Functional immune response to influenza H1N1 in children and adults after live attenuated influenza virus vaccination
Abstract
Influenza virus is a major respiratory pathogen, and vaccination is the main method of prophylaxis. In 2012, the trivalent live attenuated influenza vaccine (LAIV) was licensed in Europe for use in children. Vaccine-induced antibodies directed against the main viral surface glycoproteins, haemagglutinin (HA) and neuraminidase (NA) play important roles in limiting virus infection. The objective of this study was to dissect the influenza-specific antibody responses in children and adults, and T cell responses in children induced after LAIV immunization to the A/H1N1 virus. Blood samples were collected pre- and at 28 and 56 days post-vaccination from 20 children and 20 adults. No increase in micro-neutralization (MN) antibodies against A/H1N1 was observed after vaccination. A/H1N1 stalk-specific neutralizing and NA-inhibiting (NI) antibodies were boosted in children after LAIV. Interferon γ-producing T cells increased significantly in children, and antibody-dependent cellular-mediated cytotoxic (ADCC) cell activity increased slightly in children after vaccination, although this change was not significant. The results indicate that the NI assay is more sensitive to qualitative changes in serum antibodies after LAIV. There was a considerable difference in the immune response in children and adults after vaccination, which may be related to priming and previous influenza history. Our findings warrant further studies for evaluating LAIV vaccination immunogenicity.
1 INTRODUCTION
Influenza virus is a major respiratory pathogen causing annual epidemics leading globally to approximately 3-5 million severe infections, with 300 000-650 000 estimated deaths.1 Influenza is a vaccine preventable disease, and two main types of vaccines are available, the inactivated influenza vaccine (IIV) and the live attenuated influenza vaccine (LAIV). In 2012, the LAIV was licensed in Europe for use in children 2-17 years old, but not for adults. Trivalent live attenuated influenza vaccine (LAIV) has previously been shown to provide broader protection against influenza A infection.2-4 LAIV mimics natural infection and both the innate and the adaptive immune responses are activated after LAIV.5-7
The influenza virus has two major surface glycoproteins: the haemagglutinin (HA) and neuraminidase (NA), and antibodies directed towards both glycoproteins play an important role in protection against influenza virus. The haemagglutinin comprises an immunodominant head domain (composed of the majority of the HA1 subunit) and a stalk domain (mainly the HA2 subunit and the N- and C-terminal ends of HA1). Antibodies to the HA head inhibit viral attachment to the host cell receptors and can be measured by the haemagglutination inhibition (HI) assay. HI antibodies are measured as a surrogate correlate of protection8-10 with an HI titre of 40 providing a 50% protective threshold in adults. The micro-neutralization (MN) assay also measures mainly HA head-specific antibodies that can neutralize the influenza virus.11 The HA head-specific antibodies do not reflect the whole spectrum of protective antibodies, and the HA stalk-specific antibodies can also confer protection.12 The stalk domain is conserved and provides broad cross-protection by neutralizing antibody and through antibody-dependent cellular-mediated cytotoxicity (ADCC) by interaction of antibodies and FcγR on natural killer cells.13-16 The haemagglutinins of the influenza A viruses are divided into groups based on their amino acid sequence of mainly the head region, and the more conserved stalk region may be a promising target for universal vaccine development. A recent animal model study identified broadly protective anti-HA stalk antibodies that were induced after LAIV vaccine expressing chimeric HA.17 Furthermore, neuraminidase inhibition (NI) antibodies can also provide protection from influenza.18 NA is an important viral surface glycoprotein with enzymatic activity cleaving sialic acid on the lumen of mucosal epithelial cells of the respiratory tract. NA is important in the infection process, clearing a path for infection, lowering the pH at the cell surface and releasing of progeny virus from infected cells. Blocking NA activity is an effective way to inhibit infection and viral shedding.19, 20
During the 2014-2015 season, the USA experienced unexpectedly low protection from the H1Npdm09 strain in the LAIV, which was not observed with IIV. This led the US Advisory Committee on Immunization Practices (ACIP) to withdraw its earlier preferential recommendation of LAIV in children. However, moderate protection was observed to the H1N1 strain in Europe, with 41.5% vaccine effectiveness in the UK and 47.9% in Finland, although remaining lower than IIV.21, 22 A study in Senegal found that LAIV failed to protect against H1N1pdm09 in young children,23 whereas protection was found in a similar study in Bangladesh.24 The reason for these differences is currently unknown but could be due to the vaccines were being based on different backbones, or the population's exposure history.25, 26 The LAIV manufacturer reported that the A/H1N1 strain had a temperature sensitive mutation rendering it heat instable providing a possible explanation for the reduced protection observed, and has since updated the vaccine.27 Importantly, exposure history to influenza will influence protection and differences in vaccine recommendations could possibly affect protection observed after LAIV. The USA recommends influenza vaccination for everybody from >6 months, whilst the European vaccine recommendations primarily focus on risk groups.
