The Effect of Antifibrotic Drug Halofugine on Th17 Cells in Concanavalin A-Induced Liver Fibrosis
Summary
Anti-inflammation strategy is one of the proposed therapeutic approaches to hepatic fibrosis. T helper (Th) 17 cells, which play a detrimental role in experimental murine models of inflammatory diseases, have been demonstrated to participate in the pathogenesis of liver damage. The inhibitory effect of halofuginone (HF), an active component of extracts derived from the plant alkaloid febrifugine, on collagen synthesis has been shown in animal models of the fibrotic disease. The aim of this study was to clarify the in vivo effect of HF on Th17 cells in concanavalin A-induced fibrosis rats. Haematoxylin–eosin (HE) staining and Masson staining were performed to observe collagen deposition. The presence of INF-gamma, TNF-alpha, IL-6, IL-17, IL-1beta, IL-33 and IL-10 in serum and the presence of ROR-γt, IL-17, TGF-β1 and α-SMA in liver tissue were detected. Flow cytometry was performed to analyse the percentage of Th17 cells. We observed significantly lower levels of INF-gamma, TNF-alpha, IL-6, IL-17, IL-1beta, TGF-β1 and α-SMA in HF-treated group of rats, and the percentage of Th17 cells in splenic lymphocyte was decreased well. Histological examination demonstrated that HF significantly reduced the severity of liver fibrosis in HF-treated rats. We concluded that HF (10 mg/kg) exerts an antifibrotic impact on Th17 cells and its relative cytokines in rats with ConA-induced fibrosis.
Introduction
Hepatic fibrosis is a wound-healing response to various chronic liver injuries, including alcoholism, persistent viral and helminthic infections, and hereditary metal overload 1. Fibrosis is a complicated, multistage progressive process, often lacking a defined cause, whose different forms display different characteristics 2. Fibrotic diseases are therefore difficult to study and perplexing to treat. However, the consistent involvement of Th1, Th2 and Th17 cells suggests that communication among these cell types generally regulates fibrotic diseases. During hepatic fibrogenesis, hepatic stellate cell (HSC), which plays a crucial role in the development of liver fibrosis 3, 4, is activated by inflammatory cytokines and growth factors in a paracrine and autocrine manner 5, 6. Therefore, anti-inflammation was considered to be one of the proposed strategies to treat hepatic fibrosis.
IL-17-producing CD4+ T helper (Th17) cells, which can secrete high level of interleukin (IL)-17 and express key transcription factor RoRγt, have been identified as an unique subset of the proinflammatory helper cells 7. Their preferred differentiation up on TGF-β and IL-6, two cytokines abundantly present in injured liver, makes Th17 cells very likely contribute to hepatic inflammation 8, 9. Previous studies indicated that the number of Th17 cells increased with the severity of liver damage in CHB patients 10. The number of IL-17-positive cells correlates with the degree of liver fibrosis in patients with viral hepatitis and alcoholic liver disease as well 11. In particular, these Th17 cells in the liver spontaneously produced IL-17A, which can mobilize, recruit and activate neutrophils, lead to massive tissue inflammation and the progression of autoimmune disease and various liver diseases, including liver autoimmunity and inflammatory diseases, alcoholic liver disease (ALD) and hepatocellular carcinoma 12, 13. Indeed, initial studies in humans revealed activated Th17 cells and Th17-related cytokines in various liver diseases 9.
