Exploring the impact of diagenesis on (isotope) geochemical and microstructural alteration features in biogenic aragonite
Abstract
For the Quaternary and Neogene, aragonitic biogenic and abiogenic carbonates are frequently exploited as archives of their environment. Conversely, pre-Neogene aragonite is often diagenetically altered and calcite archives are studied instead. Nevertheless, the exact sequence of diagenetic processes and products is difficult to disclose from naturally altered material. Here, experiments were performed to understand biogenic aragonite alteration processes and products. Shell subsamples of the bivalve Arctica islandica were exposed to hydrothermal alteration. Thermal boundary conditions were set at 100°C, 175°C and 200°C. These comparably high temperatures were chosen to shorten experimental durations. Subsamples were exposed to different 18O-depleted fluids for durations between two and twenty weeks. Alteration was documented using X-ray diffraction, cathodoluminescence, fluorescence and scanning electron microscopy, as well as conventional and clumped isotope analyses. Experiments performed at 100°C show redistribution and darkening of organic matter, but lack evidence for diagenetic alteration, except in Δ47 which show the effects of annealing processes. At 175°C, valves undergo significant aragonite to calcite transformation and neomorphism. The δ18O signature supports transformation via dissolution and reprecipitation, but isotopic exchange is limited by fluid migration through the subsamples. Individual growth increments in these subsamples exhibit bright orange luminescence. At 200°C, valves are fully transformed to calcite and exhibit purple-blue luminescence with orange bands. The δ18O and Δ47 signatures reveal exchange with the aqueous fluid, whereas δ13C remains unaltered in all experiments, indicating a carbonate-buffered system. Clumped isotope temperatures in high-temperature experiments show compositions in broad agreement with the measured temperature. Experimentally induced alteration patterns are comparable with individual features present in Pleistocene shells. This study represents a significant step towards sequential analysis of diagenetic features in biogenic aragonites and sheds light on reaction times and threshold limits. The limitations of a study restricted to a single test organism are acknowledged and call for refined follow-up experiments.
Introduction and Rationale
Marine biogenic carbonates are among the most important archives of past changes in ocean chemistry, water mass circulation and the evolution of life through geological times (Bruckschen et al., 1999; Veizer et al., 1999; Mii et al., 2001; Grossman et al., 2008; Immenhauser et al., 2016). Often, the well-preserved skeletal hardparts of archive organisms for the pre-Neogene period are made of calcite because this mineralogy has a high potential to withstand post-mortem, post-depositional diagenetic alteration (brachiopods, calcitic bivalves, cephalopods and the like; Veizer et al., 1999). Conversely, many workers dealing with Neogene and Holocene archives have made use of aragonitic archives too (for example, corals: James, 1974; Ruggeberg et al., 2008; or bivalves: Krause-Nehring et al., 2012; Schöne, 2013). Due to their thermodynamically metastable nature, aragonitic biominerals and aragonite cements are prone to diagenetic alteration (Brand & Morrison, 1987; Swart, 2015). Whereas pervasive geochemical alteration or neomorphism of these materials is commonly recognized by means of petrographic or geochemical analyses, subtle alteration features are more difficult to pinpoint. More generally, mechanistic processes governing diagenetic alteration of biogenic to abiogenic aragonite and aragonite to calcite transformation (Brachert & Dullo, 2000) are underdetermined. This fact emphasizes a major obstacle in palaeoceanographic research when dealing with aragonite skeletal materials in general.
This study reports the results of a combined experimental and field study exposing aragonitic shell subsamples to different experimental diagenetic settings under controlled temperatures (100 to 200°C), fluid compositions and experimental durations (days to months). The aragonitic valves of the mollusc Arctica islandica (ocean quahog) the longest-living solitary animal known (>500 years; Butler et al., 2013) are used; these represent commonly exploited environmental archives particularly for the North Atlantic domain (Schöne, 2013). Hydrothermal alteration experiments on shell subsamples of A. islandica are combined with carbon and oxygen isotope and carbonate clumped isotope geochemistry, petrography, cathodoluminescence and fluorescence microscopy. The elevated temperatures were chosen to limit the experimental durations to weeks rather than 103 to 106 of years as is the case in natural diagenetic environments. This is because Arrhenius equation reaction rate constants are highly temperature dependent (Levine, 2009). Thus, the experiments performed herein must not be seen as an ultimate approach to directly simulate natural diagenetic reaction pathways because temperature and also rock to fluid ratios in the porewater domain are lower than in the experiments performed here and so are reaction rates in natural settings. Moreover, in natural diagenetic environments, organic compounds within bivalve shells are slowly degraded in the presence of microbial communities (Collins et al., 1992; O'Donnell et al., 2007). In the experiments performed here, water-soluble organic materials are mobilized within the shells and then decay at temperatures above about 160°C (Bénézeth et al., 1997). The above aspects are the limitations of the approach presented here. In contrast, the authors wish to emphasize that the predominant dissolution and precipitation processes for aragonite and calcite, respectively, in aqueous environments between 25°C and ≈200°C follow the same overall reaction mechanisms by considering exchange of Ca2+ and 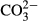 between the solid surface and the solution. Aquocomplex formation and solid surface characteristics vary as temperature increases, but most sensitive changes are related to triggering of the above processes by increasing rate constants of individual forward and backward reactions. Consistently, the observed alteration features resulting from experiments documented in the present paper (aggradational neomorphism, changes in cathodoluminescence or fluorescence properties, remobilization and redistribution of organic matter, etc.) remarkably resemble those observed in naturally altered carbonates.
between the solid surface and the solution. Aquocomplex formation and solid surface characteristics vary as temperature increases, but most sensitive changes are related to triggering of the above processes by increasing rate constants of individual forward and backward reactions. Consistently, the observed alteration features resulting from experiments documented in the present paper (aggradational neomorphism, changes in cathodoluminescence or fluorescence properties, remobilization and redistribution of organic matter, etc.) remarkably resemble those observed in naturally altered carbonates.
The aims of this study were three-fold: (i) to document the outcome of carefully constrained alteration experiments using biogenic aragonite; (ii) to discuss these findings in the context of geochemical and petrographic processes and products observed; and (iii) to compare and contrast artificially induced diagenetic features with those from naturally altered aragonitic bivalves. This work is significant for studies on marine aragonite-shelled organisms as archives of their environment and sheds light on the dominant processes during aragonite diagenesis in general.
Materials and Methods
Sample material and pre-alteration preparation techniques
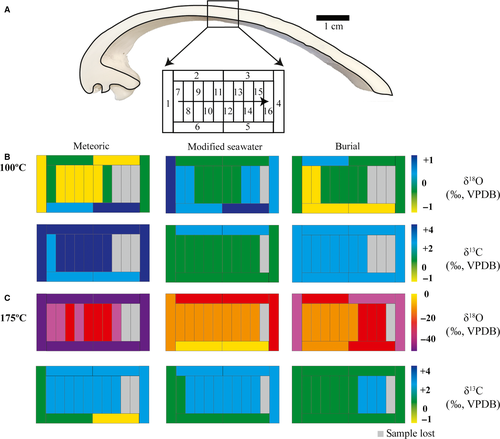
The sampling approach used here for A. islandica is illustrated in Fig. 1. Material for alteration experiments comes from a collection of about 50 A. islandica shells (each with two corresponding valves) dredged from the sea floor off north-east Iceland in 2010, at water depths between 40 m and 120 m [3 to 4°C water temperature (Locarnini et al., 2006; Jochumsen et al., 2016)]. Shells of adult specimens (ca 70 years) with shell heights (= distance umbo-ventral margin) of ca 10 cm and valve thicknesses of 2 to 5 mm were selected. Bivalves with near-identical dimensions are not a priori of the same ontogenic age because growth rates vary among specimens. Based on sclerochronological data, the age range between different specimens used here was estimated to be in the order of ≤10 years. Selected valves were divided into two halves (Fig. 1A) using a diamond saw. Subsequently, a ca 2·5 cm wide longitudinal shell section, including portions of the central axis of the bivalve and the hinge (Fig. 1B), was cut from one of the halves. This longitudinal section was further cut into eight to ten subsamples of similar dimensions (ca 2·5 × 1·0 cm; Fig. 1B and C) for incubation in the experiments.
Holocene and Pleistocene specimens of A. islandica were sampled in uplifted coastal terraces along the north-eastern coast of Iceland. Judging from direct field evidence, these shells underwent shallow marine burial, followed by uplift. Thus, they experienced the influence of marine porewater, meteoric and low-temperature hydrothermal (volcanic) fluids, respectively. As such, they constitute suitable material for a comparison with artificially induced alteration features in test organisms.