In our previous study, we found no increase in HI antibodies to the H1N1 strain after LAIV; however, most children had pre-existing antibodies.28 However, a slight trend of rising H1N1 stalk-specific antibodies was observed in children after LAIV, whereas adults had already higher levels of H1-stalk-specific IgG antibodies prevaccination and no further increase was observed after vaccination. We suggested that this was due to pre-existing antibodies present after vaccination or infection during the 2009 pandemic. The aim of this study was to further dissect the antibody responses, focusing on the functional immune response after LAIV immunization against H1N1.
2 MATERIALS AND METHODS
2.1 Patients and samples
Forty subjects (20 children [3-17 years old] and 20 adults [21-59 years old]) were intranasally immunized with 0.1 mL per nostril of the seasonal LAIV (Fluenz; AstraZeneca). LAIV (Fluenz) contained 107 fluorescent focus units (FFU) of A/California/7/2009(H1N1)pdm09-like and A/Victoria/361/2011(H3N2)-like strains in both 2012/2013 and 2013/2014 seasons, and either B/Wisconsin/1/2010-like or B/Massachusetts/2/2012-like influenza B virus strains in the 2012/2013 or 2013/2014 seasons, respectively. The study was approved by the ethical and regulatory authorities (EUDRACT2012-002848-24, www.clinicaltrials.gov; NCT01866540). Children received one (≥9 years old, n = 6) or two doses (<9 years old, n = 14) of LAIV in 2012 at a 4-week interval, whereas adults received a single dose in 2013-2014 season.5 Blood samples were collected at day 0 (prevaccination), 28 and 56 days post-vaccination and plasma aliquoted and frozen for use in the serological assays, as previously described.28 In children, cell preparation tubes (CPT, BD) were used to separate peripheral blood mononuclear cells (PBMCs) for the ELISpot assay.
2.2 Viruses and antigens
Viruses were propagated in the allantoic cavity of 10-day-old embryonated hen's eggs. Allantoic fluid was harvested, clarified and frozen at −80°C until used in the assays as described below. The reassortant A/California/7/2009(H1N1) virus (X-179A) was used for the MN assay, the chimeric cH9/1N3 virus containing the HA stalk from A/California/7/2009(H1N1) and head from A/guinea fowl/Hong Kong/WF10/99 for the virus neutralization (VN) assay, and the reverse genetics H7N1 virus (NIBRG-127 containing the NA from A/California/7/2009(H1N1) and HA from the equine A/Prague/56 (H7N7) strain) for enzyme-linked lectin assay (ELLA). The wild-type A/California/7/2009(H1N1) virus was used for the ADCC assay.
