Inhalational exposures in patients with fibrotic interstitial lung disease: Presentation, pulmonary function and survival in the Canadian Registry for Pulmonary Fibrosis
This research has been previously presented at the Annual Conference of the American Thoracic Society (ATS) 2021.
Associate Editor: Francesco Bonella; Senior Editor: Chris Grainge
Funding information: National Institute of Health, Grant/Award Numbers: K23HL146942, T32HL007605
Abstract
Background and objective
Inhalational exposures are a known cause of interstitial lung disease (ILD), but little is understood about their prevalence across ILD subtypes and their relationship with pulmonary function and survival.
Methods
Patients with fibrotic ILD were identified from the multicentre Canadian Registry for Pulmonary Fibrosis. Patients completed questionnaires regarding ILD-related occupational and environmental exposures. The relationship between exposures and the outcomes of baseline age, gender, family history, pulmonary function and survival was analysed using linear and logistic regression models, linear mixed-effect regression models and survival analysis using multivariable Cox proportional hazards along with the log-rank test.
Results
There were 3820 patients included in this study, with 2385 (62%) having ILD-related inhalational exposure. Exposed patients were younger, particularly in the idiopathic pulmonary fibrosis subgroup. Inhalational exposure was associated with male gender (adjusted OR 1.46, 95% CI 1.28–1.68, p < 0.001) and family history of pulmonary fibrosis (adjusted OR 1.73, 95% CI 1.40–2.15, p < 0.001). Patients with any inhalational exposure had improved transplant-free survival (hazard ratio 0.81, 95% CI 0.71–0.92, p = 0.001); this effect persisted across diagnostic subtypes. The relationship between exposures and annual change in forced vital capacity varied by ILD subtype.
Conclusion
Patients with fibrotic ILD report high prevalence of inhalational exposures across ILD subtypes. These exposures were associated with younger age at diagnosis, male gender and family history of pulmonary fibrosis. Identification of an inhalational exposure was associated with a survival benefit. These findings suggest that inhaled exposures may impact clinical outcomes in patients with ILD, and future work should characterize the mechanisms underlying these relationships.
INTRODUCTION
Fibrotic interstitial lung disease (ILD) represents a group of pulmonary disorders characterized by irreversible scarring and, frequently, progressive respiratory decline and early death.1 While antifibrotic medications can slow the rate of pulmonary function decline, there are currently no therapies to reverse established pulmonary fibrosis.2, 3 The aetiology of fibrotic ILD remains incompletely understood, with proposed multi-hit mechanisms including genetic predisposition, inhalational environmental exposures and accelerated lung ageing.4 Characterizing the impact of inhalational exposures on triggering or accelerating fibrotic lung disease remains a prioritized area of investigation. Given the limited treatment options and often poor outcomes, disease prevention remains a key priority where possible. Identifying risk factors for ILD development may help to inform disease pathobiology and avenues for prevention, particularly with occupational and/or environmental exposures. Furthermore, abatement of exposures in patients with ILD could improve clinical outcomes.
While inhaled agents or antigens are often associated with pneumoconiosis and hypersensitivity pneumonitis (HP), occupational and other environmental exposures are prevalent across other ILD subtypes.5 Recently, a single-centre cohort study reported a high prevalence of occupational and domestic exposures across a wide variety of ILDs.6 It is unknown how this high exposure prevalence may impact disease severity and outcomes. For example, inhaled exposure identification could translate into improved clinical outcomes if antigens and toxins can be remediated. However, studies report mixed findings as to whether exposure identification in ILD patients is associated with improved or worsened survival, and whether exposure presence modulates the trajectory of pulmonary function decline.6, 7
In this study, we used data from the multicentre Canadian Registry for Pulmonary Fibrosis (CARE-PF) to identify demographic and clinical features associated with ILD-related inhalational exposures and to characterize the relationship between exposures, pulmonary function and survival in patients with fibrotic ILD. We hypothesized that patients with a history of exposure would be younger at ILD onset, less likely to have a family history of ILD and experience accelerated loss of lung function with worse transplant-free survival compared to ILD patients without exposure.