Alteration experiments
Alteration experiments were performed in the laboratories of the Graz University of Technology (Austria). Arctica islandica subsamples were exposed to aqueous fluids (Fig. 1D) of different chemical composition representing: (i) artificial meteoric water; (ii) modified seawater from the North Sea (south-east of Helgoland); and (iii) artificial burial fluid. Essentially, aqueous fluids representing meteoric water and burial fluid contained 10 mm NaCl and 100 mm NaCl + 10 mm MgCl2, respectively. Seawater has been treated (modified) with BaCl2 to reduce SO42− concentrations from about 28 to 1 mm to suppress formation of calcium sulphate minerals during the experiments. All fluids were spiked with 18O-depleted H2O [IASON, laboratory reference water W-26; δ18O = −395‰, Vienna Standard Mean Ocean Water (VSMOW)]. The mean oxygen isotope ratios (VSMOW) of artificial meteoric and burial fluids are about −47‰ (≈1:10 mixture of 18O-depleted ultrapure Milli-Q water). Modified seawater has a δ18OVSMOW of −20·6‰ (≈1:20 mixture of 18O-depleted ultrapure Milli-Q water; Table 2). Measured isotope data of shell carbonates are reported in the conventional delta notation and normalized to Vienna Pee-Dee Belemnite (VPDB).
Individual shell subsamples were placed in Teflon™ liners and 25 ml of aqueous fluid was added. The liners were sealed with a Teflon lid, placed in a stainless steel autoclave and exposed to 100°C, 175°C or 200°C for periods between two and 20 weeks. Thereafter, a given autoclave was removed from the oven, cooled down to room temperature and opened. After passing through a 0·2 μm cellulose acetate filter, the remaining aqueous fluid was analysed for its chemical and isotopic composition. Valve subsamples were dried at 40°C overnight prior to further analyses. All 100°C samples were incubated for 20 weeks, while 175°C samples were incubated either for three (burial fluid) or for five weeks (meteoric fluid, modified seawater). The samples altered at 200°C were exposed to experimental conditions for two weeks in either marine or burial fluid.
Post-alteration preparation
After cooling, and drying, valve subsamples were halved (ca 1 × 1 cm) using a steel saw blade (Fig. 1E). One half was used for thin-section preparation and the other for geochemical and isotopic analyses. Six altered subsamples were chosen for petrographic and geochemical analyses. Each of these was representative for one experimental set-up and characterized by either a different temperature (100°C, 175°C) or a different experimental fluid. Additional data come from two samples altered at 200°C. Generally, less emphasis was placed on samples from 200°C experiments because the degree of alteration was so pervasive that, in common practice, these shells showed artificial alteration features that share only limited similarities with naturally altered shells.
For geochemical analyses, subsamples were mounted on an aluminium plate using epoxy resin and powder samples were milled using a computer-controlled milling device (CAM 100; vhf camfacture AG, Ammerbuch, Germany). The detailed sampling approach is shown in Fig. 1F. Firstly, the outer rim of each specimen (samples 1 to 6) was sampled in order to differentiate shell portions that were in direct contact with fluids from those located in the centre of the subsample. From the remaining portions of the subsamples, a series of commonly seven to ten powder samples was milled (samples 7 to end).
Analytical methods
Optical methods
Thin sections of A. islandica subsamples were analysed by means of polarization microscopy (Leica DM4500P; Leica Microsystems GmbH, Wetzlar, Germany) in order to compare petrographic properties of pristine, artificially and naturally altered specimens. Fluorescence microscopy was performed using a Leica EL6000 compact light source coupled to the microscope. Best results were achieved by using a blue light filter which yielded bright green fluorescence pictures of the analysed carbonate material (filter set I3 for blue light excitation: excitation 450 to 490 nm, emission 515 nm). The voltage of the fluorescence light source (mercury short-arc reflector lamp) varied between 100 V and 240 V AC and the frequency was 50 to 60 Hz. Fluorescence microscopy has been applied to identify changes in the organization of organic matter (OM). Cathodoluminescence microscopy has been performed on all samples using a HC1-LM hot cathode cathodoluminescence microscope developed at Ruhr-University Bochum (Neuser et al., 1995; Lumic, Dortmund, Germany). The electron beam current was 0·2 mA and accelerated with 14 keV. To investigate crystal morphology, small portions of the shell subsamples were broken off, coated with a thin carbon layer and studied under a scanning electron microscope (SEM). The device used here was a LEO/Zeiss Gemini 1530 Scanning Electron Microscope (Carl Zeiss AG, Oberkochen, Germany) with an accelerating voltage of 20 kV and a beam current of 0·3 mA. For visualization of the organic matrix, additional samples were microtome cut, polished, etched with HEDES solution for 180 sec, fixed with glutaraldehyde and critical point dried. Scanning electron microscope pictures of these specifically treated samples can show biopolymer networks and recrystallization features.
Mineralogical analyses
Powder X-ray diffraction (PXRD) was used to quantify mineralogical changes after alteration experiments. Samples were ground in an agate mortar and filled into glass capillaries (diameter: 0·3 mm, wall thickness: 0·01 mm) mounted and aligned on a rotating goniometer head. The PXRD measurements were performed on a Bruker D8 ADVANCE diffractometer using molybdenum radiation (λ1 = 0·7093 Å) equipped with a Goebel mirror and LYNXEYE detector (Bruker Corporation, Billerica, MA, USA). Scanning range was 5 to 35°2θ with a step size of 0·009° and every step lasted 2 s. Phase analysis and wt-% calculation were carried out by Rietveld refinement using the FullProf Suite 2·05 (Rodríguez-Carvajal, 1993). The number of analysed samples was limited due to the small amount of remaining material after isotope sampling.
Isotope analyses
Carbon and oxygen isotope ratios of the shell materials were analysed using a Gasbench coupled to a ThermoFinnigan MAT253 mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) in the geochemistry and stable isotope laboratory at Ruhr-University Bochum. Carbon and oxygen isotopic ratios are given in ‰ relative to the Vienna Pee-Dee Belemnite (VPDB) standard. Carbonate standards CO1, CO8, NBS19 and an internal standard were used to normalize the data. Masses of 0·35 to 0·40 mg of sample material have been weighed into 12 ml glass vials and dried in an oven (ca 105°C) for 48 h. The sample vials were heated up to 70°C and flushed with He. On average 10 drops of phosphoric acid were added to the samples and the CO2 was measured for its carbon and oxygen isotopic composition in continuous flow mode. Replicate measurements of internal standards give standard deviations of 0·1‰ for oxygen and 0·04‰ for carbon isotopes. The δ values of the solids on the VPDB scale were transformed to the VSMOW scale using the expression δ18OVSMOW = 1·03091 * δ18OVPDB + 30·91 (Coplen et al., 1983) for calculation of apparent oxygen isotope fractionation (Dietzel et al., 2016).
The oxygen isotope composition of the water used in these experiments was measured by the classical CO2–H2O equilibrium technique (Epstein & Mayeda, 1953) with a fully automated device adapted from Horita et al. (1989) coupled to a Finnigan DELTAplus Mass Spectrometer (Thermo Fisher Scientific) at JR-AquaConSol GmbH in Graz. The water–CO2 equilibration was carried out at 20°C. Normalization of raw results to the VSMOW–SLAP (Vienna Standard Mean Ocean Water–Standard Light Antarctic Precipitation) scale was achieved using a two-point calibration of in-house water standards that have been calibrated against the international standards VSMOW, GISP (Greenland Ice Sheet Projects) and VSLAP with an analytical error of ±0·05‰ for δ18O.
Additionally, seven shell subsamples [two of each alteration temperature: 100°C, 175°C (rim) and 200°C (centre) and one unaltered sample] have been analysed for their clumped isotope composition at the ETH Zurich. The samples were measured repeatedly (n = 10 to 20) in aliquots of 90 to 120 μg by reaction with 104% phosphoric acid at 70°C using a Kiel IV-MAT253 system (Thermo Fisher Scientific) following the methodology of Schmid & Bernasconi (2010) with improvements described in detail in Meckler et al. (2014). These improvements include: a pressure baseline correction of the raw beam signals; transfer of data to the absolute reference frame; correction for acid fractionation; correction for average standard offsets; and, when necessary, correction for Δ47 scale compression based on offsets among carbonate standards with different ordering state. The standards ETH-1, 2, 3 und 4 were used. The values of these standards are reported in Meckler et al. (2014) and Kele et al. (2015). The long-term reproducibility of the method based on internal standards is ±0·012 to 0·016‰ (1 SD; Meckler et al., 2014). Masses 48 and 49 were monitored to check for possible contamination according to Affek & Eiler (2006). The δ13C and δ18O results are expressed in the conventional delta notation on the VPDB scale. The Δ47 values are reported on the absolute reference frame of Dennis et al. (2011).