2.3 Micro-neutralization assay
The MN assay was conducted as previously described.28 Briefly, plasma and control sheep serum samples (NIBSC) were twofold serially diluted from 1:10 in flat-bottom 96-well cell culture plates (Nunclon Delta Surface) before incubation with one hundred 50% tissue culture infectious does (TCID50)/50 µL/well of X-179A for 1 hour at room temperature. Then, 1.5 × 105 MDCK (Mardin-Darby Canine Kidney) cells/mL was added and incubated for 16-18 hours at 37°C. The propagation of influenza virus was detected using antibody to the nucleoprotein and TMB (3,3′, 5,5′-tetramethylbenzidine; Thermo Fisher Scientific) before reading at 450 and 620 nm to obtain the final optical density (OD). The MN titres (IC50) were calculated using the Reed and Muench method.29
2.4 Virus neutralization assay
The virus neutralization assay was conducted with the cH9/1 virus using a 3-day incubation period.30, 31 Briefly, cell culture plates (Flat-bottom 96-well; Nunclon Delta Surface) were seeded with 1.5 × 104 MDCK cells/well and incubated at 37°C overnight. Next, heat-inactivated plasma samples were diluted to 1:10 and twofold serially diluted before incubation with cH9/1N3 (100 TCID50/50 µL) for 1 hour at 37°C. MDCK cells were washed with phosphate-buffered saline (PBS), and plasma/virus dilutions were added and incubated for 1 hour at 37°C. The mixture was removed, cells were washed with PBS, and 50 µL of serially diluted plasma plus 50 µL infection medium (DMEM medium containing 2.5 µg/mL tosyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin (Worthington Biomedical), PSA (100 IU/mL penicillin, 100 mg/mL streptomycin and 0.25 μg fungizone; Lonza) and 0.14% bovine serum albumin (Sigma-Aldrich) were added to each well before incubation at 37°C for 72 hours. The virus neutralization titres were measured by haemagglutination assay using the supernatant (50 µL) and 50 µL of 0.7% human red blood cells and read after 30 minutes of incubation at room temperature. The highest dilution of plasma giving complete haemagglutination was read as the neutralizing antibody titre.
2.5 Enzyme-linked lectin assay
The ELLA was used to measure antibodies inhibiting the ability of neuraminidase to cleave sialic acid as previously described32-34 using the H7N1 virus containing the HA from an equine influenza virus strain and NA from A/California/07/09. Ninety-six-well flat-bottom MaxiSorp plates (VWR) were coated overnight at 4°C with 100 µL coating solution containing 0.25 µg fetuin per well (Sigma-Aldrich; KPL; Kirkegaard & Perry Laboratories). Heat-inactivated (56°C for 45-60 minutes) serially diluted serum and H7N1 virus were added and incubated at 37°C for 16-18 hours. The virus was diluted to a titre giving 90% neuraminidase activity. The plates were developed by adding 0.1 µg/100 µL/well horseradish peroxidase (HRP)-conjugated peanut agglutinin (PNA; Sigma-Aldrich) and incubation at room temperate for 2 hours, followed by adding o-phenylenediamine dihydrochloride (OPD; Sigma-Aldrich) substrate (0.5 mg/mL) in citrate buffer to all wells. After 10-minutes incubation at room temperature in the dark, the reaction was stopped with 100 μL 1 mol/L sulphuric acid. The plates were read with a microplate reader by spectrophotometry at OD 490 nm. The anti-NA antibody titres (reported as 50% inhibition concentration, IC50) in the plasma samples were calculated as the reciprocal dilution of plasma which gave OD values equal to 50% of total OD (OD virus control + OD blank) in four-parameter non-linear regression analysis using graphpad prism.
2.6 Antibody-dependent cellular cytotoxicity assay
The ADCC Reporter Bioassay (Core Kit G7010/G7018; Promega) was used to quantify pre- and post-vaccination ADCC antibodies.15 MDCK cells as “target cells” were seeded in 96 F-well white tissue culture plates (VWR) at 1.5 × 104 cells/well. Between 18-24 hours later, cells were infected with wild-type A/California/7/2009(H1N1)pdm09 virus at a multiplicity of infection (MOI) of 3. On the day of assay, the medium was replaced with assay buffer (RPMI 1640 with 4% [vol/vol] low IgG foetal bovine serum [FBS]; Lonza) followed by the addition of fivefold (starting at 1:10) serial dilutions of plasma. The infected cells and antibodies were incubated at 37°C for 30 minutes. The effector cells (Jurkat) at 7.5 × 104 cells/well were added to the assay plates. After 6 hours of incubation at 37°C, the Bio-Glo Luciferase Assay Reagent (Promega) system was used to quantify using a plate reader with glow-type luminescence.
