophilic inflammation and modulating dendritic cells (DCs) function in AA, we appreciated the potential effect of epithelial-derived IL-25 to initiate a type 2 immune response. We presume that the initial rise of the serum IL-25 level occurs due to an increase in its secretion from damaged epithelial cells themselves after allergen exposure, which initiates eosinophilic inflammation with a further increase in serum IL-25 level.
IL-33 is an epithelial-derived cytokine and an essential mediator of type 2 inflammation, promoting bronchial hyperresponsiveness in AA. It is released to alert the immune system by first-line cells, such as tissue epithelial cells, following exposure to exogenous stimuli, including allergens.27 Importantly, high levels of serum IL-33 and IL-33 receptor genetic polymorphism were found in patients with multiple sclerosis, suggesting a relevant role of this cytokine in the pathogenesis of autoimmune disorders.28 However, our asthma patients were without history of autoimmune diseases that could influence the results. IL-33 data are controversial in recent asthma studies. On the one hand, the bronchial epithelium is a crucial IL-33 reservoir in the lung, and its expression is elevated in asthma.16 This is also confirmed by a larger scale study when a higher level of IL-33 was detected in AA patients compared with HS, determining IL-33 correlation with disease severity in the AA group.29 On the other hand, it was observed that inhaled allergen did not increase the IL-33 receptor on bone marrow DCs, although it is known that IL-33 contributes to DCs network activation.30 We found that the IL-33 serum level was twice as high as in HS and did not increase significantly after allergen challenge. Why the IL-33 level does not increase after allergen exposure remains a controversial issue. Although bronchial epithelial, smooth muscle and mast cells have been identified as key sources, the principal origin of IL-33 in allergic disease is not fully known.31, 32 One assumption may be made that elevated levels of soluble ST2 receptors for IL-33 in AA suggest localized production of this soluble form of ST2 within the airways that may act as an inhibitor of IL-33-induced inflammation.31
TSLP is produced in response to proinflammatory stimuli and drives allergic inflammatory reactions through its activity on many innate immune cells.33 TSLP is thought to cause airway and blood eosinophilia among patients with AA by activating airway DCs and by increasing the numbers of Th2 cells, resulting in the production of proinflammatory cytokines, including IL-5 and IL-13.34 Our study showed that the TSLP level did not differ between AA and HS groups; however, after allergen exposure, it significantly increased only in patients with AA. Previous studies have demonstrated the higher TSLP level in allergic and non-allergic asthmatics compared with HS.23, 35, 36 Therefore, an inverse correlation of TSLP protein expression in bronchial biopsies with the severity of bronchial obstruction was found.35 However, our study subjects were mild asthmatic and had normal lung function, which could explain the absence of TSLP difference between HS and AA. Further, it was shown that anti-TSLP after the allergen challenge in patients with stable AA attenuated allergen-induced bronchoconstriction, and decreased markers of systemic and airway inflammation, blood and sputum eosinophilia and FeNO level.33 This reveals TSLP as an important epithelial-cell–derived cytokine in AA.
Ezrin is involved in anchoring different cell-surface receptors to the cytoskeleton and can participate in asthma pathogenesis by affecting bronchial epithelium repair, T lymphocyte regulation and the contraction of the airway smooth muscle cells.18 The role of ezrin in the pathogenesis of asthma is only beginning to be explored. A study of asthmatic patients measuring the ezrin level in exhaled breath condensate and serum was carried out, finding that the levels of ezrin were decreased in the AA group compared with HS.19 However, the change in the concentration of ezrin in acute AA remains uncertain. We found an increased ezrin level at 24 hours after the bronchial allergen challenge. This can be explained by the fact that an acute episode of AA alters the function of epithelial cells and contributes to bronchial smooth muscle structural changes, which results in more biologically active substances being released (ezrin is excreted mainly by the epithelial cells and partly by the smooth muscles cells via exosomes).19, 37 Although a negative correlation between ezrin and IL-13 levels in serum has been reported,19 it should be noted that in that study, AA patients were already treated with inhaled steroids, in contrast to our study where AA patients were steroid-free. While it is known that inhaled steroids could affect cytokine levels and suppress airway inflammation, data could not be found specifically on whether the level of the ezrin can be influenced. It is also known that IL-13 can affect the release of exosomes, whose major source in the lungs of asthmatic patients is bronchial epithelial cells.38 We suppose that the initial increase in ezrin was determined by the direct effect of the allergen on the bronchial epithelium, and the initiated immune cascade led to a rise in IL-13 level, which contributed to the further increase of ezrin.