The Δ47 values of aragonites were converted to temperature using the empirical calibration established at ETH Zurich with the same analytical set-up. This calibration is based on travertine samples (calcites and aragonites) precipitated at temperatures between 6°C and 95°C and verified with biogenic samples, which fit very well on the regression line (Kele et al., 2015). Although this calibration does not cover the entire temperature range from this study, it was used because no other published calibration exists for carbonates reacted at 70°C with a Kiel IV carbonate device and because the calibration after Kele et al. (2015) has proven to yield robust results also at higher temperatures (Millán et al., 2016). This is important because the reason for different calibration slopes may be related to the sample preparation methodology complicating the comparison of data from different laboratories. In addition, using other calibrations (Dennis & Schrag, 2010) does not significantly change the temperature estimates because these overlap within uncertainties.
Results
Mesoscopic, mineralogical and petrological patterns
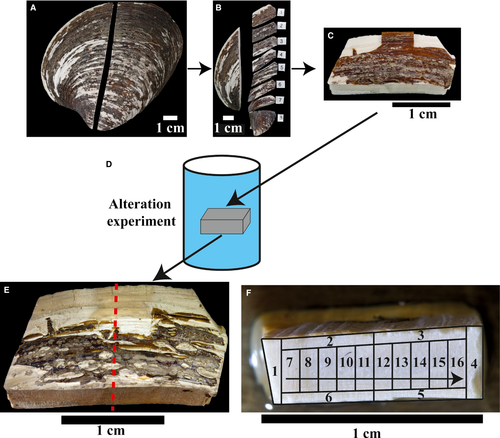
Pristine shells of A. islandica consist of two shell layers (inner and outer) separated by the pallial myostracum (Fig. 2A). The outer shell layer is covered by the organic periostracum, representing an outer protective boundary against chemical and mechanical stress (Schöne, 2013; refer to references cited in Immenhauser et al., 2016, for a discussion of the biomineralization strategies among bivalves). Thickness and preservation of the periostracum differ between subsamples. Subsamples used for alteration experiments are mainly composed of the outer shell layer (ostracum) characterized by growth lines but subordinate portions of the inner shell layer are also present. The present study deliberately targeted mainly the outer shell layer because it is more commonly exploited for palaeoenvironmental studies (Schöne, 2013). Thin sections of all pristine outer shell layers display growth lines with alternating darker and lighter colour due to variable amounts of intrabiomineral and interbiomineral organic matrices typically ranging in abundance between 0·01 and 5·0 wt-% (Fig. 2B; Marin et al., 2012).
Based on thin-section analysis, samples treated at 100°C remain largely unaltered with respect to their mineralogy and shell ultrastructure, independent of fluid type (Fig. 2C). X-ray diffraction analyses revealed a mineralogical composition of 90 to 95 wt-% aragonite in a sample incubated in the meteoric fluid at 100°C. Some of the subsamples show evidence for patchy accumulation of dark organic matter at the shell margins. Subsamples composed of both, the outer and portions of the inner shell layer, sometimes split along the pallial myostracum, documenting that the two shell layers have different mechanical properties. Other than that, no distinct microscopical fabric changes could be found in samples altered at 100°C.
Samples exposed to 175°C display mesoscopic alteration features on all surfaces. While the periostracum has vanished, shell material shows brown staining (Fig. 1E). Distinct changes in crystal morphology, size and mineralogy are visible in all samples, independent of experimental duration and fluid. The XRD analysis shows that 70 to 100% of the biogenically formed aragonitic crystals were transformed into abiogenic calcite (Table 2). Samples built by about 70 wt-% calcite and 30 wt-% aragonite were taken from the inner shell layer. Additionally, in some of the samples exposed to fluid temperatures of 175°C, the inner shell layer does not show significant recrystallization features, neither in thin sections nor under the SEM. Essentially, shell samples display evidence for mineralogical transformation and neomorphism with smaller crystals at the outer margins of samples and larger crystals (dimensions of several hundred micrometres) in more central portions. The long axis of crystals is sometimes oriented parallel to growth increments (Fig. 2D). Growth lines are still visible despite significant transformation, probably due to micro-inclusions of organic matter. Fluid chemistries and transformation patterns appear to be related in a complicated manner. Specifically, shell subsamples exposed to seawater display a more irregular size distribution relative to subsamples exposed to meteoric or burial fluids. Samples altered at 175°C in meteoric reactive fluid show the most isometric shape and average crystal diameters mostly vary between 250 μm and 400 μm. Crystals formed in seawater and burial fluid are elongated (100 to 200 × 200 to 450 μm) but also isometric crystals are found (sizes up to 350 × 400 μm). Care must be taken not to confuse sample bias (different portions of the shell, different specimen) and genuine controls of fluid chemistry on recrystallization patterns. The inner shell layer, where present, seems to be less affected by alteration compared to the thicker outer shell layer, suggesting different material properties.
Samples exposed to 200°C display pronounced mesoscopic corrosion features and patchy colour changes at sample surfaces and a complete decomposition of the periostracum. The aragonite was neomorphosed to calcite. Crystal size increases with increasing temperature. Dimensions of the largest crystals increase from 300 to 400 μm (175°C) to almost 500 μm (200°C; Fig. 2D and E). The fabric typical for 200°C alteration is characterized by a hexagonal to octagonal crystal morphology. Individual crystals contain (often translucent) triangular subcrystals. Approximately isopachous and elongated crystals (<500 μm length) form perpendicular to the outer shell surface.
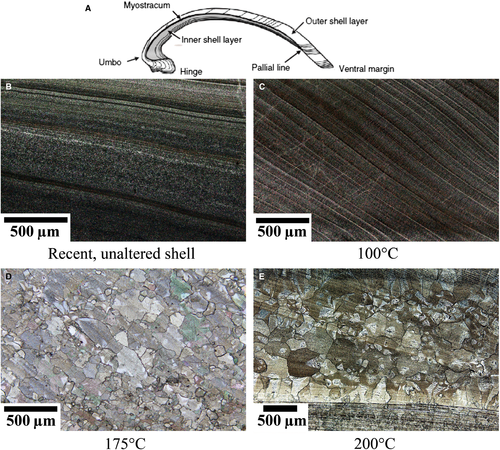
Scanning electron microscope images of different shell portions (Figs 3 and 4) show that both inner and outer shell layer exposed to 100°C (Fig. 3B) and the fossil sample (Fig. 3E) still have pristine fabrics (Fig. 3A; Ropes et al., 1984; Dunca et al., 2009). In contrast, in samples treated at 175°C, the outer shell layer is composed of large crystals, while the inner shell layer largely preserved primary features (Fig. 3C). In the sample altered at 200°C, crystal sizes further increase and the inner layer becomes blocky calcite (Fig. 3D). Elongated crystals are aligned perpendicular to the outer surface of subsamples and overlain by larger (often) octagonal crystals towards the centre of the subsample.
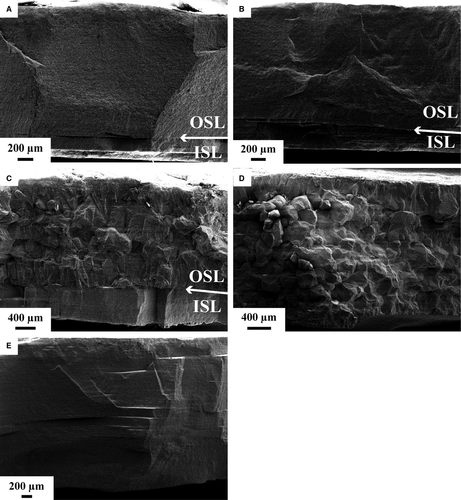
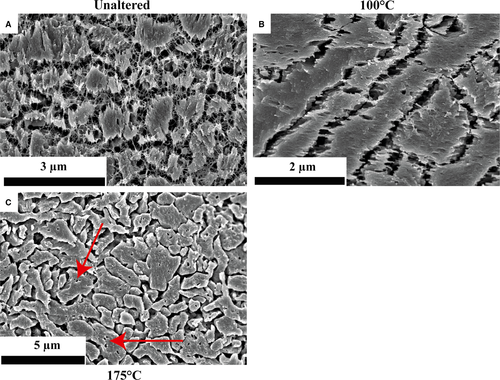
In polished and etched subsamples (Fig. 4), aragonite crystals in unaltered material (Fig. 4A) are embedded in a fibrous biopolymer network. In subsamples incubated at 100°C, the biopolymer network is only rarely preserved (Fig. 4B). Apart from that, the aragonitic samples mainly display the characteristics of unaltered biominerals. At 175°C (Fig. 4C) the size of individual neomorphosed crystals increased and the organic matrix decomposed. Small voids in the crystals mark the former position of organic matrices.