IL-17 was described as a proinflammatory cytokine produced by human T cells in 1995 14, originally termed cytotoxic T-lymphocyte antigen 8; it had first been discovered as the product of a particular clone of activated T cells 15. The remarkable increases in hepatic IL-17 correlated with the severity of the disease are seen in viral hepatitis, alcoholic liver disease, autoimmune hepatitis and hepatocellular carcinoma as well. IL-17 is able to target immune cells and many types of liver cells including hepatocytes, kupffer cells (KCs), hepatic stellate cells (HSCs), biliary epithelial cells and sinusoidal endothelial cells. By binding to the IL-17R on various types of liver cells, IL-17 upregulates the expression of a variety of proinflammatory cytokines and chemokines and subsequently promotes liver inflammation, playing a detrimental role in the pathogenesis of liver diseases. The field of IL-17 research was boosted in 2005 by the discovery of a distinct subset of proinflammatory Th17 cells, which serve as a major source of IL-17. The cytokine role of IL-17 became evident after studies showed that IL-17 can mobilize, recruit and activate neutrophils, leading to massive tissue inflammation and progression of autoimmune diseases 16. In addition, IL-17 was recently found to be extensively involved in the pathogenesis of chronic liver disease and antiviral immunity 17.
Halofuginone was an alkaloid originally isolated from the plant Dichroa febrifuga, which has been found to attenuate both collagen α1(I) gene expression and the collagen level 18. The discovery of the inhibitory effect of halofuginone on collagen synthesis and extracellular matrix (ECM) deposition has led to intensive studies that aimed to control various diseases with excessive collagen deposition, such as pancreatic fibrosis 19, liver cirrhosis 20, renal fibrosis 21, scleroderma and chronic graft-versus-host disease 22, urethral strictures 23 and ageal strictures 24. Although the precise mechanism underlying the antifibrotic effect of halofuginone is not yet well understood, it was found that halofuginone affects collagen biosynthesis probably by blocking transforming growth factor-β-mediated smad3 activation 25. Treatment with halofuginone effectively inhibited the delayed-type hypersensitivity response, indicating that it could suppress T cell-mediated inflammation in vivo 26.
Concanavalin A (ConA)-induced liver fibrosis is a widely used rat model of immune-mediated liver injury that resembles viral and autoimmune hepatitis in humans 27. The intravenous injection of ConA into rat can increase transaminase and activate T cells infiltrate in liver. The activation of T cells by ConA results in increased level of the plasma alanine aminotransferase (ALT); simultaneously, activated T cells infiltrate the liver, and the apoptosis and necrosis of hepatocytes follows. The activation of T cells by ConA results in increased expression of inflammatory cytokines, including tumour necrosis factor (TNF)-α, interferon (IFN)-γ and interleukin (IL)-6 28. On the basis of this background, with the lack of sufficient information on the effects of halofuginone in inflammatory processes of liver fibrosis, the present study was designed to examine the inhibitory effect of halofuginone on Th17 cell differentiation and related cytokine production in ConA-induced liver fibrosis.
Materials and methods
Ethics statement
All the procedures and care administered to the animals were approved by the institutional ethic committee of Qingdao University Medical College, under a permission of animal use (SCXK20090007) in the Center of Experimental Animal and Animal Experiment at Qingdao, compliance with the Experimental Animal Regulations by the National Science and Technology Commission, China.
Materials
Concanavalin A and halofuginone were purchased from Sigma-Aldrich Co. (St Louis, MO, USA) and Jilan Technology Development Co. (Shanghai, China), respectively. TGF-β1, IL-6, IL-17, IFN-γ, IL-10 enzyme-linked immunosorbent assay (ELISA) kits and α-SMA, TGF-β1, ROR-γt polyclonal antibodies were from Solarbio Science & Technology Co. (Beijing, China) and Biosynthesis Biotechnology Co. (Beijing, China). Anti-rat CD4 FITC, anti-rat CD25 PerCP and anti-rat IL-17 PE were from eBioscience (San Diego, CA, USA).
Animals
Male Wistar rats (weighing 210–230 g) were purchased from the Experimental Animal and Animal Experiment Center of Qingdao, Shandong, China. They were housed in the animal facility with a 12-h-light/dark cycle, the temperature was maintained at 22–23 °C, and the relative humidity was 60%. All animal experiments were approved by the animal committees of Qingdao Medical College.
