Determination of cell expansion and surface molecule expression on anti-CD3/28 expanded CD4+ T cells
Abstract
CD4+ T cell immunotherapy has potential for treatment in HIV-infected patients. A large number of expanded CD4+ T cells and confirmation of functional-related phenotypes are required for ensuring the successful outcomes of treatment. Freshly isolated CD4+ T cells from healthy donors were activated with anti-CD3/28-coated magnetic beads at different bead-to-cell ratios and cultured in the absence and presence of IL-2 supplementation for 3 weeks. Fold expansion, cell viability, growth kinetic and lymphocyte subset identities were determined. Data demonstrated that a 1:1 bead-to-cell ratio rendered the highest expansion of 1044-fold with 88% viability and 99.5% purity followed by the 2:1 and 0.5:1 ratios. No significant difference in proliferation and phenotypes was found between non–IL-2 and IL-2 supplementation groups. Several specific surface molecule expressions of the expanded cells including chemokine receptors, adhesion molecules, co-stimulatory molecules, activation molecules, maturation markers, cytokine receptors and other molecules were altered when compared to the unexpanded cells. This optimized expansion protocol using the 1:1 bead-to-cell ratio of anti-CD3/28-coated magnetic beads and culture condition without IL-2 supplementation provided the satisfactory yield with good reproducibility. Specific surface molecule expressions of the expanded cells presented potential roles in proliferation, differentiation, homeostasis, apoptosis and organ homing.
1 INTRODUCTION
Human immunodeficiency virus (HIV) infection causes a progressive decrease of CD4+ T lymphocytes and an increase of HIV viral load (or HIV RNA level), leading to higher susceptibility to opportunistic infections which can further develop to acquired immune deficiency syndrome.1 HIV enters target cells through the binding of viral envelope glycoproteins to CD4 receptors along with CCR5 and CXCR4 coreceptors markedly expressed on the target CD4+ T lymphocytes.2-4 Although highly active antiretroviral therapy (HAART) succeeds to control the HIV viral load into an undetectable level and recovers the CD4 counts in HIV-infected patients, the latent reservoir of virus still exists5 and the immune restoration is incomplete.6-9 A life-long treatment of HAART has also feasible consequences in cumulative drug toxicities, emergent drug-resistant viruses and unaffordable costs due to more complicated regimens. Moreover, some patients who have discordant immune responses to HAART fail to achieve target CD4 count levels despite accomplished virological control, suggesting a higher risk in mortality.10
An alternative approach, such as adoptive transfer of autologous activated CD4+ T lymphocytes, has been proposed to be a potential treatment for the benefit of both virological control and direct immune reconstitution. Its effectiveness and safety have been confirmed by in vivo studies in both simian deficiency virus-infected rhesus macaques and HIV-infected patients.11-15 To expand CD4+ T cells in vitro, anti-CD3/28-coated magnetic beads are widely used for stimulation. The anti-CD3/28-activated CD4+ T cells showed intrinsic resistance to macrophage (M)-tropic isolates of HIV-1 infection16-18 and promoted expression of RANTES, MIP-1α and MIP-1β as well as reduced expression of CCR5.11, 13, 14, 16 Furthermore, the expanded CD4+ T cells induced interferon (IFN)-γ production which is associated to type 1T helper (Th1) cell function and increased the density of variable beta (Vβ) chain T cell receptor (TCR) repertoires14 together with telomerase activity, resulting in a longer survival of the cells.11
With respect to the clinical uses, a large number of CD4+ T cells expanded in vitro was required for reinfusion in HIV-infected patients14, 15; therefore, optimization of expansion protocols is warranted. There have been established in vitro culture methods for anti-CD3/28-stimulated CD4+ T lymphocytes providing different yields19-21 which can be related to different cell isolation methods, bead-to-cell ratios used for stimulation and medium supplementation. More importantly, functional-associated phenotypic characters of the expanded cells are essential which are not only related to cell characterization but also maturation and activation stages as well as cell migration. Even so, there is limited information concerning specific cell surface molecule expressions of the expanded CD4+ T cells, such as chemokine receptors and maturation markers.
This study thus purposed to investigate the optimum bead-to-cell ratios and supplementation used for CD4+ T cell expansion as well as to explore the whole series of surface molecule expressions of the expanded cells including chemokine receptors, adhesion molecules, co-stimulatory molecules, activation molecules, maturation markers, cytokine receptors and other functional-specific molecules.
2 MATERIALS AND METHODS
2.1 Sample collection
Three healthy volunteers aged 26-30 years were enrolled in this study. The protocol was approved by the Institutional Review Board of the Faculty of Medicine Siriraj Hospital, Mahidol University, Thailand. Written informed consent was obtained from each subject prior to sample collection.
2.2 Characterization of lymphocyte subsets and specific surface molecule expressions
Monoclonal antibodies (mAbs) and their conjugated fluorochromes were obtained from Becton Dickinson Biosciences (BDB) and used at the concentrations recommended by the manufacturer. The fluorescent-labelled mAbs used for phenotypic characterization of the cells were anti-CD3 conjugated with fluorescein isothiocyanate (FITC), anti-CD4 conjugated with phycoerythrin (PE), anti-CD19 PE, anti-CD45 conjugated with peridinin chlorophyll protein (PerCP), anti-CD8 conjugated with allophycocyanin (APC), anti-CD16 APC and anti-CD56 APC. The fluorescent-labelled mAbs used for identification of specific surface molecule expression were anti-CD4 PerCP, anti-CD3 FITC, anti-CD45RO FITC, anti-CD45RA FITC, anti-CD57 FITC, anti-CD27 FITC, anti-CCR7 PE, anti-CD62L PE, anti-CD11a PE, anti-CD11b PE, anti-CD11c PE, anti-CD126 PE, anti-CD127 PE, anti-CD95 PE, anti-CD95L PE, anti-CD154 (CD40L) PE, anti-CD40 PE, anti-CD134 (OX40) PE, anti-CD278 (ICOS) PE, anti-CD71 PE, anti-HLA-DR PE, anti-GITR PE, anti-CD28 PE, anti-CD103 PE, anti-CD38 PE, anti-CD69 PE, anti-CD25 PE, anti-CD184 (CXCR4) PE, anti-CD183 (CXCR3) PE, anti-CCR10 PE, anti-CD195 (CCR5) PE, anti-PD-1 PE, anti-CXCR5 PE, anti-CCR6 PE, anti-CCR4 PE and anti-α4β7 PE.