2.7 ELISpot assay
Antigen-specific interferon gamma (IFN-γ) cytokine-secreting T cells were quantified at the single-cell level by the ELISpot assay (Mabtech).35 Optimized libraries of peptides represented unique T cell epitopes from four of the initial H1N1pdm09 circulating strains for CD4+ (originating from HA, NA, M1, NP, PB2) and CD8+ (M1, NA, PA and NS2) responses (Table S1). Briefly, 400 000 PBMCs per well were stimulated with CD4+ and CD8+ conserved peptide pools (2 μg/mL) anti-CD3 T cell activator (positive control), or lymphocyte medium alone (negative control).36 Plates were incubated overnight at 37°C with 5% CO2. After developing the following day, the plates were read using an automated reader (Advanced Imaging Devices) and spot-forming units (SFUs) were counted. The background values were subtracted from the influenza virus-specific response.
2.8 Statistics
The statistical tests and graphs were made using graphpad prism; v.7.0d for Mac (graphpad Software), where P < .05 was considered significant. Immune responses within each patient group, children or adults, were analysed using Friedman test (one-way ANOVA, non-parametric, paired test). Comparison between children and adults was performed using Mann-Whitney test (t test, non-parametric, unpaired). Correlation analysis was performed using linear regression and Spearman correlation.
3 RESULTS
Twenty children (3-17 years old) and twenty adults (21-59 years old) were intranasally vaccinated with seasonal LAIV. The children received one (≥9 years old, n = 6) or two doses (<9 years old, n = 14) of LAIV in 2012 at a 4-week interval, whilst adults received one dose of vaccine. In this study, the objective was to analyse the functional HA and NA H1N1-specific responses in children and adults, and the T cellular immune response in children.
3.1 Presence of neutralizing serum antibodies by MN assay
A MN titre of 80 is considered to protect 50% of individuals from disease.37 Sixty per cent of children had protective prevaccination MN titres (Figure 1A). Four children (20%) were boosted after 1st dose of vaccine and one of these children (5%) seroconverted after LAIV to a protective antibody titre. Only three children (15%) had no detectable MN antibodies both prevaccination and post-vaccination. Eleven adults (55%) had protective prevaccination MN titres of whom 25% increased post-vaccination (Figure 1A) and one (5%) adult seroconverted to protective antibody titres after LAIV. Of the adults with antibodies below the protective MN threshold before vaccination, 30% remained sero-negative post-vaccination. After vaccination, MN antibody titres increased <2-fold in both children and adults at day 28 and decreased in adults at day 56 (Figure 1B). Children had higher MN titres than the adults both prevaccination and post -vaccination. As expected, both pre- and post-vaccination HI and MN titres correlated well (prevaccination: children—R = .5546, adults—R = .7514; post-vaccination: children—R = .839, adults—R = .3267; Figure S2). In summary, LAIV did not significantly boost micro-neutralizing antibody titres in adults or children against the H1N1 vaccine strain.
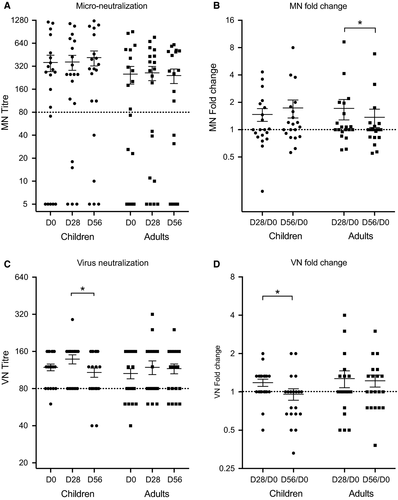
3.2 Assessing the stalk-specific neutralizing serum antibodies by ELISA
We have previously shown that only children and not adults showed a slight trend of an increase in stalk-specific antibodies measured by ELISA using chimeric HA proteins after LAIV vaccination.28 We extended these observations by investigating the HA stalk-specific virus neutralizing antibody responses using a chimeric cH9/1 virus. Nineteen (95%) children had high VN titre ≥80 prevaccination (geometric mean titre GMT: 114.2). H1N1 stalk-specific VN antibodies increased slightly after 1st dose of LAIV in 50% of children, (GMT = 129.3) but decreased after the second dose (GMT = 99.7; Figure 1C). Fifteen adults (75%) had prevaccination stalk-specific VN titres ≥80. No significant boost in stalk-specific VN antibodies was observed after LAIV, although 35% of adults had increases in VN antibodies (Figure 1C). In summary, neutralizing stalk antibodies showed a slight trend of an increase after LAIV vaccination in children after first 1st LAIV dose but were not maintained after the 2nd dose (Figure 1D). Of note, the method used for these assays is different than the standard MN assay used for wild-type virus and is—in combination with the cH9/1N3 virus—more sensitive than standard MN assays, leading to a higher baseline as well.