Experimental protocol
Halofuginone was purchased from Sigma-Aldrich Co. For in vivo experiments, HF was given in the diet at concentrations of 10 mg/kg. The animals were randomly divided into three groups (15 rats per group): rats received a weekly intravenous injection of 300 μl PBS for 8 weeks (group 1), rats received a weekly intravenous injection of ConA at a dose of 17.5 mg/kg for 8 weeks (group 2), and rats received a weekly intravenous injection of ConA with 10 mg/kg HF in diet for 8 weeks (group 3). During all experiments, rats were maintained in individually ventilated cages under specific pathogen-free conditions. Animals were sacrificed at the end of 8 weeks. Blood was collected via cardiac puncture; serum was prepared by centrifugation at 2000 g for 10 min and stored at −20 °C, while livers were taken from rats after perfusion with 4% paraformaldehyde.
Liver-to-body-weight ratio
Animals were sacrificed at indicated time points. The total body weight was measured, and the liver tissues were resected and weighed. The acquired data were expressed as percentage of the ratio between liver weight (A) and the total body weight (B) times 100 (liver-to-body-weight ratio [LBWR] (%) = A/B × 100)29.
Liver function
The serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and albumin were measured with an auto-biochemical analyzer (Roche P800, Basle, Switzerland).
Histology
After rats were sacrificed, vessels were perfused with PBS, followed by 4% paraformaldehyde. The livers were removed and fixed in 4% paraformaldehyde overnight and then embedded in paraffin. Sections (4 μm) were cut and deparaffinized, stained with haematoxylin and eosin (H&E) and Masson. Slides were evaluated using light microscopy by a pathologist on a blind basis. The representative sections were presented, and the level of fibrosis was scored according to liver fibrosis semi-quantitative scoring system (SSS) method 30.
Enzyme-linked immunosorbent assay
We examined IFN-gamma, tumour necrosis factor (TNF)-alpha, IL-6, IL-17, IL-33 and IL-10 serum concentrations using rat allele-specific ELISA kits (Solarbio Science &Technology Co.) according to the manufacturer's instructions. Briefly, diluted sera (1:5) with sample dilution were added to the wells and incubated for 30 min at 37 °C. After washing five times with wash solution, detection antibody marked with HRP was added to each well and incubated for 30 min at 37 °C. After washing five times, solutions A and B were added in sequence and incubated for 15 min at 37 °C. The reaction was terminated by adding stop solution. Immunoreactivity was determined shortly after by measuring the optical density at 450 nm with a microplate reader (RT-6100, LeiDu life science co, Shenzhen, China). All samples were run twice.
Immunohistochemistry
Paraffin blocks were cut into 4-μm sections, deparaffinized in xylene and rehydrated in graded ethanol solutions. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 20 min, washed with PBS (0.01 m, pH 7.4) for 5 min for 3 times and incubated with blocking buffer (normal goat serum) at 37 °C for 20 min. Tissue sections were incubated overnight at 4 °C with anti-TGF-β1, anti-ROR-γt polyclonal antibodies (diluted in PBS 1:200), washed and incubated with a secondary antibody conjugated to biotin for 20 min at 37 °C, washed and then incubated with avidin conjugated to HRP for 20 min at 37 °C. Then, the tissue sections were stained with 3,3-diaminobenzidine (DAB) and observed by microscope, and the sections were destained with distilled water. The slides were counterstained with haematoxylin for 5 min and dehydrated with ethanol from 70 to 80%, then 90, 95, 100%, then transformed into xylene for 10 min for 3 times. Then, the slides were covered with cover slip by neutral gums and observed by microscope.
Image Pro plus 6.0 (Media Cybernetics, Bethesda, MD, USA) was used to quantify antibody staining. This software was trained to select stained nuclei or cells based on colour intensity and nuclear shape; brown staining was considered positive. The chromatic area and strength (light density values) of positive cells were calculated and represented as the percentage of total positively stained cells with integral light density values (integral optical density).