2.3 CD4+ T lymphocyte isolation using immunorosettes formation method
CD4+ T lymphocytes can be directly isolated from whole blood by an immunorosettes formation method using RosetteSep® human CD4+ T cell enrichment cocktail (STEMCELL Technologies). Briefly, CD4+ T lymphocytes were isolated from 5 mL of whole blood by adding 250 µL of RosetteSep® human CD4+ T cell enrichment cocktail. After that, the samples were thoroughly mixed and incubated at room temperature for 20 minutes. The samples were then diluted with an equal volume of phosphate-buffered saline (PBS) containing 2% foetal bovine serum (FBS) and gently mixed. The diluted blood samples were carefully layered on top of LSM® lymphocyte separation medium and centrifuged at 1200 g with no break at room temperature for 20 minutes. After centrifugal separation, the samples were divided into four layers including plasma, enriched CD4+ T cells, LSM® lymphocyte separation medium and red blood cells (from top to bottom). Pasteur pipettes were used to remove the plasma layer and collect enriched CD4+ T cells from the layer interface. The collected CD4+ T cells were then washed with 10 mL of PBS containing 2% FBS and centrifuged twice at 450 g at room temperature for 5 minutes. The cell pellets were collected and re-suspended with a complete medium (RPMI1640 with 10% FBS, 50 µg/mL penicillin-streptomycin and 2 mmol/L l-glutamine). Cell number and viability of the enriched CD4+ T cells were determined by trypan blue exclusion using a haemacytometer.
2.4 Expansion of isolated CD4+ T lymphocytes using anti-CD3/28-coated beads
The isolated CD4+ T cells from the immunorosettes formation method were stimulated with anti-CD3/28-coated beads. Dynabeads® human T-activators (Invitrogen Dynal) were used in this study. The expansion procedures of CD4+ T lymphocytes were divided into two steps including bead washing for elimination of preservatives and cell activation. The bead number was calculated for 0.5:1, 1:1 and 2:1 bead-to-cell ratios to use for the expansion. Anti-CD3/CD28-coated beads with calculated amounts were then transferred into the tube and washed with 2 mL PBS to an original volume of beads. After that, the tube was placed on the magnet for 1 minute in order to remove the washing solvent. The washed beads remaining in the tube were re-suspended in the complete medium with an equal volume to the initial volume of beads.
For bead-to-cell ratio comparison, 1 × 106 enriched CD4+ T cells were stimulated with anti-CD3/CD28-coated beads in the absence of exogenous interleukin (IL)-2. The stimulated CD4+ T cells were then expanded in the complete medium at a concentration of 0.5 × 106 cells/mL and incubated at 37°C and 5% CO2 humidification before reactivation on day 7 at the same bead-to-cell ratio. The cells were expanded for a total of 3-week culture period, and the medium was replenished with calculated amounts of fresh media on days 4, 7, 11, 14 and 17 to maintain the cell suspension concentration at 0.5 × 106 cells/mL before transferring to appropriate culture vessels. Cell numbers and viability were observed on days 4, 7, 11, 14, 17 and 21 by using trypan blue exclusion and a haematocytometer. A fold expansion number was calculated by using the viable cell number at each indicated time point divided by the viable cell number at the beginning of cell expansion. Lymphocyte subset characters were analysed by a flow cytometer on days 0, 14 and 21.
With respect to IL-2 supplementation comparison, the similar activation and culture protocols were conducted by using only the 1:1 bead-to-cell ratio and cultures in the absence and in the presence of exogenous interleukin (IL)-2 at the low concentration of 20 units/mL (ProSpec). Cell numbers and viability were observed on days 4, 7, 11, 14, 17 and 21 by using trypan blue exclusion and a haematocytometer. Cell culture medium for both cultures with and without IL-2 supplementation was replenished on days 4, 7, 11, 14 and 17 to maintain the cell suspension concentration at 0.5 × 106 cells/mL. Lymphocyte subset characters were analysed by a flow cytometer on days 0, 14 and 21.
2.5 Immunofluorescent staining
Whole blood, purified and expanded CD4+ T cells were stained with fluorochrome-conjugated mAbs and incubated for 15 minutes. In addition, 1X FACSTM lysing solution (BDB) for red blood cell lysis was added only for the whole blood sample and purified CD4+ T cells on the blood collection day (day 0). The samples were incubated at room temperature for 15 minutes before centrifugation and removal of red cell lysis supernatant. The stained cells were then washed with PBS containing 2% FBS prior to centrifugation at 1400 rpm, 25°C for 5 minutes. Subsequently, the stained cells were re-suspended in PBS containing 1% paraformaldehyde. The stained cells were finally acquired by a BD FACSCalibur flow cytometer (BDB), and the data were analysed by using FlowJo Software (Tree Star).
A 5-(and 6)-Carboxyfluorescein succinimidyl ester (CFSE) assay was used to demonstrate the proliferation of CD4+ T cells after anti-CD3/28 stimulation. 1 × 107 purified CD4+ T cells were labelled with 5 μm of CFSE (BioLegend). The stained cells were then washed twice in PBS with 5% FBS and set in parallel cultures including unstimulation and anti-CD3/28 bead stimulation. Proliferation was kinetically evaluated via dilution of CFSE over several days of culture on a FACS Calibur flow cytometer (BDB). Data from at least 20 000 cells gated on the CD4+ T cell population were analysed by FlowJo software (Tree Star).