METHODS
Study population
This study used data from the Canadian Registry for Pulmonary Fibrosis (CARE-PF), a multicentre prospective cohort of patients with any subtype of fibrotic ILD.8 For registry inclusion, patients must be over the age of 18 years, be able to provide informed consent and complete questionnaires in English or French. ILD subtype was determined clinically at each centre site, all ILD expert centres, with multidisciplinary diagnoses established according to contemporaneous guidelines when available.9, 10 Patients meeting the proposed criteria for idiopathic pneumonia with autoimmune features were designated as having unclassifiable ILD.
Data collection
At enrolment, patients completed questionnaires regarding their environmental and occupational exposures (Figure S1 in the Supporting Information). The questionnaires emphasized repeated and regular exposure within 3 years prior to symptom onset for domestic exposures and lifetime exposure history for occupational exposures. All answers were yes/no, and addressed both domestic exposures, such as mould or birds in the home, exposures in the office setting, as well as specific occupations known to be associated with parenchymal lung disease, such as mining or working with beryllium. Patients were classified as having ‘any exposure’ if they answered yes to any exposure question. Within ‘any exposure’, the subgroup of ‘organic exposure’ was used if a patient answered yes to any question regarding water, soil, farming or birds. The subgroup of ‘inorganic exposure’ was used if a patient answered yes to any question regarding the remainder of exposures queried. Patients could have both organic and inorganic exposures simultaneously.
Data on demographics, smoking, family history, lung transplant and survival were collected from the patient's medical record. Family history was defined as either the patient reporting a family history of pulmonary fibrosis or the pulmonary physician designating a patient as having familial ILD (affected individual having one or more first-degree relatives with pulmonary fibrosis). Pulmonary function tests (PFTs) were performed as clinically indicated over the follow-up period. Baseline was considered within 6 months of first ILD clinic visit for both the survival and pulmonary function analyses. Patients were censored at the time of death, lung transplantation or last known follow-up visit with data extracted on 1 December 2020.
Statistical analysis
T-tests were used to assess differences in baseline age by exposure. Logistic regression was used to assess for differences in gender and family history by exposure, in models adjusted for baseline age and smoking status. Survival analysis assessing time to lung transplant or death by exposure was performed using multivariable Cox proportional hazards analysis along with the log-rank test in models adjusted for age, gender, smoking and baseline forced vital capacity (FVC). Mixed-effects regression modelling with a random intercept was used to analyse FVC percent predicted (%) over time by exposure status, adjusting for the fixed effects of baseline age, gender and smoking. Estimated annual change in FVC% was calculated for every ILD subtype by adding the time and exposure–time interaction coefficients from the mixed-effects model together for each exposure subgroup. Analyses were performed in the entire cohort and within major fibrotic ILD diagnostic subtypes (idiopathic pulmonary fibrosis [IPF], connective tissue disease-associated ILD [CTD-ILD], HP and unclassifiable ILD). Statistical analyses were conducted using Stata (StataCorp, 2021, Release 17).
RESULTS
Baseline characteristics
A total of 3820 patients were included. The mean age of the cohort was 64 ± 12 years, 49% were male and 61% had a history of smoking (Table 1). Thirteen percent reported a family history of ILD. Prevalence of each ILD subtype is listed in Table 1.