Cathodoluminescence
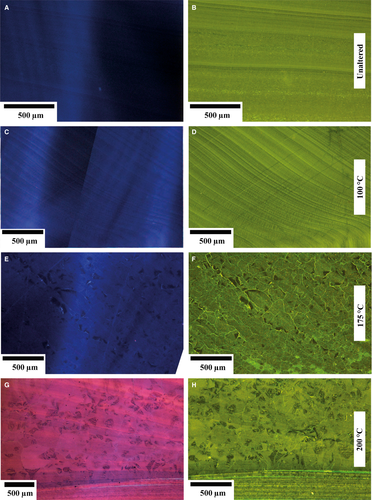
Pristine shell material and subsamples altered at 100°C (Fig. 5A and C) show intrinsic blue cathodoluminescence colours, implying that the spatial distribution of quenching and activating elements in the crystal lattice is unchanged (ten Have & Heijnen, 1985; Habermann et al., 1998; Barbin, 2013). Shells altered at 175°C mainly reveal dark blue and dull luminescence, locally with non-luminescent portions (Fig. 5E) or bright growth increments within an otherwise intrinsic blue luminescent shell (Fig. 6C). Subsamples altered at 200°C display distinct purple to blue luminescence, alternating with orange zones (Fig. 5G). Luminescence colours are spatially different not only between former growth increments, but also within individual crystals characterized by triangular-shaped subcrystals, and often showing zonation with alternating dark and purple luminescence.
Fluorescence
Fluorescence (Fig. 5) reflects organic matter and, less commonly, crystal lattice defects and solid inclusions in crystals (Wanamaker et al., 2009). Fluorescence patterns of pristine shells display different light to dark green colours tracing the darker and lighter colours of growth increments observed under thin-section microscopy as mainly induced by differential organic matter distribution (Fig. 5B). Subsamples altered at 100°C (Fig. 5D) show fluorescence patterns comparable with pristine shells. Essentially, dark and light green patterns agree well with growth increments observed under conventional light microscopy (Fig. 2). Conversely, subsamples altered at 175°C display fluorescence patterns that were induced during the alteration experiment. Examples include light green fluorescence patterns along the boundaries of secondary calcite crystals. Moreover 100 μm scale mottled patches of dark and light green fluorescence colours are present (Fig. 5F). Orientation of growth increments, even if only relictic, is still visible. Shell subsamples altered at 200°C display a generally light green fluorescence. Different patterns in fluorescence coincide with subcrystals formed within larger crystals. Despite microscopic evidence for aragonite to calcite transformation and neomorphism, the inner shell layer (Fig. 5H) preserves some attributes of the primary fluorescence patterns characteristic for unaltered shells.
Carbon and oxygen isotope ratios
Isotope variability in pristine shells
For the evaluation of the significance of geochemical alteration, natural variability in A. islandica shell δ13C and δ18O values must be taken into account. Intrashell variability is induced by seasonal or ontogenetic patterns in both δ13C and δ18O (Schöne, 2013). Thus, spatially resolved analyses were performed prior to this study. Results are shown in Table 1 and compared with published data (Schöne et al., 2004; Eagle et al., 2013; Henkes et al., 2013; Beierlein et al., 2015) from different locations. An unaltered shell was analysed for patterns in isotope ratios. In the inner shell layer of the unaltered specimen, δ13C fluctuates from +2·0 to +2·8‰ (average 2·4 ± 0·02‰) while in the outer shell layer δ13C ranges from +1·6 to +2·9‰ (average 2·4 ± 0·02‰). Oxygen isotope ratios of the inner shell layer vary between +2·2‰ and +3·1‰ (average 2·7 ± 0·06‰), while δ18O in the outer shell layer ranges from +2·0 to +3·0‰ (average 2·6 ± 0·06‰). All isotope values display a cyclical pattern (corresponding to seasonal environmental changes) with decreasing amplitudes with increasing ontogenetic age.
| Authors | δ13C [‰] | δ18O [‰] |
|---|---|---|
| Pilot experiments for this study | +1·6 to +3·0 | +2·0 to +3·1 |
| Beierlein et al. (2015) | +1·0 to +4·3 | |
| Butler et al. (2009) | −0·7 to +2·4 | |
| Butler et al. (2011) | +0·3 to +2·7 | |
| Dunca et al. (2009) | −1·68 to +3·26 | |
| Eagle et al. (2013) | −2·6 to +2·3 | −1·3 to −0·3 |
| +1·4 to +1·9 | +3·1 to +3·5 | |
| Henkes et al. (2013) | +1·4 | +3·62 |
| Schöne et al. (2004) | +0·78 to +3·55 | |
| Schöne et al. (2005a) | +2·4 to +4·0 | +0·8 to +3·5 |
| Schöne et al. (2005b) | +1·40 to +3·17 | +2·41 to +3·52 |
| Schöne et al. (2011) | +0·74 to +3·75 |
Isotope variability in experimentally altered shells
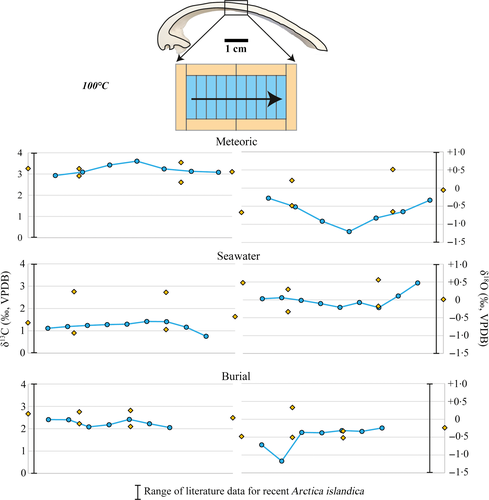
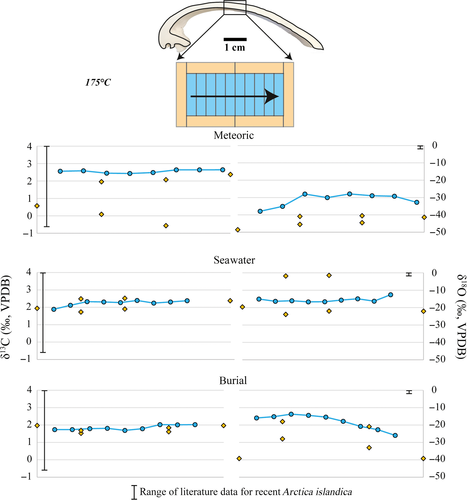
Figures 7-9 illustrate δ13C and δ18O from altered shell subsamples exposed to fluid temperatures of 100°C and 175°C and the three aqueous fluids. The δ18O composition of the experimental fluids is shown in Table 2. The δ18O composition of the fluids is – within analytical error – identical before and after the experiments (Table 2). Dissolved inorganic carbon has a δ13C value equal to that of North Sea water.