For determination of cell viability by using a viability dye, 1 × 106 expanded cells were stained with Zombie NIR™ dye (BioLegend) at a dilution of 1:1000 at 4°C for 15 minutes. The stained cells were washed with PBS containing 2% FBS and re-suspended in PBS containing 1% paraformaldehyde before sample acquisition by a BD FortessaTM flow cytometer (BDB), and the data were analysed by using FlowJo Software (Tree Star). Viable cells were determined from the cell population without fluorescent signal in a APC-Cy7 fluorescent detector.
For intracellular cytokine staining (ICS), expanded CD4+ T cells at 1 × 106 cells/mL were stimulated with 25 ng phorbol 12-myristate 13-acetate (PMA) and 1 μg ionomycin (I) in the presence of brefeldin A (BFA) at 10 μg. The samples were then incubated at 37°C/5% CO2 for 4 hours. After incubation, stimulated cells were stained with Zombie NIR™ dye (BioLegend) at 4°C for 15 minutes. A washing buffer (PBS with 2% FBS) were added, and the samples were washed by centrifugation at 450 g for 5 minutes. The samples were then surface stained with a combination of mAbs including anti-CD3 A700, anti-CD4 BV605, anti-CD8 PE/Dazzle™ 594 and anti-CD69 PerCP/Cy5.5 (all reagents were obtained from BioLegend) at 4°C for 15 minutes and washed once. The stained samples were fixed and permeabilized in 0.5 mL of BD Cytofix/Cytoperm™ Fixation and Permeabilization Solution (BDB) at 4°C for 20 minutes. After incubation, the samples were washed by adding 1X BD Perm/Wash™ Buffer (BDB) and centrifuged at 500 g for 5 minutes. ICS was performed by staining with a combination of mAbs including anti-IL-2 BV510, anti-IL-4 FITC, anti-IL-17 PE, anti-IFN-γ APC, anti-TNF-α BV650 and anti-TGF-β BV421 (all reagents were obtained from BioLegend, except anti-IL-4 was obtained from BDB) at 4°C for 30 minutes. After staining, the samples were washed with 1X BD Perm/Wash™ Buffer and re-suspended in PBS. The stained cells of at least 100 000 events were acquired for each analysis by a BD Fortessa™ flow cytometer (BDB), and the data were analysed by using FlowJo Software (Tree Star). The cytokine-producing cell subsets were determined from activated populations expressing CD69.
2.6 Flow cytometric analysis
Six-parameter analysis including forward scatter (FSC), side scatter (SSC), FITC, PE, PerCP and APC was performed using FlowJo Software (Tree Star). The stained cells were gated using lymphogate (FSC/SSC) to determine a viable lymphocyte population. After that, lymphocyte subsets were defined using two-dimensional dot plots between CD45/SSC, CD45/CD3 and CD4/CD8 or CD19/CD16+CD56. Therefore, the lymphocyte subsets were detected into CD4+ T cells, CD8+ T cells, double-positive T cells, CD16+CD56+ NK cells and CD19+ B cell populations. For detection of specific cell surface molecule, CD4+ T cells were identified on a two-dimensional dot plots between CD4 and SSC as well as the percentage of specific cell surface molecule expression was determined on a histogram plot based on a marker gate which was identified by using a fluorochrome-conjugated isotype control antibody.
2.7 Statistical analysis
All statistical analyses were performed using GraphPad Prism® version 7.02 (GraphPad Software Inc). Data sets were expressed as mean ± standard deviation (SD) and compared for statistical significance at P-value ≤ .05 with two-way ANOVA followed by Bonferroni's multiple comparisons test.
3 RESULTS
3.1 Bead-to-cell ratio comparison for anti-CD3/28 CD4+ T cell expansion
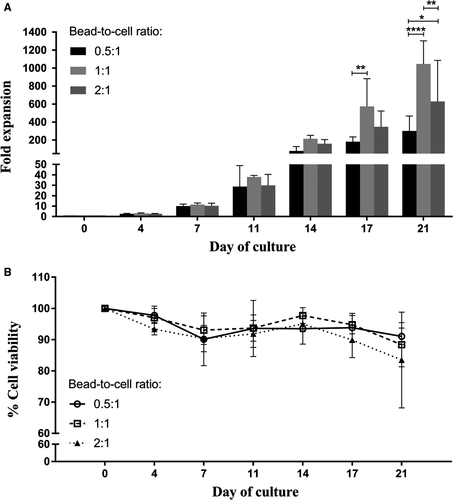
To achieve satisfactory yields of the expanded cells, it is important to determine the optimum bead-to-cell ratio used for stimulation. In this study, three healthy volunteers were recruited for blood collection. Isolated CD4+ T cells were activated with anti-CD3/28-coated magnetic beads at different bead-to-cell ratios (ie 0.5:1, 1:1 and 2:1) and cultured in the absence of IL-2 for 21 days. Fold expansion, cell viability, growth kinetic and phenotypic characters were observed for proliferation efficiency of the expanded CD4+ T cells on days 0, 4, 7, 11, 14, 17 and 21.
There was no difference in fold expansion among three different bead-to-cell ratios during the first 14 days of culture; however, the fold expansion number of CD4+ T cells expanded with the 1:1 bead-to-cell ratio on day 17 showed remarkably higher than the others (Figure 1A). On day 21 of culture, it was obvious that stimulation with the 1:1 bead-to-cell ratio provided the highest yield of the anti-CD3/28-expanded CD4+ T cells followed by the 2:1 and 0.5 bead-to-cell ratios (1044 ± 259-, 629 ± 457- and 301 ± 167-fold, respectively). Cell viabilities of the expanded cells from the three different ratios were comparable with over 90% throughout the 3-week culture period (Figure 1B). Slight decreases in viable cells were observed at the end of the culture for the 1:1 and 2:1 ratios (88 ± 7% and 83 ± 15%, respectively).