| Entire cohort (n = 3820) | Exposure history (n = 2385) | No exposure history (n = 1435) | p-value (exposure vs. no exposure) | |
|---|---|---|---|---|
| Age in years, mean ± SD | 63.8 ± 12 | 63.6 ± 12.2 | 64.0 ± 13.0 | 0.31 |
| Male, n (%) | 1889 (49) | 1271 (53) | 618 (43) | <0.001 |
| Ever smoked tobacco, n (%) | 2349 (61) | 1555 (65) | 794 (56) | <0.001 |
| Family history of PF, n (%) | 485 (13) | 354 (15) | 131 (9) | <0.001 |
| ILD diagnosis, n (%) | <0.001 | |||
| IPF | 993 (26) | 635 (27) | 358 (25) | |
| CTD | 1261 (33) | 694 (29) | 567 (40) | |
| HP | 274 (7) | 211 (9) | 63 (4) | |
| Sarcoidosis | 138 (4) | 87 (4) | 51 (4) | |
| Non-IPF IIP | 142 (4) | 105 (4) | 37 (3) | |
| Unclassifiable | 810 (21) | 505 (21) | 305 (21) | |
| Other | 202 (5) | 148 (6) | 54 (4) | |
| Baseline FVC, % predicted, mean ± SD | 77 ± 20 | 77 ± 20 | 76 ± 20 | |
| Baseline DLCO, % predicted, mean ± SD | 60 ± 20 | 61 ± 20 | 59 ± 21 | |
| Died, n (%) | 795 (21) | 436 (19) | 359 (26) | |
| Transplanted, n (%) | 173 (5) | 122 (7) | 51 (5) | |
| Follow-up in years, median (IQR) | 3.0 (1.6–5.0) | 2.9 (1.6–4.8) | 3.1 (1.6–5.4) |
- Abbreviations: CTD, connective tissue disease; DLCO, diffusing capacity for carbon monoxide; FVC, forced vital capacity; HP, hypersensitivity pneumonitis; IIP, idiopathic interstitial pneumonia; ILD, interstitial lung disease; IPF, idiopathic PF; IQR, interquartile range; PF, pulmonary fibrosis.
Exposures
Overall, 2385 patients (62%) reported any environmental or occupational exposure. Mould (38%) and bird (37%) were the most common exposures, with asbestos and other inorganic dust exposures present in 12% and 17% of patients, respectively. Notably, 40% of patients reported more than one exposure. The prevalence of each individual exposure is described in Figure S2 in the Supporting Information. Exposures were present in over 50% of patients in each diagnostic category.
While patients with HP had the highest proportion (52%) reporting an organic-only exposure, over one third of patients with either IPF, CTD-ILD or unclassifiable ILD reported an organic-only exposure (Figure 1). Patients with IPF had the highest prevalence of inorganic exposure at 10%. Both organic and inorganic exposures were reported in 22% of patients with HP, 19% of patients with IPF, 18% of patients with unclassifiable ILD and 11% of patients with CTD-ILD.
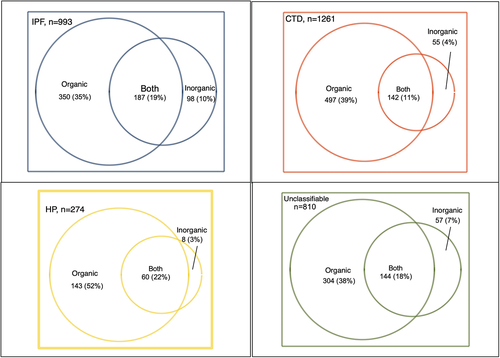
Exposures and baseline features
Patients with any exposure were overall younger than unexposed patients, particularly in the IPF subtype (p < 0.001, Table S1 in the Supporting Information).
In the entire cohort, men had higher odds of exposure compared to women (adjusted OR 1.46, 95% CI 1.28–1.68, p < 0.001, Table 2), particularly in patients with CTD-ILD and unclassifiable ILD. This association was driven by inorganic exposures; inorganic exposures were associated with male gender in patients with IPF (OR 6.03, 95% CI 2.69–13.50, p < 0.001), CTD-ILD (OR 8.58, 95% CI 4.56–16.13, p < 0.001) and unclassifiable ILD (OR 8.61, 95% CI 3.73–19.90, p < 0.001), while organic exposures were negatively associated with male gender in patients with IPF (OR 0.70, 95% CI 0.51–0.95, p = 0.02), HP (OR 0.52, 95% CI 0.28–0.96, p = 0.04) and unclassifiable ILD (OR 0.63, 95% CI 0.45–0.88, p = 0.007). Reporting both organic and inorganic exposure was strongly associated with male gender in all four diagnostic subtypes (OR for entire cohort 5.31, 95% CI 4.22–6.69, p < 0.001).
| OR of exposure, men compared to women (95% CI) | p-valuea | OR of exposure, family history versus no family history (95% CI) | p-valuea | |
|---|---|---|---|---|
| All ILD patients | 1.46 (1.28–1.68) | <0.001 | 1.73 (1.40–2.15) | <0.001 |
| IPF | 1.30 (0.97–1.75) | 0.08 | 1.17 (0.82–1.67) | 0.38 |
| CTD | 1.80 (1.39–2.33) | <0.001 | 3.00 (1.82–4.94) | <0.001 |
| HP | 0.89 (0.50–1.58) | 0.70 | 1.75 (0.64–4.75) | 0.273 |
| Unclassifiable | 1.39 (1.04–1.87) | 0.03 | 1.64 (1.06–2.57) | 0.03 |
- Abbreviations: CTD, connective tissue disease; HP, hypersensitivity pneumonitis; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis.