3.3 Neuraminidase inhibiting assay showed increases titres in children and adults
Neuraminidase inhibiting antibodies also play an important protective role in influenza virus infection. A NI titre of ≥40 reduced influenza illness in the human challenge model and has been recommended as protective titre.38, 39 Eight children had prevaccination NI titres above the protective titre, two children had low titres, and the remaining 10 children did not have detectable titres (<10; Figure 2A). The median NI titre increased significantly at 28 days post-vaccination in children, and most children responded with higher NI titres, although not all responses were ≥40.
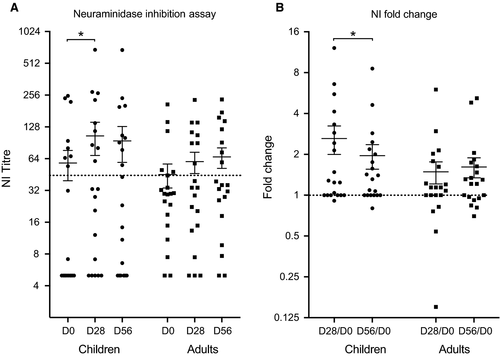
In adults, 30% had protective NI antibody titres prevaccination, whilst 60% had low NI titres and 10% had non-detectable NI titres (<10) before vaccination (Figure 2A). Vaccination boosted the NI titres in most of the adults, and the number of adults with protective level increased to 40%. Overall, the NI titres increased in most of the subjects, and very few had a decrease in titre (Figure 2B).
3.4 Children had stronger ADCC levels than adults after LAIV
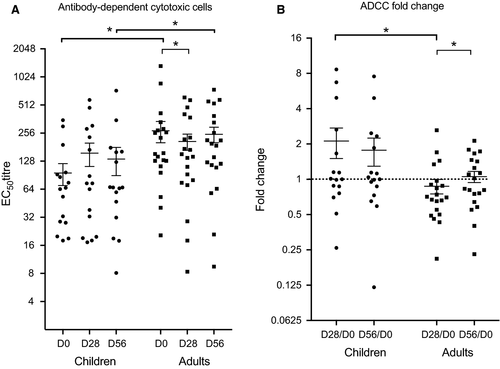
We examined the ability of antibodies to elicit ADCC activity in children and adults to the H1N1pdm09 virus after LAIV vaccination. Adults had higher ADCC antibody titres compared to children both prevaccination and post-vaccination. The mean ADCC titre was 95 prevaccination in children and increasing to 156 after 28 days, but this change was not significant (Figure 3A). The adults had a mean ADCC titre of 271 prevaccination, and this dropped significantly to 147 after 28 days (Figure 3A). Significantly higher fold changes were found in children compared to adults after 1st dose of vaccine (Figure 3B).