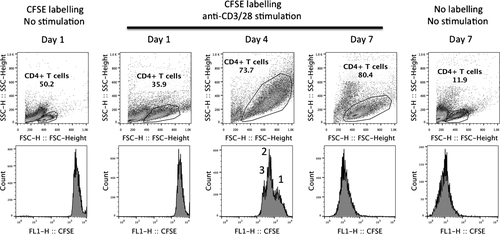
Proliferation based on a decrease in CFSE fluorescent intensity was also determined on 1 representative sample with anti-CD3/28 stimulation at 1:1 bead-to-cell ratio. As shown in Figure 2, while a blast transformation was observed on a cell scattered pattern starting on day 1 after cell stimulation, a decrease in CFSE fluorescent intensity began on day 4 of cell stimulation with a high degree of blast transformation. The result also showed mix populations of non-divided cells (ie the highest CFSE intensity observed on the first histogram peak on the far right) and cells with 1-2 division cycles (ie the second and third peaks, respectively). More importantly, a polyclonal expansion of CD4+ T cells was observed after 7 days of anti-CD3/28 stimulation as shown by a decrease in CFSE intensity to a level similar to unlabelled cells.
Lymphocyte subset characters of the anti-CD3/28-stimulated CD4+ T cells were analysed by a flow cytometer (Table 1). It was clearly demonstrated that the major population of the expanded cells was CD3+ T cells (>99% of lymphocytes) with the dominant subset of CD4+ T cells for all bead-to-cell ratio groups over the culture period (>98% of lymphocytes). The purity of the expanded cells was also confirmed with low frequencies of CD8+ T cells, double-positive T cells, CD19+ B cells and CD16+CD56+ NK cells (<3.5% of expanded cells).
| Cell population | Day of culture | Bead-to-cell ratio | ||
|---|---|---|---|---|
| 0.5:1 | 1:1 | 2:1 | ||
| CD3+ | 0 | 99.3 ± 0.4 | 99.3 ± 0.4 | 98.8 ± 1.2 |
| T cells | 14 | 99.8 ± 0.2 | 99.9 ± 0.1 | 99.9 ± 0.1 |
| 21 | 99.6 ± 0.5 | 99.9 ± 0.1 | 99.9 ± 0.2 | |
| CD3+CD4+CD8− | 0 | 97.8 ± 1.3 | 98.1 ± 1.4 | 97.5 ± 1.7 |
| CD4+ T cells | 14 | 99.1 ± 0.6 | 99.8 ± 0.1 | 99.7 ± 0.1 |
| 21 | 98.7 ± 1.5 | 99.5 ± 0.3 | 99.3 ± 0.4 | |
| CD3+CD4−CD8+ | 0 | 0.0 | 0.0 | 0.0 |
| CD8+ T cells | 14 | 0.0 ± 0.1 | 0.0 | 0.0 |
| 21 | 0.0 | 0.0 | 0.0 | |
| CD3+CD4+CD8+ | 0 | 0.0 | 0.0 | 0.0 |
| Double-positive | 14 | 0.0 | 0.0 | 0.0 |
| T cells | 21 | 0.0 ± 0.1 | 0.0 ± 0.1 | 0.0 |
| Othersa | 0 | 0.9 ± 0.5b | 0.7 ± 0.4b | 1.1 ± 0.9b |
| 14 | 1.7 ± 0.4b | 3.5 ± 1.5b | 2.4 ± 0.3b | |
| 21 | 1.3 ± 0.7b | 1.3 ± 0.4b | 1.2 ± 0.4b | |
- a Other cell populations include CD19+ B cells, CD16+CD56+ NK cells, CD3+CD16+CD56+ and CD3+CD19+.
- b A sum of average percentages of frequencies of the other cell populations.
3.2 Effects of IL-2 supplementation on cell expansion
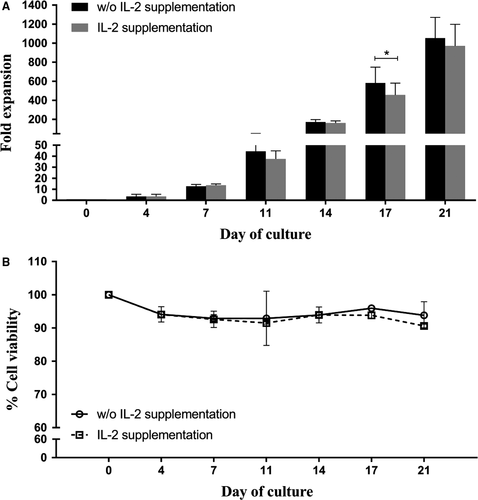
IL-2 supplementation has been generally used to promote cell proliferation in addition to the autocrine/paracrine IL-2 production by the activated T cells. High concentrations of IL-2 (100 and 300 IU/mL) have been reported to predominantly affect CD8+ T cell development,22 whereas the low concentration of IL-2 (20 IU/mL) was used for CD4+ T cell expansion.15, 21 This study, therefore, used a low concentration of IL-2 at 20 IU/mL to support cell expansion and compared this expansion effect of IL-2 with the autocrine/paracrine IL-2 production (ie cell culture in the absence of IL-2). Fold expansion, cell viability, growth kinetic and lymphocyte subset characters were observed for proliferation efficiency of the expanded CD4+ T cells on days 0, 4, 7, 11, 14, 17 and 21.
Data showed that fold expansion numbers between the culture without and with IL-2 supplementation were similar throughout the 21-day culture period (Figure 3A). Only the expanded cells cultured in the absence of IL-2 supplementation on day 17 proliferated significantly higher than those in the presence of IL-2 supplement (582 ± 166- and 455 ± 125-fold, respectively). At the end of the culture, there was no significant difference in proliferation between the two culture groups. With respect to cell viability, the cultures without or with IL-2 supplementation maintained great numbers of viable cells with over 90% throughout the 3-week culture period (Figure 3B).