- a Adjusted for age and smoking.
Patients with a family history of ILD had higher odds of exposure compared to those without a family history (adjusted OR 1.73, 95% CI 1.40–2.15, p < 0.001, Table 2). This persisted across all diagnostic subtypes, with highest odds in patients with CTD-ILD and unclassifiable ILD. This finding also persisted across exposure subgroups (only organic exposure OR 1.66, 95% CI 1.32–2.10, p < 0.001; only inorganic exposure OR 2.48, 95% CI 1.74–3.55, p < 0.001; both exposures OR 1.61, 95% CI 1.20–2.16, p = 0.001).
Exposures and clinical outcomes
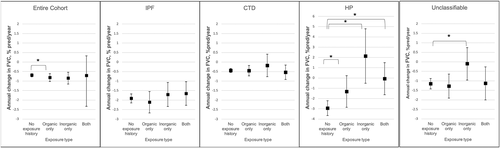
Three thousand four hundred and ninety-one patients with follow-up PFTs were included in the longitudinal pulmonary function analysis. The average number of PFTs analysed per patient was 6.1. Overall, organic exposures were associated with a slight but significantly increased FVC decline per year across the entire cohort; no differences were seen in patients with inorganic exposure or both organic and inorganic exposures (no exposure −0.69%/year, organic exposure −0.82%/year, p = 0.04, Figure 2). However, the association between exposure and adjusted FVC% decline varied across ILD subtypes; patients with HP and known exposure had less FVC decline and in some cases FVC improvement across all exposure subgroups compared to no known exposure (no exposure −2.94%/year; organic only −1.32%/year, p < 0.001; inorganic only 2.14%/year, p < 0.001; both −0.06%/year, p < 0.001), while no differences between exposure and FVC decline were seen in patients with IPF or CTD-ILD. For patients with unclassifiable ILD, inorganic exposure was associated with less FVC decline compared to no known exposure (−0.01%/year compared to −1.15%/year with no exposure, p < 0.001).
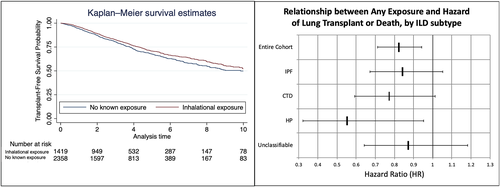
Transplant-free survival was longer in patients with any exposure compared to those without exposure after adjustment for age, gender and smoking status (hazard ratio [HR] 0.82, 95% CI 0.71–0.94, p = 0.004, Figure 3). This effect persisted across all ILD subtypes, including patients with CTD-ILD (HR 0.77, 95% CI 0.59–1.01, p = 0.06) and HP (HR 0.55, 95% CI 0.32–0.95, p = 0.03). Within the subgroup of organic exposures, transplant-free survival was also improved across the entire cohort (HR 0.81, 95% CI 0.70–0.95, p = 0.010, Figure 4, Table S2). Inorganic exposures were associated with a survival benefit in the entire cohort compared to no exposure (HR 0.73, 95% CI 0.55–0.97, p = 0.03); this effect did not persist when stratified within individual ILD diagnoses. Having both organic and inorganic exposures was associated with improved transplant-free survival only in patients with HP (HR 0.40, 95% CI 0.19–0.87, p = 0.02).