Cell preparation and flow cytometry
Splenic lymphocytes were prepared from rats using density gradient centrifugation with Ficoll-Cardiografin (tbdscience.co.Tianjin, China) and were stimulated with 25 ng/ml PMA (Beyotime Institute of Biotechnology, Jiangsu, China), 1 μg/ml ionomycin (Beyotime) and 10 μg/ml brefeldin A (BioLegend, San Diego, CA, USA) for 4 h at 37 °C under 5% CO2 environment. The cells were washed with washing buffer and then stained with FITC-conjugated anti-CD4 Ab (clone eBioOX35; eBioscience) in the dark on ice for 30 min. After surface staining, the cells were resuspended in fixation/permeabilization solution (eBioscience) and then stained intracellularly with PE-conjugated anti-IL-17 (clone eBio17B7; eBioscience) in the dark at room temperature for 20 min. After extensive washing, stained cells were analysed using a FACS Calibur flow cytometer (BD Biosciences, Mountain View, CA, USA). Isotype controls and unstimulated controls were utilized to confirm antibody specificity and to enable correct compensation. Stained cells were analysed using Flowjo7.6.5 software (TreeStar, Inc., Ashland, OR, USA).
Statistics
All statistical analyses in this study were carried out using Sigma Plot 10 (Systat Software Inc., San Jose, CA, USA) and SPSS 19.0 (SPSS Inc., IBM, Armonk, NY, USA), and results were expressed as the means ± SEM. A one-way analysis of variance (anova) followed by the post hoc Dunnett's test was used to analyse statistical significance among multiple groups. P < 0.05 was considered significant.
Results
HF reduces rat mortality and improves LBWR
All 15 rats in G1 were alive when they were sacrificed after 8 weeks and only 9 and 13 left in G2 and G3. As shown in Fig. 1, HF can significantly improve the survival rate. The body and liver weights after 8 weeks have a statistically significant difference between these three groups. The LBWR was highest in G2, while it was significantly lower in G3 than in G2, which indicates that HF can improve LBWR in ConA-induced liver fibrosis (Table 1).
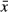 ± s)
± s)- *P < 0.05, **P < 0.01,***P < 0.001 versus G1 group;#P < 0.05 versus G2 group.

HF rescued liver function impairment in ConA-treated rats
Haematoxylin–eosin staining was applied to evaluate the pathologic injuries. It showed that repeated ConA administration induced the formation of necrotic area in the liver with substantial amount of inflammatory cells infiltration surrounding the centrilobular veins of the liver. Treatment with HF attenuated the severity of necrosis induced by ConA. Histopathological findings of the liver tissue are shown in Fig. 2A. HE staining showed fibrosis and distorted tissue architecture in the G2 liver with collagen bundles surrounding the lobules and a large number of inflammatory cell infiltrations. In comparison with G2, fibrous strips and inflammatory cell infiltration were remarkably attenuated in G3, demonstrating that HF improved liver histology.
To investigate the effect of HF on liver function, plasma ALT, AST and albumin levels were determined. Studies revealed that ConA stimulation significantly increased plasma ALT, AST and decreased albumin levels in 9 ConA-treated rats compared to 15 normal control rats, but treatment with HF resulted in significantly lower levels of ALT, AST and higher level of albumin, indicating that HF could dampen the abnormal expression of ALT, AST and albumin induced by ConA treatment (Fig. 2B). Consistently, the semi-quantitative scoring system (SSS) value for G3 was also lower than that for G2 (Fig. 2C).