Predominant phenotypes of the expanded cells from both culture groups were CD3+ T cells (>97% of expanded cells) with the major CD3+CD4+CD8− subset (>94% of expanded cells) as presented in Table 2. The minor cell populations including CD3+CD4-CD8+, CD3+CD4+CD8+, CD3-CD19+, CD3-CD16+CD56+, CD3+CD16+CD56+ and CD3+CD19+ were also found with very low frequencies (<2% of expanded cells), suggesting a specific expansion of purified CD4+ T cells.
| Cell population | Day of culture | Supplementation | |
|---|---|---|---|
| w/o IL-2 | IL-2 | ||
| CD3+ | 0 | 99.5 ± 0.2 | 99.2 ± 0.7 |
| T cells | 14 | 96.7 ± 5.7 | 99.8 ± 0.1 |
| 21 | 99.9 ± 0.1 | 99.9 ± 0.0 | |
| CD3+CD4+CD8− | 0 | 98.3 ± 0.6 | 97.9 ± 1.7 |
| CD4+ T cells | 14 | 94.4 ± 5.2 | 99.6 ± 0.1 |
| 21 | 99.2 ± 0.1 | 99.0 ± 0.9 | |
| CD3+CD4−CD8+ | 0 | 0.0 | 0.0 |
| CD8+ T cells | 14 | 0.0 | 0.0 |
| 21 | 0.1 ± 0.1 | 0.0 | |
| CD3+CD4+CD8+ | 0 | 0.0 | 0.0 |
| Double-positive | 14 | 1.4 ± 2.4 | 0.1 ± 0.1 |
| T cells | 21 | 0.0 | 0.0 |
| Othersa | 0 | 0.7 ± 0.4b | 0.8 ± 0.6b |
| 14 | 2.0 ± 1.0b | 1.9 ± 0.6b | |
| 21 | 1.3 ± 0.4b | 0.6 ± 0.4b | |
- a Other cell populations include CD19+ B cells, CD16+CD56+ NK cells, CD3+CD16+CD56+ and CD3+CD19+.
- b A sum of average percentages of frequencies of the other cell populations.
Since high viabilities were observed in the expanded cell cultures without IL-2 supplementation, additional cell expansions were conducted on three different samples and the viability results at the end of 3-week culture period were confirmed by using the viability dye. As shown in Figure 4A, high frequencies of viable cells were observed with an average frequency of 90.1%. Cytokine production ability of these expanded cells was also determined on 1 representative sample by using ICS, and a cytokine-producing profile was showed in Figure 4B. While the majority was cells producing TNF-α, the results also showed that 95.5% of these expanded cells expressed IL-2, suggesting an autocrine function of IL-2 on these expanded cells.
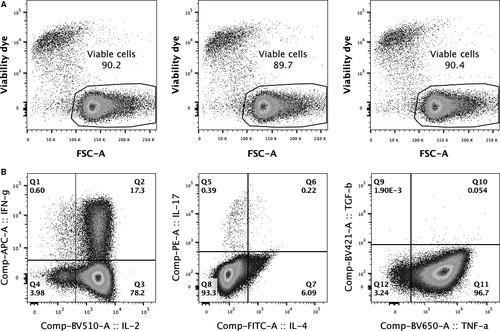
3.3 Specific cell surface molecule expressions of anti-CD3/28 CD4+ T cells
As surface molecule expressions are related to cell maturation, activities and functions, this study thus explored several cell surface molecule expressions of anti-CD3/28-expanded CD4+ T cells which were divided into seven groups according to the molecules’ functions and roles. The cell surface molecules included (a) chemokine receptors: CCR4, CCR5, CCR6, CCR7, CCR10, CXCR3, CXCR4 and CXCR5; (b) adhesion molecules: CD11a, CD11b, CD11c, CD103 and α4β7; (c) co-stimulatory molecules: CD27, CD28, CD40, CD40L, CD134, PD-1 and ICOS; (d) activation molecules: CD25, CD38, CD69, CD71 and HLA-DR; (e) maturation markers: CD45RO, CD45RA and CD62L; (f) cytokine receptors: CD126 and CD127; and (g) other molecules: CD57, CD95, CD95L and GITR. Frequencies of the expanded cells expressing these surface molecules in the cultures without and with IL-2 supplementation on day 21 were observed and compared with those of unexpanded cells on day 0 as a baseline control. Representative histograms of selected molecules were presented in Figure 5.
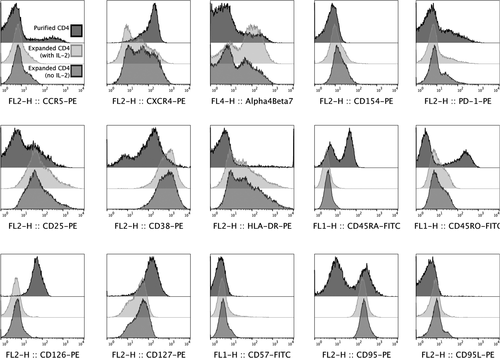
For chemokine receptors, the expanded cells from cultures whether IL-2 supplementation or not exhibited significant lower expressions of CCR6, CCR7 and CXCR4 and dramatic higher expression of CXCR3 when compared to the unexpanded cells as seen in Figure 6A. Other molecules, CCR4, CCR5, CCR10, CXCR5, remained similar after the expansion. While a low level of CCR5 expressing cells was observed, expression kinetic showed transient downregulation with re-expression at the end of the culture period (Figure 1). Low levels of CCR5 expression on expanded cells were also confirmed by using different clones of anti-CCR5 mAbs (Figure 2). The expanded cells also had marked increases in expressions of adhesion molecules, CD11b, CD11c and α4β7 when compared to the unexpanded cells (Figure 6B). There was also no change in expressions of CD11a and CD103 between the expanded and unexpanded cells.