DISCUSSION
This multicentre study found that exposures were present in two thirds of patients across all ILD subtypes and associated with male gender. A family history of ILD was associated with higher odds of exposure across all ILD subtypes, and exposed patients with IPF were younger. Exposure was differentially associated with longitudinal pulmonary function dependent on ILD subtype; patients with HP and an identified exposure had slowed FVC decline across all types of exposures compared to those unexposed, an effect not seen in other diagnoses. Notably, a history of exposure was associated with improved survival, and consistent with prior reports, organic exposure was associated with improved transplant-free survival in patients with HP.
Our findings that exposures were present in a majority of ILD patients and associated with male gender are consistent with a prior single-cohort study at the University of Chicago.6 In the current study, this finding was mainly driven by the relationship between male gender and inorganic exposure. Given that multiple occupations exposed to inorganic dust asked in our questionnaire were male-predominant, such as silica and asbestos,11 this gender differential may simply be a reflection of the types of questions asked in ILD-related exposure questionnaires. Concordant with this hypothesis, when more general queries regarding occupation were examined in a study of death certificates in sarcoidosis, female-predominant occupations such as banking, teaching and childcare were associated with sarcoidosis mortality.12 Alternatively, this finding could reflect that male-predominant occupations, particularly those involving inorganic dust, increase the risk for development of ILD.13 Systematically surveying all ILD patients more thoroughly, such as with a complete occupational history, could reveal relevant female-predominant occupations that may be associated with ILD incidence and outcome.
This study is the first to associate family history of ILD with higher odds of prior exposure. While recall bias in relatives of ILD patients may contribute to this observation, previous work has suggested that genetics are not the sole mechanism by which patients with a family history develop ILD. For example, multiple ILD subtypes have been described within the same family, including HP, and smoking has been associated with the development of familial ILD.15, 14 Additionally, computed tomography features of patients with familial ILD do not conform to classic patterns associated with sporadic ILD; in one cohort, most patients with familial ILD did not have usual interstitial pneumonia (UIP) or nonspecific interstitial pneumonia (NSIP), the two most common radiological patterns in ILD generally.16 Multiple mechanisms could explain this association, including familial clustering of occupation or hobbies predisposing to exposure or a ‘two-hit’ mechanism of disease requiring both genetic predisposition and environmental insult. Regardless of mechanism, patients with a family history of ILD represent a population at risk that may benefit from more aggressive exposure assessment, remediation and counselling.
Our study is also the first to demonstrate a varying effect of exposure on pulmonary function based on ILD subtype. In HP particularly, patients with a known exposure had a slower FVC decline compared to patients without a known exposure; this effect was not seen in other diagnoses with the exception of unclassifiable ILD and history of inorganic exposure. This is in contrast to prior work by De Sadeleer and colleagues that did not find a difference in longitudinal pulmonary function in patients with HP or IPF and mould or bird exposure.17 This difference in the relationship between HP, exposure and pulmonary function could be related to a more inflammatory phenotype in patients with HP that may be treatment responsive. Additionally, this phenomenon could reflect differential remediation of exposures by ILD subtype; while no remediation information was available in our database, clinicians could be more attuned to looking for and counselling patients on remediating exposures in the setting of HP. Furthermore, the higher rate of organic exposures reported in HP patients could have contributed to this group's attenuated pulmonary function decline, as these exposures may be more easily identified and remediable compared to inorganic antigens.
Patients with ILD and a history of exposure had improved transplant-free survival in our cohort across all disease subtypes. This finding is in contrast to a prior study that found a trend towards worse survival in patients with exposure in all-comers with ILD.6 However, our findings are consistent with those in an HP cohort by Fernández Pérez et al., reporting improved survival in patients with an identified antigen compared to those without.7 In our cohort, when both organic and inorganic exposures were reported, only patients with HP experienced a survival benefit. These differences in clinical outcome could either be associated with the timing of exposure identification and remediation, that is, inorganic exposures combined with others may be remote and less intervenable, or a difference in clinical phenotype based on the type or combination of exposures encountered. Exposed patients may present earlier, as demonstrated by their higher baseline FVC and diffusing capacity for carbon monoxide and younger age at presentation.