HF treatment attenuated liver fibrosis parameters and collagen accumulation
In order to assess the liver fibrosis index associated with fibrogenesis, we determined the levels of hyaluronic acid (HA), type III precollagen (PCIII) and transforming growth factor (TGF)-β1 in the serum. HA is one of the best serum predictors of liver fibrosis and a main component of the extracellular matrix. In the liver, HA is synthesized by stellate cells and is involved in fibrogenesis 31. PCIII is closely correlated with the degree of liver fibrosis, reflecting the status of liver fibre synthesis and inflammatory activity. Transforming growth factor (TGF)-β1 is one of the strongest profibrotic cytokines in the long pathological progression of chronic hepatic injury to fibrosis. The levels of HA, PCIII and TGF-β1 were significantly higher in ConA-treated rats. However, simultaneous administration of HF resulted in reduced levels of all the three parameters in ConA+HF-treated rats (Table 2).
 ± s)
± s)| Group | N | TGF-β1/(ng/l) | HA/(ng/l) | PCIII/(μg/l) |
|---|---|---|---|---|
| G1 | 15 | 97.86 ± 9.39 | 210.33 ± 33.11 | 19.72 ± 2.15 |
| G2 | 9 | 129.83 ± 20.90** | 365.01 ± 54.08** | 29.64 ± 4.37** |
| G3 | 13 | 112.24 ± 12.07*,# | 264.06 ± 51.66**,## | 23.02 ± 3.33*,## |
- *P < 0.05, **P < 0.01,*** P < 0.001 versus G1 group;#P < 0.05, ##P < 0.01 versus G2 group.
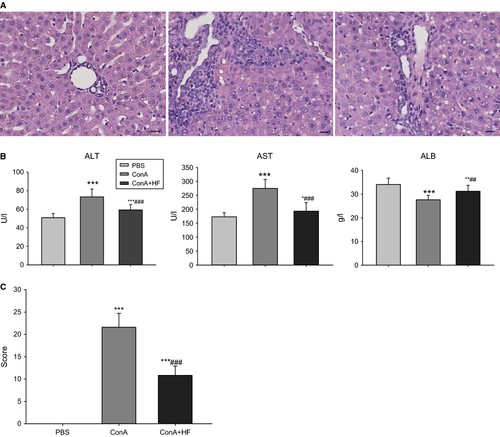
The collagen deposition in liver tissue was determined by appearance of the blue-stained collagen fibres with the Masson's trichrome method (Fig. 3A). G1 liver showed normal foliages and cell structures were found in Masson staining, while fibrosis for a distance and a large number of inflammatory cell infiltrations was found together with hepatocellular necrosis in the G2 liver tissue. The deposited collagen was significantly increased in both the pericellular area and along the central vein in the G2 liver. Noticeably, fibrous strips and inflammatory cell infiltration were remarkably attenuated in the G3 liver compared with those seen in the G2, which was in accordance with the observations made on the sections. Supportively, the mean score of fibrosis in the G3 was significantly lower than that of G2 (P < 0.05).
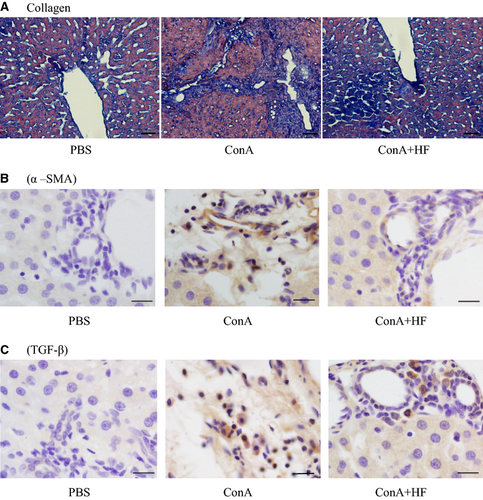
HF treatment inhibited the profibrotic factors in liver tissue
To assess the effect of HF on liver fibrosis induced by ConA, we measured the expression of α-SMA and TGF-β1, the two important profibrotic factors that play important roles in the process of liver fibrosis 32. TGF-β1, one of the most potent profibrotic cytokines, is believed to facilitate the production of collagen and other extracellular matrix components usually in an autocrine fashion 33. As shown in Fig. 3B–C, we found that the expression of both α-SMA and TGF-β1 was much higher in the 9 rats treated with ConA (P < 0.05) compared to the controls. More importantly, HF treatment inhibited the overexpression of those profibrotic factors induced by ConA, indicating that HF treatment downregulated profibrogenic markers.