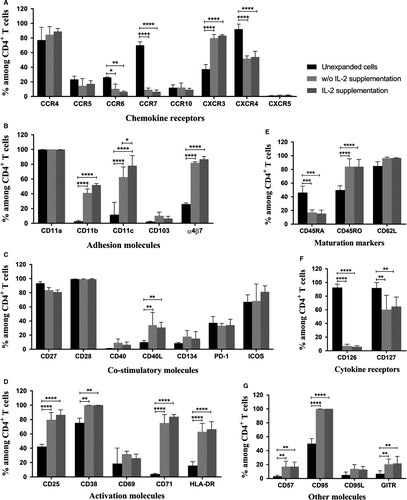
With respect to co-stimulatory molecules, only frequencies of CD40L of the expanded cells from both expansion groups were significantly increased when compared to the unexpanded cells (Figure 6C). All other molecules including CD27, CD28, CD40, CD134, PD-1 and ICOS remained unchanged after the expansion. Furthermore, expressions of all activation molecules, except CD69, on the expanded cells were significantly upregulated when compared to those of the unexpanded cells (Figure 6D). The expanded cells also showed significant lower frequencies of CD45RA and higher frequencies of CD45RO, while their CD62L expression was similar to the unexpanded cells (Figure 6E).
The expanded cells also exhibited notable downregulation of cytokine receptor, CD126, and upregulation in CD127 when compared to the unexpanded cells (Figure 6F). Expressions of other molecules including CD57, CD95 and GITR were dramatically raised after the expansion, whereas there were slight increases in CD95L (Figure 6G). Moreover, no significant difference in numbers of any chemokine receptors, adhesion molecules, co-stimulatory molecules, activation molecules, maturation markers, cytokine receptors and other molecules was found between the two culture groups with and without IL-2 supplementation (Figure 6A-G).
4 DISCUSSION
In this report, we have described effects of bead-to-cell ratio of anti-CD3/28-coated magnetic beads for stimulation and IL-2 supplementation on the growth of expanded CD4+ T cells as well as their specific surface molecule expression changes after the expansion. Ones of the important concerns are quantity of anti-CD3/28-coated magnetic beads used for expansion and medium supplementation. Levine et al20 used immobilized anti-CD3/28 coated beads at 1-3 beads/cell for a long-term proliferation of polyclonal CD4+ T cells from normal donors. The expanded cells were able to maintain the exponential growth of 2.3 × 105-fold over 40 days of culture without exogenous cytokines or feeder cells added to the culture. However, the fold expansion was dramatically increased to 109- to 1011-fold when supplemented with IL-2 at the late culture (~ days 28-106).20 The previous method was also implemented for a large-scale production of CD4+ T cells from HIV-infected patients by using anti-CD3/28-coated beads at 3 beads/cell for stimulation and the culture method with IL-2 supplementation. The cell numbers were increased by 37-fold after a 14-day culture.21 Later, our previous study showed that CD4+ T cell stimulation with anti-CD3/28-coated magnetic beads to a 1:1 bead-to-cell ratio and culture in the absence of IL-2 were able to prolong the cell expansion throughout a 3-week culture with the good yield of 1000-fold.19 During the cell harvest, the higher number of beads used for expansion, the higher number of beads remaining in the final products which leads to the bead accumulation in the body after the cell reinfusion tentatively causing toxicity. It is then warranted to compromise between the bead quantity for the highest activation efficiency and the treatment safety for patients.
We then compared different bead-to-cell ratios (ie 0.5:1, 1:1, and 2:1) to minimize the number of beads used for expansion without sacrificing their promotion for the suitable yield. Our data showed that the 1:1 bead-to-cell ratio provided the highest fold expansion (1044 ± 259-fold) which was 1.7 and 3.5 times greater than the 2:1 and 0.5:1 ratios, respectively. Hence, cell stimulation at the 1:1 bead-to-cell ratio was sufficient to achieve the satisfactory yield with good viability (88 ± 7%) and high purity (>98% of lymphocytes) within the reasonable time (ie a 3-week culture). Autocrine cytokines produced from the expanded cells themselves were also adequate to supply the proliferation throughout the culture period. This was confirmed by our comparison between the cultures without and with IL-2 supplementation. No significant difference in fold expansion and cell viability was found between the two groups.
Changes in specific cell surface molecule expressions of the anti-CD3/28 CD4+ T cells were also observed in this study. Several chemokine receptors have been identified as coreceptors for the HIV entry, such as CCR5, CXCR4, CCR4, CCR6 and CCR10. The in vitro expanded CD4+ T cells with anti-CD3/28 activation were proved to be resistant to HIV-1 infection via the reduction in frequencies and densities of CCR5 molecules.18, 23 Our expanded cells also rendered a low frequency in CCR5 (<15% of CD4+ T cells) which presumably maintain at this low level because the recovery of CCR5 expression was low when activation with anti-CD3/28-coated beads compared to stimulation with anti-CD3/28 immobilized on the surface of a tissue culture plate.23 Our anti-CD3/28 activation protocol also rendered the expanded CD4+ T cells with twice as less CXCR4 expression than the unexpanded cells. Although a high number of the expanded cells still expressed CXCR4 (52% of CD4+ T cells), the chance of viral entry when switching coreceptor usage from CCR5 to CXCR424 will feasibly diminished when compared to the unexpanded cells.
CCR4, CCR6 and CCR10 were also reported to be other HIV-1 coreceptors of primary HIV-1 isolates.25-27 Our expanded cells showed that CCR4 was highly expressed throughout the expansion period (84% of CD4+ T cells), whereas a significant downregulation in CCR6 by 2.5-fold and low frequency of CCR10 (12% of CD4+ T cells) were found. Although CCR4 expression was high, soluble viral protein gp120 had greater affinity to CCR5 than CCR4.27 CCR4+ T cells are also able to be defined as Th2 cells, while Th1 cells are classified by CXCR3+.28 In our study, the frequency of CXCR3+ T cells was twofold higher than that of the unexpanded cells (~83% of CD4+ T cells) which was almost equal to CCR4+ T cells. However, we cannot specify that our CXCR3+ T cells are absolutely purified Th1 cells as they need to be further characterized with expressions of CCR4−, CCR5+ and CXCR6+.28 These evidences clearly demonstrate that our expanded cells are possibly suitable for reinfusion due to their potential ability for HIV-1 resistance in vivo.