3.5 LAIV elicited CD4+ T cell activation in children but not CD8+ cell activation
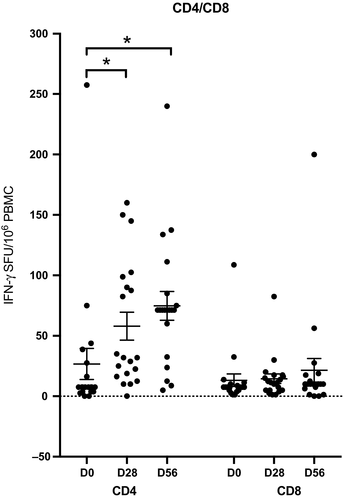
As only children responded after LAIV, we further extended this work by investigating the ability of LAIV vaccine to elicit IFN-γ producing T cells in children using H1N1pdm09 strain-specific CD4 or CD8 peptides. These CD4+ and CD8+ peptides (Table S1) represented unique T cell epitopes from four of the initial H1N1pdm09 circulating strains. Previous work has shown that 100 spot-forming units (SFUs)/106 PBMCs provided protection from laboratory-confirmed influenza after LAIV immunization in children,40 although a recent population-based study in the UK showed that T cell responses to NP ≥20 SFU/106 PBMC were associated with protection in adults.41
Most children had less than 100 IFN-γ secreting CD4+100 SFUs per million PBMCs, and only 1 child (5%) had above this level before vaccination. LAIV significantly boosted the CD4+ IFN-γ response (Figure 4A) with 20% of children had more than 100 IFN-γ CD4+ T cells at 28 days post-vaccination. Two of these children (10%) maintained high IFN-γ secreting CD4+ T cell after 2nd dose of LAIV. Two further children had increases in IFN-γ to >100 SFUs/106 PBMCs after the 2nd dose of LAIV. However, we observed no changes after LAIV in IFN-γ secreting H1N1-specific CD8+ T cells, except one (5%) child who showed an increase at day 56 (Figure 4B).
4 DISCUSSION
Earlier, the LAIV showed poor protective efficacy against the H1N1pdm09 strain and was therefore not recommended used by the ACIP; however, in the 2018/2019 season the vaccine was reinstated.42 Several European studies showed other results, and therefore, the EU continued to recommend use of LAIV in children.43 We have previously shown that LAIV immunization did not boost H1N1 HI titres in adults or children.28 Although children had significantly higher H1N1 HI titres than adults prior to and up to 56 days after vaccination, probably due to previous infection or the pandemic vaccination during the 2009 mass vaccination campaign. In this study, we evaluated the humoral (children and adults) and the cellular immune response (children only) after LAIV immunization to further understand the lower immune responses to H1N1pdm09. Our results demonstrate that the majority of children had relatively high prevaccination MN titres, perhaps due to either infection from circulating H1N1pdm09 or the previous pandemic vaccination. Although no boosting in MN antibodies was found after vaccination, in agreement with a previous report,44 the children had low but significant increases in neuraminidase-specific antibody levels and CD4+ T cell responses, supporting findings that LAIV induces a broad immune response, which could have a protective effect.
4.1 No boost in H1N1-specific MN antibodies in children or adults after LAIV
The immune response to LAIV is multifaceted, and the HI titre underestimates protection achieved from LAIV.45 Overall, no significant changes in MN antibodies were observed after LAIV, although a trend of an increase in MN titres was observed in both adults and children. Children have experienced less influenza virus exposure in their lifetime compared to adults and therefore may have a stronger recall response to antigenically matched strains,46-48 perhaps explaining the higher MN antibody observed in the children (Figure 1C,D). Our study reveals that HI titres correlated well with the MN titres in both children and adults, pre- and 28 days post-vaccination, with adults and children with HI titres >40 also having higher MN titres. Previously the MN assay has been shown to have a higher sensitivity to H1N1pdm09 than the HI assay.37 Interestingly, HI titres of 40 were associated with the geometric mean of MN titres over 160 in both children and adults in agreement with a previous study that suggested that MN titres of 160 are associated with protective HI titres,47 but this threshold may be different from study to study.