This study has limitations, including its retrospective nature. The lack of a control group without ILD prevents us from characterizing these inhaled exposures as risk factors for ILD, an important focus for future study. We cannot exclude recall bias or exposure misclassification; however, the standardized approach with exposure surveys systematically administered at enrolment for all clinic patients is a unique feature of this study compared to other multicentre ILD registries. More information on intensity, frequency and duration of exposure will be essential in future studies. Patients with non-HP ILD and inhalational exposure could also have been mis- or differentially classified given their exposure history or type of exposure itself, although the coexistence of inhalational exposures and non-HP ILDs has been previously described.6, 11, 17 Additionally, information on treatment was limited and would be an important variable in determining survival in ILD patients. Key strengths of this study include its size, breadth of ILD diagnoses and inclusion of patients from eight ILD centres across Canada.
In summary, this large registry-based study identified a high prevalence of inhalational exposures across all ILD subtypes and an association between exposure and family history of ILD. These findings suggest that exposures may be risk factors for all ILD diagnoses and contribute to disease development. In addition, we found improved survival among all patients with exposure, and slowed FVC decline among patients with HP and identified exposure. Future work should use control groups to assess relationships between exposure and ILD incidence, provide more granular detail on specific exposure–disease phenotypes and characterize the impact of exposures on clinical outcomes in ILD patients. Exposure identification may be an avenue to improve both pulmonary function and survival in fibrotic ILD, and help better understand the pathobiology of disease and risk of ILD development.
ACKNOWLEDGEMENTS
Research funding: Cathryn T. Lee has received grant funding from the National Institute of Health (T32HL007605). Ayodeji Adegunsoye has received grant funding from the National Institute of Health (K23HL146942).
CONFLICT OF INTEREST
CARE-PF is supported by Boehringer Ingelheim. The study sponsor had no input on the research question, study design, data analysis, interpretation of results or production of the manuscript. Cathryn T. Lee has received grant funding from the National Institute of Health (T32HL007605). Ayodeji Adegunsoye has received grant funding from the National Institute of Health (K23HL146942), American College of Chest Physicians and the Pulmonary Fibrosis Foundation, and honoraria from Boehringer Ingelheim. Mary E. Strek has received grants from Boehringer Ingelheim and Galapagos, honoraria from the American College of Chest Physicians, served on an advisory board for Fibrogen, served on committees for the American Thoracic Society and received medical writing support from Boehringer Ingelheim. Alyson W. Wong has received honoraria from Boehringer Ingelheim and AstraZeneca. Deborah Assayag has received a research grant from Boehringer Ingelheim Canada and served on advisory boards for Hoffman LaRoche Canada and Boehringer Ingelheim Canada. Charlene D. Fell has received personal fees from Brigham & Women's Hospital, Novartis and Galapagos; honoraria from Boehringer Ingelheim and Roche Canada; and has leadership roles with the Canadian Pulmonary Fibrosis Foundation and the Canadian Thoracic Society. Jolene H. Fisher has received grants from the Canadian Pulmonary Fibrosis Foundation and the University of Toronto, consulting fees from Boehringer Ingelheim and AstraZeneca and is a member of the Canadian Pulmonary Fibrosis Advisory Board. Andrew J. Halayko is a member of the American Thoracic Society Board of Directors, including the Finance Committee. Nathan Hambly has received grants and personal fees from Boehringer Ingelheim, Roche, Janssen and Bayer. Martin Kolb has received grants from Boehringer Ingelheim, Pieris and Roche; consulting fees from Boehringer Ingelheim, Roche, Horizon, Cipla, Abbvie, Belerophon, Algernon and CSL Behring; honoraria from Novartis, Boehringer Ingelheim and Roche; payment for expert testimony from Roche; served on advisory boards for Covance and United Therapeutics; and receives a Chief Editor allowance from the ERJ. Stacey D. Lok has received honoraria from Boehringer Ingelheim. Hélène Manganas has received research grants from Boehringer Ingelheim, Galapagos and BMS, and participates in an advisory board