| Temperature | Incubation time [weeks] | Fluid | Sample number (see Fig. 1F) | δ13CPDB [‰] | ± s | δ18OPDB-carbonate [‰] | ± s | δ18OSMOW-fluid [‰] | Mineralogy determined via XRD (wt-%) |
|---|---|---|---|---|---|---|---|---|---|
| 100 | 20 | Meteoric | 1 | 3·26 | 0·03 | −0·67 | 0·03 | −47·43 | 95% aragonite |
| 4 | 3·11 | 0·05 | −0·05 | 0·08 | −47·43 | ||||
| 3 | 3·54 | 0·03 | −0·65 | 0·07 | −47·43 | ||||
| 2 | 3·25 | 0·06 | −0·49 | 0·11 | −47·43 | ||||
| 5 | 2·61 | 0·03 | 0·51 | 0·04 | −47·43 | 95% aragonite | |||
| 6 | 2·91 | 0·05 | 0·21 | 0·09 | −47·43 | ||||
| 7 | 2·93 | 0·05 | −0·28 | 0·07 | −47·43 | ||||
| 8 | 3·09 | 0·03 | −0·52 | 0·04 | −47·43 | ||||
| 9 | 3·42 | 0·04 | −0·91 | 0·05 | −47·43 | ||||
| 10 | 3·61 | 0·03 | −1·20 | 0·08 | −47·43 | ||||
| 11 | 3·24 | 0·04 | −0·83 | 0·02 | −47·43 | ||||
| 12 | 3·13 | 0·04 | −0·65 | 0·04 | −47·43 | ||||
| 13 | 3·08 | 0·03 | −0·33 | 0·05 | −47·43 | 90% aragonite | |||
| 175 | 5 | Meteoric | 1 | 0·57 | 0·04 | −48·60 | 0·07 | −47·43 | |
| 4 | 2·38 | 0·03 | −41·40 | 0·09 | −47·43 | ||||
| 3 | 2·07 | 0·03 | −40·54 | 0·05 | −47·43 | ||||
| 2 | 1·96 | 0·06 | −45·53 | 0·11 | −47·43 | ||||
| 5 | −0·57 | 0·03 | −44·53 | 0·08 | −47·43 | ||||
| 6 | 0·09 | 0·06 | −40·90 | 0·14 | −47·43 | ||||
| 7 | 2·56 | 0·04 | −37·92 | 0·07 | −47·43 | ||||
| 8 | 2·59 | 0·02 | −35·12 | 0·07 | −47·43 | ||||
| 9 | 2·45 | 0·04 | −27·99 | 0·07 | −47·43 | ||||
| 10 | 2·43 | 0·03 | −30·06 | 0·11 | −47·43 | ||||
| 11 | 2·49 | 0·04 | −27·91 | 0·06 | −47·43 | ||||
| 12 | 2·64 | 0·03 | −28·96 | 0·05 | −47·43 | ||||
| 13 | 2·64 | 0·03 | −29·25 | 0·05 | −47·43 | ||||
| 14 | 2·64 | 0·02 | −32·74 | 0·05 | −47·43 | ||||
| 100 | 20 | Burial | 1 | 2·67 | 0·02 | −0·48 | 0·07 | −47·17 | |
| 4 | 2·49 | 0·04 | −0·24 | 0·06 | −47·17 | ||||
| 3 | 2·82 | 0·02 | −0·33 | 0·04 | −47·17 | ||||
| 2 | 2·76 | 0·05 | 0·33 | 0·07 | −47·17 | ||||
| 5 | 2·10 | 0·04 | −0·52 | 0·10 | −47·17 | ||||
| 6 | 2·22 | 0·02 | −0·51 | 0·03 | −47·17 | ||||
| 7 | 2·41 | 0·02 | −0·72 | 0·03 | −47·17 | ||||
| 8 | 2·40 | 0·04 | −1·17 | 0·08 | −47·17 | ||||
| 9 | 2·08 | 0·02 | −0·37 | 0·06 | −47·17 | ||||
| 10 | 2·18 | 0·04 | −0·38 | 0·05 | −47·17 | ||||
| 11 | 2·42 | 0·03 | −0·32 | 0·08 | −47·17 | ||||
| 12 | 2·22 | 0·04 | −0·34 | 0·06 | −47·17 | ||||
| 13 | 2·05 | 0·04 | −0·24 | 0·04 | −47·17 | ||||
| 175 | 3 | Burial | 1 | 1·97 | 0·04 | −39·23 | 0·13 | −47·17 | |
| 4 | 1·97 | 0·04 | −39·23 | 0·13 | −47·17 | ||||
| 3 | 1·62 | 0·04 | −33·01 | 0·04 | −47·17 | ||||
| 2 | 1·53 | 0·02 | −27·95 | 0·04 | −47·17 | 68% calcite | |||
| 5 | 1·84 | 0·02 | −20·90 | 0·02 | −47·17 | 87% calcite | |||
| 6 | 1·69 | 0·03 | −18·03 | 0·10 | −47·17 | ||||
| 7 | 1·74 | 0·04 | −15·91 | 0·08 | −47·17 | ||||
| 8 | 1·74 | 0·04 | −15·19 | 0·06 | −47·17 | ||||
| 9 | 1·79 | 0·04 | −13·73 | 0·06 | −47·17 | ||||
| 10 | 1·81 | 0·05 | −14·43 | 0·10 | −47·17 | ||||
| 11 | 1·69 | 0·03 | −15·42 | 0·04 | −47·17 | 100% calcite | |||
| 12 | 1·79 | 0·04 | −17·82 | 0·14 | −47·17 | ||||
| 13 | 2·02 | 0·05 | −20·75 | 0·05 | −47·17 | ||||
| 14 | 2·01 | 0·03 | −22·68 | 0·11 | −47·17 | ||||
| 15 | 2·02 | 0·06 | −25·95 | 0·13 | −47·17 | ||||
| 100 | 20 | Marine | 1 | 1·36 | 0·05 | 0·48 | 0·14 | −20·61 | |
| 4 | 1·64 | 0·05 | 0·01 | 0·15 | −20·61 | ||||
| 3 | 2·72 | 0·06 | −0·18 | 0·07 | −20·61 | ||||
| 2 | 2·75 | 0·03 | −0·33 | 0·05 | −20·61 | ||||
| 5 | 1·05 | 0·02 | 0·56 | 0·03 | −20·61 | ||||
| 6 | 0·90 | 0·05 | 0·29 | 0·06 | −20·61 | ||||
| 7 | 1·11 | 0·02 | 0·03 | 0·03 | −20·61 | ||||
| 8 | 1·19 | 0·04 | 0·06 | 0·07 | −20·61 | ||||
| 9 | 1·24 | 0·04 | −0·01 | 0·09 | −20·61 | ||||
| 10 | 1·28 | 0·05 | −0·10 | 0·03 | −20·61 | ||||
| 11 | 1·30 | 0·02 | −0·21 | 0·02 | −20·61 | ||||
| 12 | 1·42 | 0·03 | −0·07 | 0·09 | −20·61 | ||||
| 13 | 1·41 | 0·03 | −0·22 | 0·06 | −20·61 | ||||
| 14 | 1·16 | 0·02 | 0·11 | 0·04 | −20·61 | ||||
| 15 | 0·76 | 0·03 | 0·47 | 0·09 | −20·61 | ||||
| 175 | 5 | Marine | 1 | 1·94 | 0·05 | −19·60 | 0·06 | −20·61 | |
| 4 | 2·38 | 0·03 | −22·03 | 0·02 | −20·61 | ||||
| 3 | 2·52 | 0·03 | −21·98 | 0·05 | −20·61 | ||||
| 2 | 2·49 | 0·04 | −23·89 | 0·06 | −20·61 | ||||
| 5 | 1·90 | 0·03 | −1·41 | 0·02 | −20·61 | ||||
| 6 | 1·73 | 0·02 | −1·78 | 0·03 | −20·61 | ||||
| 7 | 1·88 | 0·04 | −15·03 | 0·07 | −20·61 | ||||
| 8 | 2·12 | 0·03 | −16·34 | 0·06 | −20·61 | ||||
| 9 | 2·32 | 0·05 | −16·03 | 0·08 | −20·61 | ||||
| 10 | 2·31 | 0·05 | −16·67 | 0·07 | −20·61 | ||||
| 11 | 2·27 | 0·02 | −16·61 | 0·04 | −20·61 | ||||
| 12 | 2·39 | 0·02 | −15·66 | 0·05 | −20·61 | ||||
| 13 | 2·24 | 0·02 | −14·91 | 0·05 | −20·61 | ||||
| 14 | 2·31 | 0·05 | −16·26 | 0·08 | −20·61 | ||||
| 15 | 2·38 | 0·03 | −12·56 | 0·04 | −20·61 | ||||
| 200 | 2 | Marine | − | 1·20 | 0·01 | −18·73 | 0·07 | −20·61 | 100% calcite |
| Burial | − | 2·89 | 0·02 | −38·79 | 0·06 | −47·17 | 100% calcite |
Alteration experiments with all three fluids at 100°C show the same range in δ13C and δ18O as pristine valves (Tables 1 and 2). The small differences observed do not exceed natural background variability (Table 1). In the 175°C alteration experiments, δ18O is significantly altered, with values for the neomorphosed calcite ranging from −48·6 to −1·4‰. The data point with a δ18O value of −1·4‰ stems from the inner shell layer. Experiments with less 18O-depleted modified seawater (δ18Osw = −20·6‰) coincide with less 18O-depleted isotope values in the altered shell material (δ18Omean = −15·4‰) compared to experiments with the more depleted meteoric and burial experimental fluids (δ18Omet = −47·4‰; δ18Obur = −47·2‰; Table 2). The sample altered at 200°C in seawater yielded a δ18O ratio of −18·7‰ (±0·07) and the sample altered in the burial fluid (200°C) of −38·8‰ (±0·06). Shell subsamples from all experiments did not show a significant alteration of δ13C (Tables 1 and 2).