During the 2009 pandemic, Norway conducted a mass vaccination campaign with AS03 adjuvanted pandemic vaccine and nearly half of the population was vaccinated. In our study, half of the children and adults were previously vaccinated with adjuvanted pandemic vaccine and these vaccinated subjects had higher MN antibody titres compared to the unvaccinated group, in agreement with previous findings.49, 50 It has been shown that H1N1pdm09-specific HI antibodies persist after a single adjuvanted pandemic vaccination beyond the 2012 and 2013 seasons.51 Interestingly, the adults vaccinated with the pandemic vaccine had significantly higher MN titres than unvaccinated adults, whereas this was not observed in the children many of whom may have had H1N1pdm09 as their first influenza virus infection which circulated in Norway in the 2009/2010 and 2010/2011 seasons (Figure S3). In children, influenza imprinting by early exposure to influenza virus influences subsequent influenza immunity,52, 53 but whether the imprinting is specific to the first infection, or a result of the cumulative effect of repeated exposures and boosting, remains unclear, although a child's first encounter with influenza virus (HA imprinting) has a large impact on future immune status.54, 55
4.2 An elevated H1N1 stalk-specific neutralizing antibody titre was observed in children post-LAIV vaccination
Micro-neutralization antibodies prevent infection and therefore are a direct measure of protection, although HA stalk-specific antibodies may also confer protection12 through neutralization or by ADCC through Fc–FcγR interactions.13 We have previously reported a slight trend of an increase in group 1 HA stalk-specific IgG in children after LAIV, but not in adults.28 Stalk-specific neutralizing antibodies also showed a slight boost after the first dose in children, suggesting that LAIV could be used as a priming vaccine for inducing broadly cross-reactive stalk responses. However, no change in stalk neutralizing responses after LAIV vaccination was found in adults, irrespective of previous vaccination history. Furthermore, the stalk-specific VN antibody response did not correlate with HI titre in either children or adults and confirm the different specificity of the assays (data not shown).
Overall children had more head-specific neutralizing MN antibodies, whereas adults had higher stalk-specific virus neutralizing antibodies (data not shown). Interestingly, we found both children and adults previously vaccinated with AS03 pandemic vaccination had elevated head-specific MN antibodies, whereas elevated stalk-specific VN antibody responses were observed in non-vaccinated children and adults (Figure S5). Infection does generally stimulate stronger antibody responses to stalk antigens than vaccination. This suggests induction of stalk-specific neutralizing antibodies depends on previous exposure history but is not related age related.15, 56
4.3 LAIV boosts NI response in children
Neuraminidase inhibition antibodies can reduce release of new viruses from infected cells,57, 58 and NA-specific antibodies may be a correlate of protection38, 39 in man, independently of HA antibodies. Here, we found significant increases in NI antibodies after LAIV in children although titres often remained below the protection level (<40). In contrast, in the UK no increase in NI antibodies was found after LAIV4 perhaps because of the H1N1pdm09 having the lowest replication of the four LAIV strains.44, 59 Here, LAIV boosted the NI response after the 1st dose in children which continued to increase after the 2nd dose in the previously vaccinated group, whereas antibodies only increased after 1st dose in the previously unvaccinated group. Further analysis demonstrated that both children and adults with high HI or MN antibody titres (>40) also had protective NI antibody titres, although these NI antibodies were only boosted significantly in children. Surprisingly, the NI antibody fold change after LAIV seemed to increase in children with increasing age, whilst decreasing in adults.18
4.4 No boost in ADCC-inducing antibodies
We and others have previously shown that A(H1N1)pdm09 vaccination and infection induced HA-specific ADCC mediating antibodies, with the stalk-specific antibodies better at mediating ADCC than head-specific antibodies.30 In the current study, we evaluated the ADCC activity to wild-type H1N1pdm09 with the aim of detecting both H1 stalk and NA antibodies. Half of the children were vaccinated with AS03 pandemic vaccine in 2009, and these children had high stalk-specific functional antibodies, as hypothesized previously.60-62 But we also found high titres of stalk-specific VN antibodies in the unvaccinated children who may have experience natural infection with H1N1pdm 09 (Figure S4). Higher pre-existing ADCC-inducing antibodies may limit replication of the LAIV strains and therefore limiting the ability of the vaccine to boost antibody responses.63 Interestingly, our study illustrated that both children and adults had pre-existing ADCC antibodies, although the children had significantly lower antibody titres than adults, perhaps explaining the lower efficacy seen after LAIV in adults. Concurrently, the adults had lower HA (HI and MN)- and NA-specific (NI) antibody titres than the children despite having higher ADCC titres pre- and post-vaccination. No significant changes in ADCC reporter activity were found after LAIV, in agreement with an earlier study.64