HF decreases the differentiation of Th17 cells in ConA-treated rats
To demonstrate whether augmented Th17 (CD4+IL-17+T cell) infiltration was responsible for the exacerbated liver fibrosis, single monocytes from spleen were prepared. By analysing the number of IL-17-positive cells in CD4+T subset, we could learn the frequency of circulating Th17 cells from various subjects. As shown in Fig. 4A–B, the number of Th17 cells dramatically decreased in HF-treated rats than in control ConA-treated ones (P < 0.05). Thus, our results provided evidence that HF treatment can suppress Th17 cells.
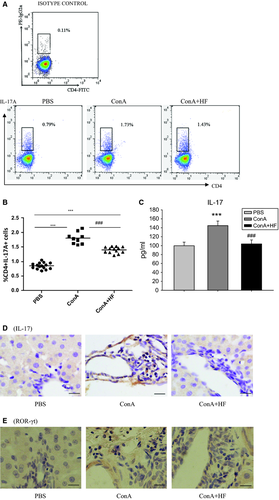
The expression of IL-17 protein in serum and liver tissue
Because previous studies have demonstrated that HF decreases the differentiation of Th17 cells in rat, we further detected whether IL-17 was changed after treatment with HF. As shown in Fig. 4C, IL-17A protein level in serum was significantly suppressed after chronic HF treatment. Consistently, the expression of IL-17A in liver tissue also decreased after HF treatment (Fig. 4D).
Expression of ROR-γt protein in liver tissue of rat
Retinoic acid-related orphan receptor γt (RORγt) is essential for the development of lymphoid tissues and innate and adaptive immune cells such as Th17 cells 34. To test whether the inhibitory effects of HF on Th17 differentiation were related to RORγt, we detected the expression of RORγt in liver tissues from 15 control rats, 9 ConA-treated rats and 13 ConA+HF-treated rats by immunohistochemistry. Our result showed that there was no difference between ConA-treated and HF+ConA-treated rats (Fig. 4E). Therefore, it seemed that RORγt was not the intervention target of HF and was not sufficient to drive the effector function of Th17 cells.
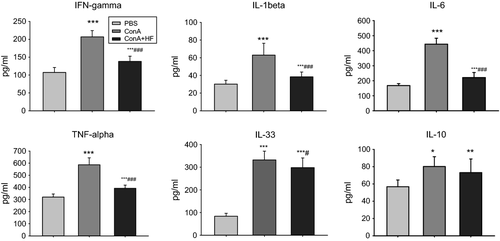
Decline of Th17 cells was associated with decreased serum inflammatory cytokines
Previous studies have demonstrated that inflammatory cytokines play important roles in the pathophysiology of alcoholic liver disease 35. We compared serum levels of IFN-γ, TNF-α, IL-6, IL-1β, IL-33 and IL-10 in 3 groups. IFN-γ, an antiviral cytokine, exerts antifibrotic effects in vitro via blunts profibrotic TGF-β signalling 36. TNF-α, a primary immune and inflammatory regulator, stimulates fibroblast chemotaxis and proliferation 37. IL-6, another inflammatory cytokine, may affect the differentiation of fibroblast to myofibroblast and hence plays an important role in fibrosis diseases 38. IL-1β, a ubiquitous and pleiotropic cytokine, inhibits collagen production and enhances fibroblast proliferation. IL-33, which is released from liver cells in the context of hepatocellular stress, is a key mediator of hepatic fibrosis in vivo 39. IL-10, an important cytokine with anti-inflammatory actions, plays anti-immune and antifibrotic functions in the process of liver fibrosis. We found that ConA treatment led to increases in the serum levels of IFN-γ, TNF-α, IL-6, IL-1β and IL-33 (G2, n = 9) compared to the controls (G1, n = 15), while HF treatment significantly reduced those expressions (G3, n = 13). Interestingly, serum levels of IL-10 representing Treg-type cytokines increased after ConA treatment; however, HF treatment had no effect on such overexpression of IL-10 (Fig. 5). It is well known that there is a cross-regulation of Treg and Th17 cells; the relationship between IL-17 and IL-10 is a complex one 40. In summary, we observed in this study that HF treatment decreased ConA-induced IL-17 expression without change in IL-10 expression in serum. These results may indicate that HF impaired Th17 differentiation and did not affect Treg cells.