Clumped isotope thermometry
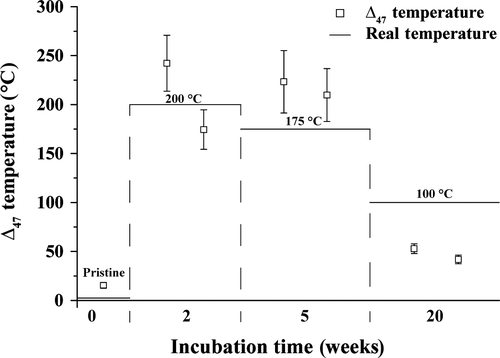
Clumped isotope data are shown in Table 3 and Fig. 10. The amount of sample material available only allowed for the measurement of 10 aliquots per sample; thus, the analytical error is relatively large. However, differences between the samples are significant. The pristine shell yielded a Δ47 value of 0·731‰. Samples incubated at 100°C (20 weeks) gave Δ47 values of 0·615‰ and 0·646‰. The two samples altered at 175°C show Δ47 values of 0·379‰ and 0·389‰, whereas those heated to 200°C range from 0·366 to 0·420‰, significantly lower than the original and within error of the experimental temperature.
| Alteration temperature | Incubation time [weeks] | Fluid | Standard C/O isotope measurements | Clumped isotope measurements | Δ47 | SE | Temperature (Kele et al., 2015) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δ18O [PDB] | SD | δ13C [PDB] | SD | δ18O [PDB] | SD | δ13C [PDB] | SD | ||||||
| 100°C | 20 | Burial | −0·33 | 0·04 | 2·82 | 0·02 | 0·07 | 0·08 | 2·86 | 0·04 | 0·646 | 0·016 | 41·2 |
| 20 | Burial | −0·52 | 0·10 | 2·10 | 0·04 | 0·26 | 0·04 | 2·29 | 0·06 | 0·644 | 0·013 | 41·8 | |
| 175°C | 5 | Burial | −18·03 | 0·10 | 1·69 | 0·03 | −18·26 | 0·24 | 1·82 | 0·02 | 0·379 | 0·012 | 223·4 |
| 5 | Burial | −33·01 | 0·04 | 1·62 | 0·04 | −30·03 | 0·80 | 1·86 | 0·02 | 0·389 | 0·011 | 209·8 | |
| 200°C | 2 | Marine | −18·73 | 0·07 | 1·20 | 0·01 | −18·74 | 0·17 | 1·32 | 0·02 | 0·366 | 0·007 | 242·1 |
| 2 | Burial | −38·79 | 0·06 | 2·89 | 0·02 | −32·27 | 0·25 | 2·51 | 0·02 | 0·420 | 0·011 | 174·4 | |
| Pristine, unaltered | – | – | 2·51 | 0·05 | 3·17 | 0·03 | 3·578 | 0·042 | 2·753 | 0·026 | 0·731 | 0·016 | 14·9 |
Diagenetic features in naturally altered (Pleistocene) shells of Arctica islandica
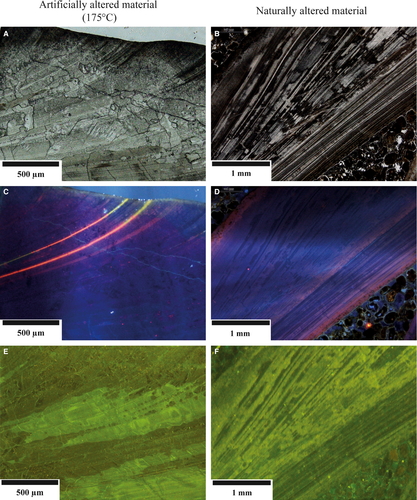
Pleistocene specimens of A. islandica display the following alteration features: the periostracum has been disintegrated/abraded in all cases, probably due to mechanical (waves) and biochemical (microborer) erosion. Thin-section images display cloudy patterns and spatially confined bleaching of the inner and outer shell layers and darkening of organic matter along growth increments (Fig. 6B).
Cathodoluminescence displays alternating dark and lighter blue intrinsic colours tracing growth increments. The shell surface displays a pale blue-red luminescence, often prominent in portions of the valves that are penetrated by microborings. Pale blue-red luminescence tracing growth increments are present in the inner and in transition zones from outer to inner shell layer. Individual growth increments show brighter luminescence (Fig. 6D).
Fluorescence images display patterns typical for pristine shells (Figs 5B and 6F) superimposed by secondary features. Fissures, oriented at angles between 5° and 50° to growth increments, show light green fluorescence. Irregular patches of darker and lighter green fluorescence and generally, cloudy fluorescence patterns are common. The outer valve is characterized by sub-vertical elongated light green fluorescence features in part coinciding with microborings.
Interpretation and Discussion
Experimentally induced mineralogical and ultrastructural patterns
Compared to calcite, aragonite weathering or dissolution receives only moderate attention in the literature. Several researchers have taken an experimental approach to the problem (Plummer & Busenberg, 1982; Busenberg & Plummer, 1985; Walter, 1985; Walter & Morse, 1985; Cubillas et al., 2005; Morse et al., 2007; Finneran & Morse, 2009; Romanek et al., 2011). To the knowledge of the authors, however, alteration experiments using aragonitic bivalves with a focus on diagenetic processes and products have not yet been reported.
Table 4 provides a general overview of the different alteration features observed and their comparison to naturally altered (fossil) samples. With the exception of minor surficial alteration features, the aragonitic mineralogy and ultrastructure of shell samples does not significantly respond to 100°C alteration experiments, independent of fluid types and experimental durations. In contrast, high-resolution SEM images reveal initial disintegration of organic matrices (Fig. 4). These observations document that a differentiation of early diagenetic processes in inorganic (mineralogy, ultrastructure) and organic matter-related processes is required. Moreover, ambient temperature and exposure time along diagenetic pathways are critical. Shallow burial diagenetic pathways exposing carbonates to non-marine fluids over geological timescales may induce significant alteration at even lower temperatures (Swart, 2015). Typical features of early-stage aragonite diagenesis in natural settings include a series of processes such as: (i) the colonization of the carbonate surface by biofilms resulting in the formation of micritic envelopes (Bathurst, 1966); (ii) microboring by fungi and other endolithic organisms aiming at organic matrices in these biominerals (Glover & Kidwell, 1993); (iii) the near-sea floor dissolution or transformation of aragonitic shell material resulting in mouldic porosity; or (iv) freshwater aragonite diagenesis including dissolution (Budd, 1988). Many of these processes are not present in the experiments discussed here and these limitations must be kept in mind.
| Naturally altered | Pristine, unaltered | Artificially altered | |||
|---|---|---|---|---|---|
| 100°C | 175°C | 200°C | |||
| Macroscopic features | Beige to light brownish colour | Beige colour | Beige colour | Light brownish colour | Brownish, fractured |
| Shell structure | Laminated | Laminated | Laminated | Mostly blocky crystals, partly with remaining laminated bands | Very big crystals |
| Cathodoluminescence | Intrinsic (with orange bands) | Intrinsic | Intrinsic | Intrinsic (with orange bands) | Pink with orange bands |
| Fluorescence | Laminated and/or patchy | Laminated | Laminated | Patchy, partly with remaining laminated bands | Patchy |
| δ13C | Differs depending on, for example, age, locality or diagenetic history | 1·6 to 1·9‰ * | Similar to pristine | Similar to pristine | Similar to pristine |
| δ18O | 2·0 to 3·1‰ * | Similar to pristine | Up to -50‰ | Up to -50‰ | |
| Δ47 and temperature Mineralogy (XRD) | Differs depending on, for example, age, locality or diagenetic history | ca 15°C Aragonite (ca 100%) | ca 40°C Aragonite (≥90%) | >200°C Calcite (70 to 100%) | 174 to 242°C Calcite (ca 100%) |
With regard to samples exposed to 175°C and 200°C experiments, a series of features requires discussion:
- At 175°C, the shell fabric is largely, and at 200°C completely, neomorphosed and transformed to calcite. This is best explained by the dominant control of temperature on reaction rates and mineral solubility (Morse et al., 2007) at least within the experimental durations applied here. The Arrhenius approach predicts that the reaction rate constants are about double in value considering a temperature rise of 10°C (Arrhenius, 1889) and the high fluid to solid ratio in the present experiments – relative to pore-fluid diagenesis in natural environments – is expected to result in fast reaction rates too. Additionally, it is conceivable that organic matrices surrounding individual biominerals in the primary shell initially protect the crystals from alteration. The fact that organic matter starts to decompose at ca 160°C (Martell et al., 1975; Motekaitis et al., 1982; Boles et al., 1987; Crossey, 1991; Bénézeth et al., 1997) may explain why major alteration features did not take place at fluid temperatures lower than 175°C. Along natural diagenetic pathways, however, much of the organic material in these shells decomposes at ambient temperatures and in the presence of microbial communities within months to years (Glover & Kidwell, 1993). Moreover, it is important to note that recent work (Jonas et al., 2017) has clearly documented that the geochemistry of the fluid at the reaction front within the pore space of the carbonate undergoing alteration is potentially more significant than that of the bulk aqueous medium. The interaction between organic material and pore fluid within the sample is an aspect that clearly deserves more attention in forthcoming work. These are important aspects that merit an in-depth assessment but are rather complex (Dupraz et al., 2009) and beyond the aims of the present paper. Assuming that neomorphism is not instantaneous, transitional stages are expected at intermediate temperatures with shell portions altered to calcite and others preserving the primary texture and aragonitic mineralogy. Similar to the issues of organic matrices, this hypothesis will be validated in forthcoming work.