4.5 LAIV boosts CD4 T cells but not CD8 T cells in children
T cells play an important role in coordinating the immune response and are also critical for the control of influenza infection. Early human studies showed an inverse correlation between influenza virus shedding and CD8+ T cells in sero-negative adults.65, 66 The importance of CD8 cells was confirmed during the 2009 pandemic when pre-existing late-effector CD8+ IFN-γ T cells with lung-homing and cytotoxic potential were associated with milder symptoms and less severe illness67 in the absence of H1N1pdm09 specific antibody. In human challenge studies, pre-existing CD4+ T cells resulted in lower virus shedding and less severe influenza symptoms in sero-negative subjects.68 We have previously shown that LAIV induces durable increases in IFN-γ–positive T cells to H1N1pmd09 in children and that pre-existing cross-reactive CD8 T cells are boosted in young children.69 Here, we aimed to further understand the T cell response in children using optimized libraries of CD4 and CD8 peptides representing unique T cell epitopes from the H1N1pdm09 virus. In the children, we found that only H1N1pdm09-specific CD4 responses were boosted after LAIV and no change in CD8 responses was observed.70
Our results suggest that the H3N2 LAIV strain may be responsible for our earlier observation of boosting of CD8 cross-protective responses but will need to be confirmed using conserved CD8 peptides. In earlier LAIV studies, we found that 25% had significant increases in virus-specific T cell responses after vaccination although no increase in serum responses was detected.5 This study further extends these findings to show that although the children did not respond by traditional MN antibodies, they responded with increase in CD4 T cells, which may provide clinical protection. LAIV has been shown to induce broader protection in ferrets71 and in children when used in a school setting.72 Furthermore, the inclusion of LAIV in the childhood vaccination programme in the UK has shown the benefits of herd immunity, which are thought to be mediated by T cells.73, 74 Therefore, the use of LAIV in children could be an important step in the protection against novel drifted or pandemic strains.
This study has focused on the functional systemic immune responses. The LAIV vaccine is intended to mimic a natural infection and stimulate the local immune response. We have in a previous study, shown that LAIV induces local influenza-specific IgA production.70 As there are some degree of compartmentalization of the immune system, we cannot expect to observe the same degree of immune response systemically as with parenteral administered vaccines. Using established immune response thresholds validated for systemic vaccines may have to be used with caution when evaluating LAIV. This warrants further work and development of improved evaluation tools for mucosal vaccines.
In conclusion, children and adults have different previous exposure histories to the influenza virus through infection and vaccination and this can impact upon the generation of immune responses after LAIV. The LAIV vaccine is administered mucosally requiring replication of the vaccine viruses; therefore, pre-existing antibodies may limit replication and subsequent induction of immune responses as observed in the adults with relatively high prevaccination antibody levels. Although the children also had high MN antibodies, they responded with significant increase in NI and CD4 T cells after vaccination. This indicates that the NI assay may be more sensitive to detect boosting in antibody responses after mucosal LAIV vaccination which needs further investigation. The multifaceted immune response after LAIV in children supports the continued recommendation of LAIV for use in children.
5 ACKNOWLEDGMENT
We thank the children and their parents and adults who participated altruistically in this study; all of the staff at the Ear, Nose and Throat Department; and the Children's clinical trial unit at Haukeland University Hospital and the Influenza Centre for assistance with the clinical trial and to Dr Fredrik Oftung at the Norwegian Institute for Public Health, Oslo, for providing the peptides used in this study. The study was supported intramurally by the Influenza Centre at the University of Bergen. The Influenza Centre is funded by the Ministry of Health and Care Services, Norway, the European Union (Univax 601738, EU IMI115672 FLUCOP), Helse Vest, and the KG Jebsen Centre for Influenza Vaccine Research. Support for the Krammer laboratory came from the NIAID Centers of Excellence for Influenza Virus Research and Surveillance (CEIRS) contract HHSN272201400008C.
6 CONFLICT OF INTEREST
The authors declare no conflict of interest.