Discussion
Concanavalin A (ConA)-induced liver fibrosis characterized by the activation of T cells is considered to be a better model to study the liver fibrosis because it resembles the progression of liver fibrosis originated from viral and autoimmune hepatitis in humans 28. Repeated intravenous injection of ConA impaired the liver function and induced hepatic fibrosis in rats. Simultaneously, activated T cells infiltrate the liver, and the apoptosis and necrosis of hepatocytes follows.
Our study reported for the first time that HF could slow down the process of liver fibrosis by suppressing Th17 differentiation. Previous studies have shown that HF, a derivative of febrifugine, has anticancer effects in haematological malignancies 41. It mainly inhibits collagen type I synthesis and extracellular matrix formation, via downregulation of TGF-beta signalling, matrix metalloproteinase 2 and angiogenesis with doses ranging from 5 to 400 ng/ml 25, 42, 43. In vivo studies have reported that intraperitoneal injection of HF had significant antitumoral effects with doses varying from 50 to 300 μg/kg without the significant toxicity 41. Feeding the thioacetamide-induced cirrhotic rats with a halofuginone-containing diet from 5 to 10 mg/kg can reduce a-SMA, TIMP-2 and collagen deposition 44. Therefore, the doses we chosen in the experiment were within the range that was reported previously to be effective and non-toxic. In addition, low concentration of HF suppresses the activity of T cell and the production of pro-inflammatory cytokine in peripheral blood through the inhibition of NF-kB activation and p38 MAPK phosphorylation, making it an attractive immunomodulator and anti-inflammatory agent 42. Very recently, Sundrud et al. reported that HF selectively inhibited the development of Th17 cells with a median inhibitory concentration of 3.6 ± 0.4 nm. Low concentration of HF that impaired Th17 differentiation did not influence Th1, Th2 or iTreg differentiation and had no impact on T cell receptor (TCR)-induced cytokine secretion by naive T cells 45. They further demonstrated that HF relieved the pathology in a mouse model of autoimmunity. However, no data have been published so far to study the possible actions of HF on Th17 differentiation in liver fibrosis. Because the development of oral medications against liver injury is desirable 46, our study was designed to check the effect of oral treatment with HF on ConA-induced liver fibrosis. We found that HF treatment significantly improved liver histology and declined the high values of collagen a 1(I) protein. We also found that the decline in collagen deposition was accompanied by a reduction in numbers of a-SMA-positive cells, suggesting that halofuginone may affect collagen levels by more than one mechanism.