- The 175°C experiments produce different patterns in crystal size and orientation when compared to the 200°C experiments (Figs 2, 3, 5 and 11). Specifically, the average secondary calcite crystal diameter increases with increasing reaction temperature. The systematic presence of oriented crystals at some, and the absence of oriented crystals at other surfaces exposed to reactive fluids, suggests a strong crystallographic control related to the ultrastructure of the primary shell. In the 175°C experiments, a possible relation between crystal size and fluid properties is observed but the present data are insufficient to draw statistically relevant conclusions.
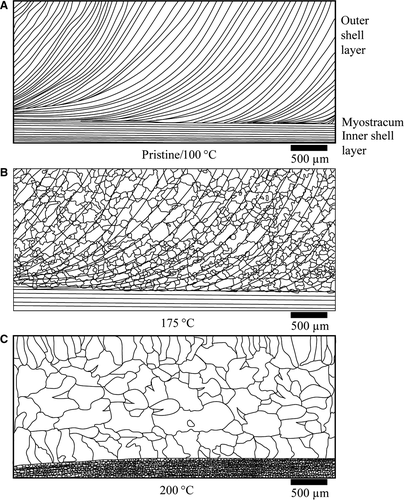 Figure 11Schematic of valve thin-section features for different alteration parameters. (A) Microstructure of pristine shells as well as samples altered at 100°C. Note the lack of alteration features. (B) Microstructure representative for samples altered at 175°C. Aggradational neomorphism has led to large secondary calcite crystals. Relictic primary lamination is still visible as inclusions. The inner shell layer often retains primary features. (C) Microstructure observed in samples altered at 200°C. Note the large, irregularly shaped crystals.
Figure 11Schematic of valve thin-section features for different alteration parameters. (A) Microstructure of pristine shells as well as samples altered at 100°C. Note the lack of alteration features. (B) Microstructure representative for samples altered at 175°C. Aggradational neomorphism has led to large secondary calcite crystals. Relictic primary lamination is still visible as inclusions. The inner shell layer often retains primary features. (C) Microstructure observed in samples altered at 200°C. Note the large, irregularly shaped crystals. - The inner shell layer is more resistant towards transformation and neomorphism compared to the outer shell layer (Fig. 3C). Subtle spatial differences in shell crystal fabrics and organic compounds (Ropes et al., 1984; Dunca et al., 2009), as well as geochemical patterns (Perkins et al., 1991; Fuge et al., 1993; Carell et al., 1995), have been described and seem significant on the level of reaction kinetics. Essentially, the interaction of organic matter with the surfaces of carbonate (bio)minerals is complex. Pokrovsky et al. (2005, 2009) and Ryu et al. (2010) document that different organic compounds strongly affect the dissolution kinetics of carbonates. Conversely, examples of preserved organic matter in carbonates deposited some 100 Ma have been reported too (Neuweiler et al., 1999). As discussed for the 100°C experiments, the separation of organically mediated processes (representing early diagenetic environments) and thermodynamically mediated processes (representing burial diagenetic domains) requires attention.
Experimentally induced fluorescence and cathodoluminescence patterns
Cathodoluminescence microscopy is a commonly applied screening tool in carbonate diagenesis research. Often, the working assumption is that increasingly luminescent carbonates point to alteration and fluid–crystal exchange under reducing porewater conditions favouring Mn2+ (activator) incorporation in the crystal lattice (Bruhn et al., 1995; Kaufmann & Wendt, 2000). Nevertheless, other factors including kinetics (for example, growth rates of crystals; Barbin et al., 1991) or elements other than Mn or Fe (quencher) affect luminescence patterns in carbonates (ten Have & Heijnen, 1985; Ritter et al., 2015). Additional complexity with reference to biogenic carbonates comes from the observation that pristine (recent) material can show luminescence regardless of its mineralogical composition or habitat (Barbin et al., 1991).
None of the pristine shells investigated here revealed luminescence patterns other than the typical intrinsic blue luminescence (Fig. 5A). Consequently, all luminescence features other than blue intrinsic colours were probably induced by alteration experiments. This is clearly documented by the blue-purple luminescence with orange bands found in shells exposed to 200°C (Fig. 5G). The authors are not aware of comparable luminescence patterns and colours in fossil biogenic carbonates and it is proposed that these features represent an experimental bias that is not yet well-constrained with regard to its significance for the understanding of aragonite diagenesis.
Thin luminescent zones observed in some of the 175°C samples (Fig. 6C) are similar to those observed in fossil A. islandica shells (Fig. 6D) and therefore of direct interest for archive research. The fact that these features are most prominent in modified seawater experiments might be related to the higher Mn concentration of this fluid type (up to 11 p.p.m. Mn) compared to the others (<0·005 p.p.m.). Nevertheless, given that shell material exposed to 175°C has seen pervasive aragonite–calcite transformation, it is conceivable that at least portions of the Mn from the shell aragonite is recycled via dissolution/reprecipitation into secondary calcite. The oxidation of organic matter contained in the biominerals led to locally oxygen-depleted fluids. In reducing porewaters, Mn is typically present as Mn2+ and incorporated into the secondary, luminescent calcitic minerals.
Wanamaker et al. (2009) applied fluorescence microscopy to shells of A. islandica and concluded that internal growth patterns fluoresce in the blue light spectrum. At present, the complex array of factors that induce fluorescence in bivalves is not sufficiently studied. According to Wanamaker et al. (2009) and Pérez-Huerta et al. (2008), fluorescence is triggered by organic macromolecules associated with chitin polysaccharides and proteins. Dark fluorescence colours commonly refer to portions of the shells that are relatively organic matter-depleted and indicative for periods of slow shell growth. Bright green or blue fluorescence colours typify areas with increased organic matter accumulation (Wanamaker et al., 2009). Fluorescence patterns induced in the 175°C and 200°C experiments represent (water-soluble) organic matter redistribution within the valve subsamples (Fig. 5). Accumulation of organic matter at the boundary of secondary calcite crystals (Fig. 5F and H) is documented by bright fluorescence colours. This feature may represent displacement of organic macromolecules during crystal growth. Care must be taken, however, because crystal boundaries represent three-dimensional surfaces within the thin section that can also reflect fluorescence. Interestingly, the innermost portions of valve subsamples seem less affected by organic matter remobilization (Fig. 5H) even under fluid temperatures of 200°C. A first implication of these results is that organic matter redistribution as observed under fluorescence microscopy deserves attention as an indicator for early-stage diagenetic alteration.
Experimentally induced isotope geochemical patterns
The primary δ13C variability of A. islandica shells, as reported in previous studies (−0·7 to +4·0‰; Table 1; Butler et al., 2009, 2011; Schöne et al., 2005b; Schöne et al., 2011) is very similar to that found in the specimen analysed here (−0·6 to +3·6‰). A first observation is that δ13C remains unchanged in all experiments. This is due to the fact that the carbon pool in these experiments is dominated by the carbon contained in the shells, which is recycled and incorporated into the neomorphosed calcite crystals without significant carbon isotope fractionation.
Oxygen isotope values measured from individual valves (+2·0 < δ18Ovalve < +3·1‰; Table 1) are within the range of literature data (excluding aquaria cultured specimens) that range from −1·7 to +3·5‰ (Table 1) and correspond to biomineral secretion from seawater at ambient temperatures (Schöne et al., 2011). Due to the low δ18O of the experimental fluids (δ18Osw = −20·6‰; δ18Omet = −47·4‰; δ18Obur = −47·2‰) compared to sea-water (δ18O = 0‰), the progress of exchange reactions between oxygen in fluids and the solid carbonate can be traced. Subsamples exposed to 100°C reveal no significant fluid–solid interaction, in agreement with the lack of petrographic alteration features (Figs 7 and 8).
At 175°C (Figs 7 and 9), a strong decrease in δ18Ovalve is observed, indicating an aragonite to calcite alteration by a dissolution/reprecipitation process leading to local oxygen isotope exchange between solid and fluid. Interestingly, the oxygen isotopic composition of samples altered at 200°C is in rather good agreement with some of the samples from the 175°C experiments.