As the gut compartment is a major portal for HIV entry, the considerable depletion in mucosal CD4+ T cells was observed during acute infection.29 Our study shows that the expanded cells had a preferential increase in α4β7 expression to 82% of CD4+ T cells. In contrary, the level of another gut mucosal-specific adhesion molecule, CD103,30 was low at the end of the culture (10% of CD4+ T cells) even though it was fivefold higher expressed in the expanded cells. It is then worth proposing that these cells feasibly migrate to the site of depletion and improve immune response at the gut-associated lymphoid tissues.
Other surface adhesion molecules on T lymphocytes are β2 integrins (CD11/CD18 family) including CD11a, CD11b and CD11c. These molecules facilitate the T cell engagement with target cells and endothelial cells. CD11a is generally highly expressed in CD4+ T cells, whereas CD11b and CD11c are more prevalent in CD8+ T cells.31 Our results affirm this evidence as the unexpanded CD4+ T cells exhibited high expression of CD11a (100% of CD4+ T cells) and low expressions of CD11b and CD11c (3% and 12% of CD4+ T cells, respectively). Interestingly, after a 3-week culture, the expression of CD11a on the expanded cells remained high. Results also showed significant upregulation in CD11b (14.6-fold) and CD11c (5.3-fold). These expanded cells with the notable high CD11a expression are suitable for survival due to ability for T cell localization to areas rich in cytokines for promoting their homeostasis.32
With respect to co-stimulatory molecules, a high frequency of CD28 in our study remained unchanged during the expansion (99% of CD4+ T cells). This is in agreement with the previous report showing the constitutive expression of CD28 on CD4+ T cells,33 suggesting that the function for an effective antigen-specific immune response is still active. However, our results are in contrary to another study reporting that its expression was transiently down-regulated following T cell activation and progressively declined due to in vitro senescence.34 For CD40 expression, it is not surprisingly to find its low level on the expanded CD4+ T cells (9% of CD4+ T cells) as they are commonly identified and functionally characterized on B cells.35 On the other hand, CD40L is only expressed on activated CD4+ T cells35 which is in accordance with our results presenting a significantly increased CD40L expression by 3.4-fold, proposing that the expanded CD4+ T cells can probably promote isotype switching, maturation and survival of B cells.36
We also found that the expression of CD134 (OX40) was slightly increased after the expansion (18% of CD4+ T cells), possibly promoting survival of T cell numbers and accumulation of developed memory CD4+ T cells over time.37 For ICOS, it was highly expressed and comparable to that of the unexpanded cells (68% of CD4+ T cells), even though this ICOS has to be de novo induced on the T cell surface 33 and augmented by CD28 co-stimulation together with TCR engagement.38 It was also reported to be matching CD28 in potency and enhancing all basic T cell responses33 as well as T-cell–dependent B cell help.39 Overall, all co-stimulatory molecules of the anti-CD3/28 CD4+ T cells were endurable over the expansion period. Only CD40L was markedly higher expressed in the expanded cells. It is then suggested that these in vitro expanded cells with consistent co-stimulatory molecules will be able to have normal proliferation and differentiation in vivo when reinfusion to HIV-infected patients in which some co-stimulatory molecules are dysregulated following the disease progression.
Expression kinetics of the CD25, CD69, CD38, CD71 and HLA-DR were previously described.40, 41 The results showed that while CD25 and CD69 expression on CD4+ T cells reached over 90% at 24 hours after soluble anti-CD3/28 stimulation, whereas CD38 and CD71 reached their maximum levels at 72 hours.41 Our activation molecules including CD25, CD38, CD71 and HLA-DR were also dramatically increased (80%, 100%, 75% and 63% of CD4+ T cells, respectively), while CD69 was slightly increased to 32% of CD4+ T cells. It is then indicating that our expanded cells expressed full ranges of activation molecules in both early- (ie CD25 and CD69) and late-activation markers (ie CD38, CD71 and HLA-DR). Interestingly, the previous study also demonstrated that CD25 expression maintained at high level, whereas CD69 was significantly dropped to ~50% after 72 hours.41 The low level of CD69 expression on our expanded cells revealed similar expression kinetics since the data were observed 2 weeks after re-stimulation.
Maturation stage of CD4+ T cell subsets including naïve, effector, effector memory and central memory cells is generally based on expression of CD45RA with CD62L or CD45RA with CCR7.42 We found that the unexpanded cells had equal numbers of naïve (CD4+CD45RA+) and memory (CD4+CD45RO+) subsets with ~50% of CD4+ T cells. After a 3-week culture, CD45RA+ cells were notably declined by ~2.7-fold, whereas CD45RO+ cells were twofold increased (84% of CD4+ T cells). Furthermore, there were contradict expressions between CD62L and CCR7 which were supposed to be correlated each other. CD62L remained highly expressed (97% of CD4+ T cells), whereas CCR7 was prominently decreased to 9% of CD4+ T cells. However, this study had a limitation in T cell subset characterization due to those molecules were not determined simultaneously. Therefore, we cannot precisely specify T cell subsets. Even so, our results can assume that the expanded cells were probably in the transition towards memory cells either central memory (CD45RA−CCR62L+ or CD45RA−CCR7+) or effector memory (CD45RA−CCR62L− or CD45RA−CCR7−) cells. A great number of CD27+ T cells (84% of CD4+ T cells) were also found, indicating that the cells have not been differentiated into effector cells which finally lose this CD27 expression.43
Additionally, the expression of CXCR5 on CD4+ T cells indicates a distinct memory T cell subset with B cell helper function (ie follicular B helper T cells [TFH]).44 Our data exhibited a very low frequency of CXCR5+ T cells (1.7% of CD4+ T cells), revealing that our expanded cells are not TFH. A low frequency of GITR+ cells designated as regulatory T (Treg) cells was also significantly increased but still <20% of CD4+ T cells. This small augmented population of Treg may help preventing an aberrant HIV-induced chronic T cell hyperactivation, leading to retardation of disease progression.45 Therefore, it is then suggested that our expanded cells may pose a regulatory function.