Th17 cells may regulate local immune responses in liver by secreting inflammatory cytokines, which may in turn mediate tissue damage 10. Studies further confirmed that Th17 cells and the secretion of IL-17a (IL-17), IL-17F and IL-6 can promote inflammation and immune-related liver disease 9, 10, 47, 48. IL-17 was recently found to be extensively involved in the pathogenesis of chronic liver disease and antiviral immunity 17. Consistent with this, level of IL-17 is strongly correlated with the degree of fibrosis 49. In recent study, we observed that the expression of IL-17 in serum and liver tissues was significantly reduced in rats with hepatic fibrosis induced by ConA injection. The percentage of CD4+IL-17+T cells decreased dramatically in HF+ConA-treated recipients, as compared with that of ConA-treated ones (P < 0.05). Semi-quantitative scoring system showed that blockade of Th17 cells differentiation ameliorated liver fibrosis. Consistent with our observations, Th17 cells have been shown to be involved in ConA-induced liver fibrosis 9, 50. In the liver, IL-17-secreting cells contributed to inflammatory infiltrates in alcoholic cirrhosis, and alcoholic hepatitis foci disclosed many IL-17-positive cells, including T lymphocytes and neutrophils. In alcoholic liver disease, liver IL-17-positive cell infiltrates correlated with the end-stage liver disease score, and in alcoholic hepatitis with modified discriminant function 13. In addition, Thimme et al. identified high levels of CD26 expressed by Th17 cells in the intrahepatic T cell compartment in patients with chronic hepatitis C virus infection 51, suggesting that Th17 cells also play an important role in hepatitis C virus infection. There was some experimental evidence showing that HF did not directly inhibit signalling induced by TGF-β or IL-6, the two principal cytokines mediating Th17 differentiation, while it selectively inhibits Th17 differentiation and associated autoimmune inflammation via the cytoprotective AAR pathway 45. Thus, the activation of the AAR by HF may contribute to the repression of Th17 differentiation. Our study demonstrated the decrease in liver fibrosis along with the inhibition of Th17 cell differentiation. This may be an important pathway by which halofuginone inhibits hepatic fibrosis; however, further studies are still needed to explore the underlying mechanisms.
RORγt has been detected exclusively in cells of the lymphoid lineage 52. It is a molecule that was originally discovered to regulate gene expression during the development of T cells in the thymus 52, 53. Littman et al. demonstrated that RORγt is the key transcription factor in the differentiation programme of Th17 cells, although the mechanisms by which RORγt is regulated is unknown yet 54. In our study, the RORγt expression in the liver has no statistical change, suggesting that HF manipulates the differentiation of Th17 cells by other pathways rather than RORγt.
Because ConA-induced liver fibrosis is caused by the infiltration of repeated activated T cells, the potential immunomodulatory effect of HF may be responsible for its suppressive effect. Batra et al. showed that pretreatment of T cells with halofuginone resulted in a marked, although partial, decrease in the secretion of TNF-α and IFN-γ, as well as IL-4, IL-13 and TGF-β. These cytokines are known to regulate collagen synthesis 55 and thus are likely to be involved in the antifibrotic effect of halofuginone. Otherwise, IL-1β, IL-6 and IL-23 can enhance murine Th17 cell differentiation 56, 57, while IL-10 shows antifibrogenic and anti-inflammatory activities in animal models 58. We showed that HF effectively downregulated the production of TNF-α, IFN-γ, IL-6, IL-1β and IL-33 in chronic liver injury, but the change in IL-10 level is not significant, which lead to a reduced inflammatory response. The attenuated inflammatory response by HF treatment reduced the activation of hepatic stellate cells, which inhibits the release of profibrogenic factors and extracellular matrix. Although we showed that the treatment with HF reduced the expression levels of α-SMA and TGF-β1 compared with the ConA group, the exact mechanism for this occurrence is not known. Therefore, the reduced secretion of TNF-α, TGF-β1, IL-1β, IL-6 and IL-33 may contribute to the suppression action of HF on Th17 differentiation in liver fibrosis.
In summary, our study demonstrated, for the first time, the potential in vivo effect of HF on Th17 cell differentiation in ConA-induced liver fibrosis model rats. Th 17 cells play a pathogenic role in liver fibrosis. Interference with the development of Th17 can influence the severity of liver fibrosis in rat. This study provides additional information towards elucidating its mode of action and therapeutic potential. However, this study only demonstrated that HF can impact Th17 differentiation and its related cytokines production, thus reducing the severity of liver damage. The underlying mechanism of this has to be explored further.
Acknowledgment
This study was funded by grant-in-aids for The National Natural Science Foundation of China, code no: 81072398.
Disclosure
None of the authors has any conflict of interest to report.