Calculated clumped isotope temperatures obtained from shell samples treated at 100°C are between 44°C and 50°C (Table 3; Fig. 10), thus significantly lower than the measured experimental fluid temperature. This result is in good agreement with the observations of Staudigel & Swart (2016) who performed dry heating experiments both under vacuum and under a CO2 atmosphere. These authors observed rapid decrease of the Δ47 of aragonite after 2·5 h heating at 125°C, but also found that none of the aragonites heated at temperatures lower than 375°C reached the equilibrium value within the three day duration of the experiments. Because only one time duration was available for the present study, it is not possible to evaluate the kinetics of the reaction. It is possible that the change is due to annealing to an apparent equilibrium, as postulated for the experiments by Staudigel & Swart (2016) and also observed in calcite (Passey & Henkes, 2012; Stolper & Eiler, 2015). The heating duration was insufficient to completely re-equilibrate the aragonite Δ47; thus, more experiments with both shorter and longer duration and lower temperatures are needed to determine the behaviour of aragonite during temperature alteration. Summarizing these results confirms earlier studies showing that metastable aragonite is more susceptible than calcite to bond reordering (Passey & Henkes, 2012; Stolper & Eiler, 2015; Staudigel & Swart, 2016) and that fossil aragonite material will only preserve an original signature if the burial fluid temperature remained well below 100°C. Thus, the clumped isotope thermometer is a sensitive indicator for early-stage diagenesis. Importantly, conventional petrographic and geochemical proxies (fluorescence or cathodoluminescence microscopy, C and O isotope analysis) do not show any significant alteration pattern.
The TΔ47 values derived from aragonite shells heated to 175°C and 200°C broadly reflect the expected temperatures. The observed scatter is related to partial transformation to calcite and could also be related to disequilibrium as indicated by δ18O. Forthcoming work will test the homogeneity of clumped isotope data within one valve and between different valves exposed to the same fluid temperatures.
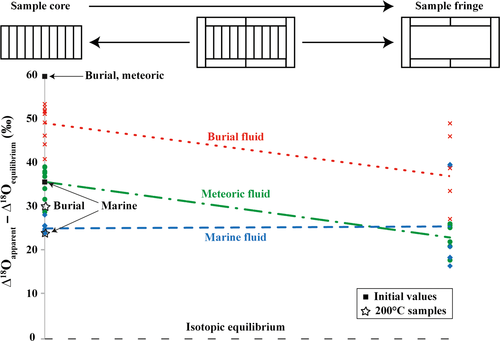
In order to investigate deviations from isotopic equilibrium and spatial patterns in oxygen isotopic compositions of the 175°C samples, Δ18Oequilibrium values between calcite and water (Δ18O = δ18Ocalcite to δ18OH2O) have been calculated following the equation of Coplen (2007). Figure 12 shows the spatial variability of the isotopic fractionation (apparent Δ18O value minus equilibrium Δ18O value). This figure illustrates that the samples altered in both meteoric (incubated for five weeks) and burial fluid (incubated for three weeks) exhibit a spatial pattern, with a trend towards calculated equilibrium values from the centre to the margin. This implies that rim material changes oxygen isotope composition with the experimental fluid and becomes closer to isotopic equilibrium, whereas material from central portions still exhibits significantly stronger deviation from it. It is possible that this pattern will disappear under increasing experimental duration, and bulk δ18O equilibrium of the solid with the ambient experimental fluid will be reached. Importantly, no such trend is found in the specimen altered in modified seawater (incubated for five weeks), and this sample deviates from theoretical equilibrium by ca 25‰. Specifically, isotope data at 175°C are similar to those from experiments at 200°C. This might be due to local transformation and neomorphism of aragonite to calcite, for example along fissures, crystal boundaries or growth increments. At high local solid to liquid ratios, the oxygen isotopic composition of the migrating fluids within the pore space of the sample seems altered through intense oxygen exchange during carbonate dissolution and reprecipitation. Therefore, the oxygen isotopic composition of the migrating fluid within the sample does not necessarily represent the bulk isotopic composition, and the oxygen isotopic composition of the final calcite is determined by the bulk fluid and its 18O/16O exchange conditions at a microscale to nanoscale with the carbonate material where transformation occurs. Isotope exchange conditions are controlled by – next to solid to fluid ratios – local physicochemical conditions, such as fluid composition, temperature and reaction time (Coplen, 2007; Dietzel et al., 2009; Jonas et al., 2017). This outcome is of significance for the interpretation of ancient carbonates. The assumption that – at some stage along diagenetic pathways – equilibrium between diagenetic fluid and the altered solid is reached might represent an oversimplification of a significantly more complex pattern.
Comparison of natural and experimental alteration features
Naturally altered shells studied here were exposed to marine and meteoric fluids with temperatures in the order of 5 to 10°C or hydrothermal fluids with temperatures of 40 to 120°C related to volcanic activity on Iceland. Artificially altered samples have seen temperatures in the range of 100 to 200°C. In this sense, all experiments performed here represent the burial realm with respect to fluid temperature independent of fluid chemistry. Applying a temperature gradient of approximately 2·5°C per 100 m (Fridleifsson et al., 2008), the experiments performed here represent the temperature range in burial depths of between 4 km and 8 km. At these depths, both lithostatic and hydrostatic pressure are potentially significant. In the experiments performed here, only vapour pressure is applied.
Despite issues limiting the alteration experiment approach as applied here, the resulting diagenetic features share important similarities with those observed in fossil shells. These include colour alteration of intrashell organic matter (Figs 2 and 6) in naturally and experimentally altered samples. Similar colour alterations have been described from conodonts and other skeletal hardparts containing significant amounts of organic matrices (Harris, 1979). With respect to cathodoluminescence features, luminescent zones are oriented parallel to the valve surfaces particularly where, in the case of ancient shells, microborings are present. A second pale-orange luminescent zone is found in the outer, specifically at the boundary to the inner shell layer (Fig. 6D). Otherwise, valves display the same blue intrinsic colours as found in experimentally altered subsamples (175°C; Figs 5E and 6C). With respect to the shell ultrastructure, none of the naturally altered samples displays the neomorphic features observed in 175°C and 200°C experiments (Figs 2 to 6). This is not surprising, given that the ancient shells from Iceland have seen comparably cool hydrothermal temperatures only.
Conclusions
Aragonitic Arctica islandica shells were experimentally altered to assess different diagenetic processes and products (100°C, 175°C, 200°C, meteoric, marine and burial fluids). Natural diagenetic pathways differ in several important ways from the experiments shown here: (i) the experimental duration (weeks to months versus 103 to 106 years); (ii) the natural early-stage microbial degradation of organic matrices in shells absent in experiments; (iii) the lack of lithostatic pressure in experiments; or (iv) the high fluid to solid ratio of experiments relative to natural pore-fluid settings. Acknowledging all of these issues, one of the key results of this study is that altered shell subsamples show important features that are directly comparable to those observed in naturally altered (diagenetic) material. This allows for a quantitative assessment of processes and products during aragonite alteration pathways.
(i) Traditional petrographic and geochemical proxies (cathodoluminescence and fluorescence images, mineralogy, carbon and oxygen isotopes) do not show significant alteration features in samples exposed to 100°C. In contrast, clumped isotopes show most likely initial annealing processes rather than bond reordering and indicate elevated fluid temperatures. (ii) Samples exposed to 175°C show petrographic and geochemical evidence for significant aggradational neomorphism, and petrographical patterns are comparable to fossil shells. (iii) Samples exposed to 200°C show petrographic features that are increasingly dissimilar from those found in natural samples and may represent an endmember of diagenetic alteration at the boundary to the anchimetamorphic domain. (iv) Despite significant aragonite to calcite neomorphism in the 175°C and 200°C experiments, carbon isotope ratios remained conservative due to a carbonate-buffered closed system behaviour. (v) Care must be taken because at microscale to nanoscale, fluid compositions and conditions within the carbonate might differ from those of the ambient fluid. Therefore, deducing the degree of alteration and the temperature of diagenetic fluid from the isotope data of altered material alone may be misleading. This study shows that important alteration steps take place between fluid temperatures of 100°C and 175°C. Thus, future work will target a higher resolved approach using 20°C increments between 80°C and 160°C using different biogenic and abiogenic aragonites. The issues of organic matrices and the separation of organic and inorganic processes deserve significant attention in future work. Another important aspect is the incorporation of high-resolution analytical tools. For example, NanoSIMS δ18O analyses might decipher conditions and mechanisms limiting oxygen exchange between calcite and bulk solution during aragonite–calcite transformation within these test materials.
Acknowledgements
This is a contribution to the DFG Forschergruppe 1644 (CHARON). B. Schöne, Mainz, provided us with some of the naturally altered shell material from Iceland. Experiments were conducted at the Central Laboratory for Water, Minerals and Rocks (NAWI Graz). The authors acknowledge important scientific and editorial guidance by Sedimentology reviewers A. Strasser and an anonymous colleague, and by T. Frank and S. Lokier acting as Editor and Associate Editor, respectively.