for Boehringer Ingelheim. Veronica Marcoux has received grants from AstraZeneca and Roche, consulting fees from Boehringer Ingelheim Canada and Roche LTD and honoraria from Boehringer Ingelheim. Julie Morisset has received consulting fees from Hoffman-La Roche and Boehringer Ingelheim, honoraria from Hoffman-La Roche and Boehringer Ingelheim and participated on advisory boards for Hoffman-La Roche and Boehringer Ingelheim. Shane Shapera has received honoraria from Hoffman-LaRoche Canada, Boehringer Ingelheim and AstraZeneca Canada; participated in advisory boards for Hoffman-La Roche Canada and Boehringer Ingelheim; and participated in clinical trial research for Hoffman-La Roche Canada, Boehringer Ingelheim, Galapagos, Galecto and Gilead Pharmaceuticals. Pearce Wilcox has received consulting fees from Boehringer Ingelheim, honoraria from Vertex and Glaxo Smith Kline and served on an advisory board for the Cystic Fibrosis Foundation. Christopher J. Ryerson has received grant funding from Boehringer Ingelheim and Hoffmann-La Roche; consulting fees from Boehringer Ingelheim, Hoffman-La Roche, AstraZeneca and Veracyte; honoraria from Boehringer Ingelheim and Hoffmann-La Roche; and travel support from Cipla Ltd and Boehringer Ingelheim. Kerri A. Johannson has received grants from Three Lakes Foundation, Chest Foundation, University of Calgary CSM and University Hospital Foundation; consulting fees from Boehringer Ingelheim, Hoffman-La Roche Ltd, Pliant Therapeutics, Blade Therapeutics, Theravance and Three Lakes Foundation; honoraria from Boehringer Ingelheim and Hoffman-La Roche Ltd; and served on an advisory board for PFOX trial. Gerard Cox, Andrea S. Gershon, Nasreen Khalil, Mohsen Sadatsafavi and Teresa To have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION
Cathryn T. Lee: Conceptualization (lead); formal analysis (lead); investigation (lead); methodology (equal); writing – original draft (lead); writing – review and editing (equal). Mary E. Strek: Conceptualization (supporting); supervision (equal); writing – review and editing (equal). Ayodeji Adegunsoye: Formal analysis (supporting); methodology (supporting); writing – review and editing (equal). Alyson W. Wong: Data curation (equal); resources (equal); writing – review and editing (equal). Deborah Assayag: Data curation (equal); resources (equal); writing – review and editing (equal). Gerard Cox: Data curation (equal); resources (equal); writing – review and editing (equal). Charlene D. Fell: Data curation (equal); resources (equal); writing – review and editing (equal). Jolene H. Fisher: Data curation (equal); resources (equal); writing – review and editing (equal). Andrea S. Gershon: Data curation (equal); resources (equal); writing – review and editing (equal). Andrew J. Halayko: Data curation (equal); resources (equal); writing – review and editing (equal). Nathan Hambly: Data curation (equal); resources (equal); writing – review and editing (equal). Nasreen Khalil: Data curation (equal); resources (equal); writing – review and editing (equal). Martin Kolb: Data curation (equal); resources (equal); writing – review and editing (equal). Stacey D. Lok: Data curation (equal); resources (equal); writing – review and editing (equal). Hélène Manganas: Data curation (equal); resources (equal); writing – review and editing (equal). Veronica Marcoux: Data curation (equal); resources (equal); writing – review and editing (equal). Julie Morisset: Data curation (equal); resources (equal); writing – review and editing (equal). Mohsen Sadatsafavi: Data curation (equal); resources (equal); writing – review and editing (equal). Shane Shapera: Data curation (equal); resources (equal); writing – review and editing (equal). Teresa To: Data curation (equal); resources (equal); writing – review and editing (equal). Pearce Wilcox: Data curation (equal); resources (equal); writing – review and editing (equal). Christopher J. Ryerson: Conceptualization (supporting); data curation (equal); formal analysis (supporting); resources (equal); supervision (equal); writing – review and editing (equal). Kerri A. Johannson: Conceptualization (lead); data curation (equal); formal analysis (equal); resources (equal); supervision (lead); writing – review and editing (equal).
HUMAN ETHICS APPROVAL DECLARATION
This study was approved by all CARE-PF centres with the coordinating centre at the University of British Columbia (REB-H20-00191). Informed consent was received from all patients.
Open Research
DATA AVAILABILITY STATEMENT
The data are not publicly available due to privacy or ethical restrictions.