Aberrant production of the prototypical type 2 cytokines, IL-4 and IL-13, has long been associated with the pathogenesis of allergic disorders.39 IL-13 and IL-4 share the same receptor and signaling pathways, and both are involved in eosinophil activation, IgE synthesis, mucus production, airway remodeling and innate cell recruitment to sites of inflammation. IL-4/IL-13 signaling plays a critical role in orchestrating the recruitment and activation of effector cells of the asthmatic response and driving the pathophysiology of AA.40, 41 The data we have obtained in our study overlap existing results,40, 42-48 showing that serum levels of these cytokines increase after allergen challenge, and demonstrating aggravation of allergic inflammation. This, therefore, strengthens the potential link between epithelial-derived cytokine levels and the intensity of airway inflammation. We found significant differences in both cytokine levels in AA and HS groups, and the allergen challenge triggered an even greater increase in them. This reveals the importance of IL-4/IL-13 signaling in airway type 2 inflammation during AA.
Although the treatment of asthma is moving forward with the onset of the biological therapy era, leading to greater opportunities for individualized treatment, identification of new molecular targets and biomarkers reflecting them is needed. Currently, assessment of airway type 2 inflammation is related with validated biomarkers of eosinophilic airway inflammation, such as blood eosinophil count, sputum eosinophilia, FeNO, serum periostin and IgE.49-51 We also checked blood eosinophil count and FeNO, and observed that following the allergen exposure, these biomarkers only exhibited a significant increase in the AA group, without changes in HS. However, type 2 inflammation is associated with a whole range of type 2 inflammatory cytokines, especially epithelial-derived, directly involved in the development of the immune response. According to our study data, epithelial-derived mediators, such as IL-25, IL-33, TSLP and ezrin, as well as prototypical cytokines IL-4 and IL-13 of type 2 airway inflammation, could be molecular targets, while IL-25, TSLP, ezrin, IL-4 and IL-13 could act as the potential biomarkers in AA.
There are some limitations to the study, namely, the small size of the studied population and gender disproportion. Most of the subjects were women, but to date, it has not been determined whether IL-4, IL-8, IL-13, IL-25, IL-33, ezrin or TSLP differ by gender. Moreover, the results obtained should not be distorted as the gender distribution in the asthma group was proportional. As the design of the study was focused on epithelial-derived mediators initiating airway type 2 inflammation and biomarkers in acute AA, a limitation could be the lack of evaluation of their level at subsequent time points.
5 CONCLUSION
In this study, we found that the epithelial-derived mediators IL-25, TSLP and ezrin are involved in early-onset airway inflammation in acute AA. Enhanced IL4/IL13 signaling demonstrates that epithelial-derived mediators IL-25, TSLP and ezrin could be alarmins, initiating airway type 2 inflammation in AA. Therefore, the increased serum levels of IL-25, TSLP and ezrin after contact with a specific allergen indicate that these bioactive substances could be the potential biomarkers of bronchial epithelial dysfunction, whereas IL-4 and IL-13 indicate type 2 inflammation activity in AA. All these findings may be useful for the search for new therapeutic targets for the treatment of asthma.
ACKNOWLEDGMENT
We are grateful to Airidas Rimkunas, Beatrice Tamasauskaite for their help with laboratory experiments of all participants.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHORS CONTRIBUTIONS
Kestutis Malakauskas, Virginija Kalinauskaite-Zukauske, Andrius Januskevicius and Ieva Janulaityte conceived and designed the experiments. Virginija Kalinauskaite-Zukauske, Ieva Janulaityte and Andrius Januskevicius performed the experiments. Kestutis Malakauskas, Virginija Kalinauskaite-Zukauske, Andrius Januskevicius and Ieva Janulaityte analysed the experimental data. Virginija Kalinauskaite-Zukauske take care of patients and analysed clinical data. Ieva Janulaityte, Andrius Januskevicius and Virginija Kalinauskaite-Zukauske contributed reagents/materials/analysis tools. Kestutis Malakauskas, Virginija Kalinauskaite-Zukauske, Andrius Januskevicius and Ieva Janulaityte revised the manuscript for intellectual content. All authors have read and approved the final manuscript.