According to cytokine receptors, CD126 expression was markedly low in our expanded CD4+ T cells compared with the unexpanded cells (7% vs 93% of CD4+ T cells). This downregulation might be resulted from TCR cross-linking in vitro, indicating a non-naïve stage of T cell differentiation.46 This supports our cell maturation stage discussed previously. Our study also shows that CD127 was markedly decreased over the expansion by 1.5-fold (from 92% to 60% of CD4+ T cells). The reduced expression possibly cause from prolonged CD127 suppression via TCR stimulation.47
During the expansion, activation-induced cell death of CD4+ T lymphocytes can occur via Fas-dependent apoptosis by triggering Fas (CD95) with its ligand (FasL or CD95L).48 We found that the expanded cells expressing CD95+ were significantly increased to 100% of CD4+ T cells, whereas CD95L+ cells remained at low level (14% of CD4+ T cells). The results in this study showed high cell viabilities even though high CD95 expression was observed, suggesting that the engagement of CD95 and CD95L on the same cell is limited due to low numbers of CD95L expressing cells. It is then assumed that the apoptosis following cell-to-cell contact between CD95+ and CD95L+ CD4+ T cells will be limited as well. We also investigated the expression of CD57 which is associated to functions in termination of cell differentiation and submission to apoptosis.49 A significant increase in CD57+ cells was found, but the level was low at 17% of CD4+ T cells, indicating some of the expanded cells may be in the exhaustion stage. However, the expression of PD-1 which is also a critical mediator for T cell exhaustion50 was not changed during the expansion (33% of CD4+ T cells). Our expanded cells thus possibly retain normal function in proliferation.
Moreover, a low concentration of IL-2 supplementation did not affect surface molecule expressions of any chemokine receptors, adhesion molecules, co-stimulatory molecules, activation molecules, maturation markers, cytokine receptors and other molecules of the expanded cells. These findings then support the previous experiments from Onlamoon et al.19
From the time when autologous transfusion of anti-CD3/28-expanded CD4+ T cells was proposed as an alternative treatment strategy for HIV-infected patients, only limited numbers of studies were conducted. Although the question regarding a long-term risk has never been addressed, no adverse event was observed in both animal models and HIV-infected patients.12, 14, 15 However, the stage of viral resistance occurs as a result of the downregulation of CCR5 expression on anti-CD3/28-expanded cells. These cells were thus still susceptible to viral strain using CXCR4 as its coreceptor.16-18 Interestingly, a study showed that CCR5 inactivation may trigger HIV envelope mutations that shift viral receptor usage from CCR5 to CXCR4.51 Therefore, transfusion of anti-CD3/28-expanded CD4+ T cells may drive the occurrence of CXCR4 usage virus. Moreover, since CXCR4-binding virus can be detected in more than 50% of patients with severe immunodeficiency,52 a careful selection of patients to exclude those with the presence of viral strain using CXCR4 should be considered before starting the treatment.
While an original application of anti-CD3/28-expanded CD4+ T cells was intended to be used for autologous transfusion in HIV-infected patients, a development of new antiretroviral drugs allows an effective treatment due to the ability to select and use a variety of drugs for a treatment as well as using them in a highly effective combination. However, since 10%-27% of patients treated with HAART showed stable or low CD4 levels after the treatment (ie referred as immunological discordant patients),53, 54 an alternative treatment such as autologous transfusion of anti-CD3/28-expanded CD4+ T cells may provide a beneficial role in this group of patients by increasing the level of CD4 count. More importantly, a current development in chimeric antigen receptor (CAR) T cell application may also get an advantage from the expansion protocol demonstrated in this study since recent studies showed that a defined ratio of CD4 to CD8 in a CAR T cell product can enhance the effectiveness of the treatment.55, 56 A large-scale expansion of CD4+ T cells can be applied for an expansion of CAR CD4+ T cells during the manufacturing process.
This study demonstrated that the 1:1 bead-to-cell ratio of anti-CD3/28-coated magnetic beads for CD4+ T cell expansion was the most optimum bead quantity to achieve the satisfactory yield of the expanded cells. The autocrine cytokines, mainly IL-2, produced by the expanded cells themselves are also adequate for a 3-week proliferation without additional IL-2 supplementation. After the expansion, phenotypic profiles of the expanded cells were changed. The expanded cells likely become more resistant to HIV-1 via downregulation of dominant coreceptors for HIV entry, CCR5 and CXCR4, as well as migrate to the site of depletion and improve immune response at the gut-associated lymphoid tissues due to higher expressions of gut-homing molecules, α4β7 integrin. Furthermore, other specific surface molecule expressions related to activation, proliferation, differentiation, homeostasis and apoptosis revealed certain functions of the expanded cells. It is thus worth suggesting that these expanded cells following our optimized protocol could be suitable for CD4+ T cell immunotherapy used in HIV-infected patients, even though further investigation on CD4+ T cells from HIV-infected patients and a large-scale production are required.
5 ETHICS STATEMENT
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable standards. The study was approved by the Institutional Review Board of the Faculty of Medicine Siriraj Hospital at Mahidol University. Informed consent was obtained from all individual participants recruited in the study.
6 ACKNOWLEDGMENT
This work was supported by the Faculty of Medicine Siriraj Hospital, Mahidol University. PA is sponsored by Chulalongkorn University Centernary Academic Development Project. The authors gratefully thank all volunteers who donated their blood for this study.
7 CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
8 AUTHOR CONTRIBUTIONS
PT performed the experiment, data analysis and manuscript writing; PP, VT and PA: performed the experiment and data analysis; NO involved in research idea formation, research monitoring and manuscript editing. All authors read and approved the final manuscript.